Therapeutic Potential of Chlorogenic Acid in Chemoresistance and Chemoprotection in Cancer Treatment
Abstract
:1. Introduction
2. Methodology
3. Functional Foods High in Chlorogenic Acid
4. Biosynthesis of Chlorogenic Acid
5. Absorption, Distribution, and Metabolism of Chlorogenic Acid
6. Toxicological Profile of CGA
7. Role of Chlorogenic Acid in Chemoresistance
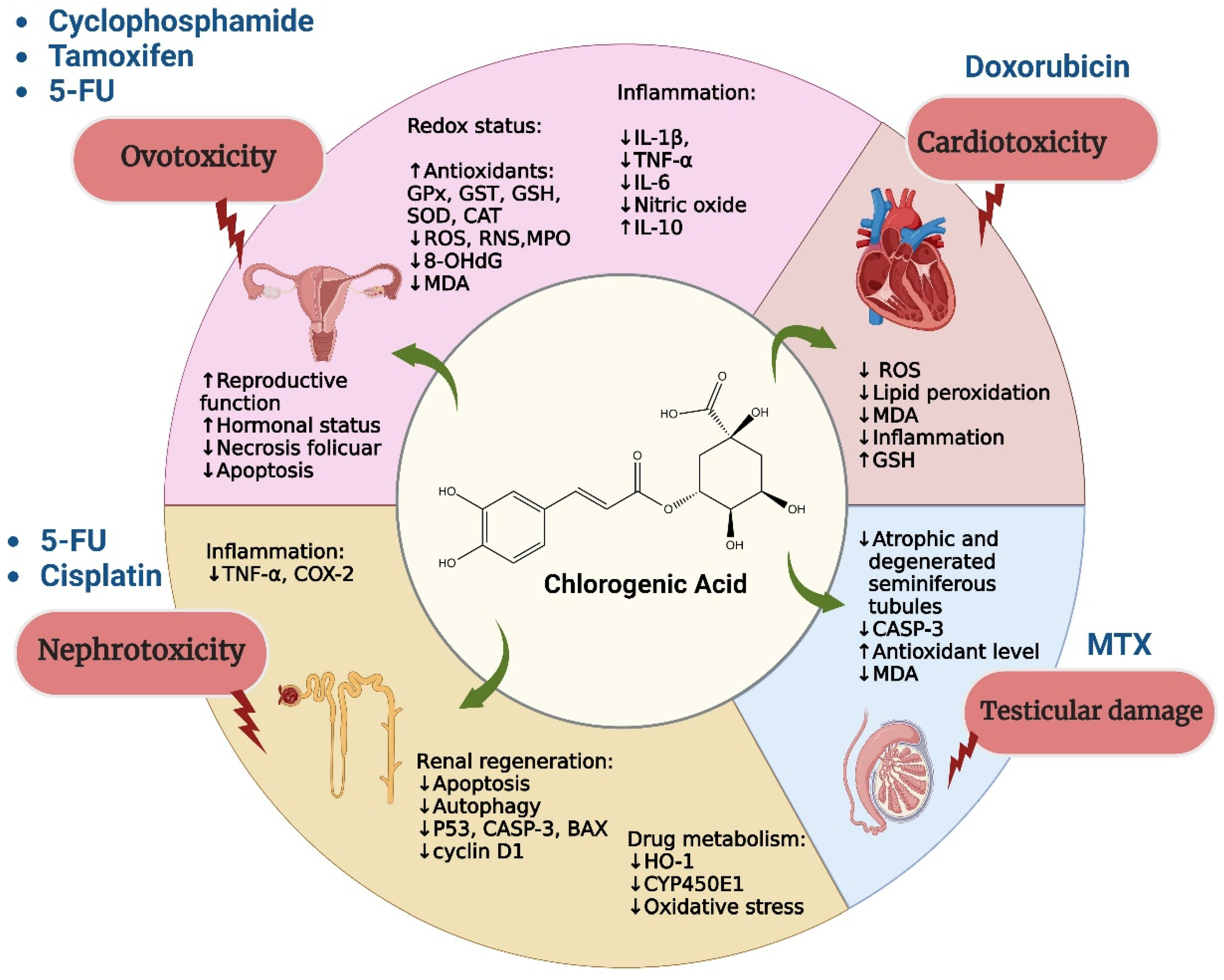
8. Protective Role of Chlorogenic Acid against Toxicity Induced by Chemotherapy
9. Adjuvant Role of Chlorogenic Acid in Radiotherapy
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar] [PubMed]
- Gupta, A.; Atanasov, A.G.; Li, Y.; Kumar, N.; Bishayee, A. Chlorogenic acid for cancer prevention and therapy: Current status on efficacy and mechanisms of action. Pharmacol. Res. 2022, 186, 106505. [Google Scholar] [CrossRef] [PubMed]
- Murai, T.; Matsuda, S. The Chemopreventive Effects of Chlorogenic Acids, Phenolic Compounds in Coffee, against Inflammation, Cancer, and Neurological Diseases. Molecules 2023, 28, 2381. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950–59964. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Boulikas, T.; Vougiouka, M. Recent clinical trials using cisplatin, carboplatin and their combination chemotherapy drugs (review). Oncol. Rep. 2004, 11, 559–595. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Orditura, M.; Galizia, G.; Sforza, V.; Gambardella, V.; Fabozzi, A.; Laterza, M.M.; Andreozzi, F.; Ventriglia, J.; Savastano, B.; Mabilia, A.; et al. Treatment of gastric cancer. World J. Gastroenterol. 2014, 20, 1635–1649. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Livshits, Z.; Rao, R.B.; Smith, S.W. An approach to chemotherapy-associated toxicity. Emerg. Med. Clin. N. Am. 2014, 32, 167–203. [Google Scholar] [CrossRef]
- Cleeland, C.S.; Allen, J.D.; Roberts, S.A.; Brell, J.M.; Giralt, S.A.; Khakoo, A.Y.; Kirch, R.A.; Kwitkowski, V.E.; Liao, Z.; Skillings, J. Reducing the toxicity of cancer therapy: Recognizing needs, taking action. Nat. Rev. Clin. Oncol. 2012, 9, 471–478. [Google Scholar] [CrossRef]
- Domitrović, R.; Cvijanović, O.; Šušnić, V.; Katalinić, N. Renoprotective mechanisms of chlorogenic acid in cisplatin-induced kidney injury. Toxicology 2014, 324, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Hassin, O.; Oren, M. Drugging p53 in cancer: One protein, many targets. Nat. Rev. Drug Discov. 2023, 22, 127–144. [Google Scholar] [CrossRef]
- Badr, N.M.; McMurray, J.L.; Danial, I.; Hayward, S.; Asaad, N.Y.; Abd El-Wahed, M.M.; Abdou, A.G.; Serag El-Dien, M.M.; Sharma, N.; Horimoto, Y.; et al. Characterization of the Immune Microenvironment in Inflammatory Breast Cancer Using Multiplex Immunofluorescence. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2023, 90, 31–43. [Google Scholar] [CrossRef]
- Lee, S.; Shin, S.; Kim, H.; Han, S.; Kim, K.; Kwon, J.; Kwak, J.H.; Lee, C.K.; Ha, N.J.; Yim, D.; et al. Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF-κB pathways. J. Inflamm. 2011, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Salama, S.A.; Maghrabi, I.A. Camel Milk Ameliorates 5-Fluorouracil-Induced Renal Injury in Rats: Targeting MAPKs, NF-κB and PI3K/Akt/eNOS Pathways. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 46, 1628–1642. [Google Scholar] [CrossRef]
- Mentese, A.; DemİR, S.; TÜRkmen, N.; AlİYazicioĞLu, Y.; DeĞEr, O. The effect of chlorogenic acid on 5-fluorouracil-induced oxidative damage in rat ovarian tissue. Farabi Tıp Derg. 2022, 1, 1–7. [Google Scholar]
- Rashid, N.; Koh, H.A.; Baca, H.C.; Lin, K.J.; Malecha, S.E.; Masaquel, A. Economic burden related to chemotherapy-related adverse events in patients with metastatic breast cancer in an integrated health care system. Breast Cancer 2016, 8, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Sultana, S. Chlorogenic acid represses 5-fluorouracil induced renal oxidative stress, apoptosis and inflammation in murine model. Free Radic. Biol. Med. 2016, 100, S128–S129. [Google Scholar] [CrossRef]
- Li, R.; Zhan, Y.; Ding, X.; Cui, J.; Han, Y.; Zhang, J.; Zhang, J.; Li, W.; Wang, L.; Jiang, J. Cancer Differentiation Inducer Chlorogenic Acid Suppresses PD-L1 Expression and Boosts Antitumor Immunity of PD-1 Antibody. Int. J. Biol. Sci. 2024, 20, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Thakur, K.; Sharma, V.; Saini, M.; Sharma, D.; Vishwas, S.; Kakoty, V.; Pal, R.S.; Chaitanya, M.V.N.L.; Babu, M.R.; et al. Exploring the multifaceted potential of chlorogenic acid: Journey from nutraceutical to nanomedicine. S. Afr. J. Bot. 2023, 159, 658–677. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Terashima, H.; Aizawa, S.; Taga, A.; Yamamoto, A.; Tsutsumiuchi, K.; Kodama, S. Simultaneous Determination of Trigonelline, Caffeine, Chlorogenic Acid and Their Related Compounds in Instant Coffee Samples by HPLC Using an Acidic Mobile Phase Containing Octanesulfonate. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2015, 31, 831–835. [Google Scholar] [CrossRef]
- Heck, C.I.; Schmalko, M.; Gonzalez de Mejia, E. Effect of growing and drying conditions on the phenolic composition of mate teas (Ilex paraguariensis). J. Agric. Food Chem. 2008, 56, 8394–8403. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.M.; Muzquiz, M.; Garcia-Vallejo, C.; Burbano, C.; Cuadrado, C.; Ayet, G.; Robredo, L.M. Determination of caffeic and chlorogenic acids and their derivatives in different sunflower seeds. J. Sci. Food Agric. 2000, 80, 459–464. [Google Scholar] [CrossRef]
- Sasaki, K.; Oki, T.; Kobayashi, T.; Kai, Y.; Okuno, S. Single-laboratory validation for the determination of caffeic acid and seven caffeoylquinic acids in sweet potato leaves. Biosci. Biotechnol. Biochem. 2014, 78, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; de Jesus Filho, M.; da Silva, L.C.; da Silva Constant, L.; Teixeira Filho, J.; Wagner, R.; Teixeira Godoy, H. Study of new sources of six chlorogenic acids and caffeic acid. J. Food Compos. Anal. 2019, 82, 103244. [Google Scholar] [CrossRef]
- Singh, S.; Singh, B.; Alam, T. Evaluation of shelf-life, antioxidant activity and nutritional quality attributes in carnauba wax coated eggplant genotypes. J. Food Sci. Technol. 2019, 56, 4826–4833. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Simon, P.W.; Tanumihardjo, S.A. Antioxidant phytochemicals and antioxidant capacity of biofortified carrots (Daucus carota L.) of various colors. J. Agric. Food Chem. 2009, 57, 4142–4147. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, E. The influence of organic and conventional cultivation systems on the nutritional value and content of bioactive compounds in selected tomato types. J. Sci. Food Agric. 2012, 92, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
- Mehari, B.; Redi-Abshiro, M.; Chandravanshi, B.S.; Atlabachew, M.; Combrinck, S.; McCrindle, R. Simultaneous determination of alkaloids in green coffee beans from Ethiopia: Chemometric evaluation of geographical origin. Food Anal. Methods 2016, 9, 1627–1637. [Google Scholar] [CrossRef]
- Gebrekidan, M.; Redi-Abshiro, M.; Chandravanshi, B.; Ele, E.; Mohammed, A.; Mamo, M. Influence of altitudes of coffee plants on the alkaloids contents of green coffee beans. Chem. Int. 2019, 5, 247–257. [Google Scholar] [CrossRef]
- Macheiner, L.; Schmidt, A.; Schreiner, M.; Mayer, H.K. Green coffee infusion as a source of caffeine and chlorogenic acid. J. Food Compos. Anal. 2019, 84, 103307. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Sentkowska, A.; Pyrzyńska, K.; De Peña, M.P. Chlorogenic acids, caffeine content and antioxidant properties of green coffee extracts: Influence of green coffee bean preparation. Eur. Food Res. Technol. 2016, 242, 1403–1409. [Google Scholar] [CrossRef]
- Bobková, A.; Jakabová, S.; Belej, Ľ.; Jurčaga, L.; Čapla, J.; Bobko, M.; Demianová, A. Analysis of caffeine and chlorogenic acids content regarding the preparation method of coffee beverage. Int. J. Food Eng. 2021, 17, 403–410. [Google Scholar] [CrossRef]
- Ishida, K.; Yamamoto, M.; Misawa, K.; Nishimura, H.; Misawa, K.; Ota, N.; Shimotoyodome, A. Coffee polyphenols prevent cognitive dysfunction and suppress amyloid β plaques in APP/PS2 transgenic mouse. Neurosci. Res. 2020, 154, 35–44. [Google Scholar] [CrossRef]
- Lee, A.H.; Tan, L.; Hiramatsu, N.; Ishisaka, A.; Alfonso, H.; Tanaka, A.; Uemura, N.; Fujiwara, Y.; Takechi, R. Plasma concentrations of coffee polyphenols and plasma biomarkers of diabetes risk in healthy Japanese women. Nutr. Diabetes 2016, 6, e212. [Google Scholar] [CrossRef]
- Miranda, A.M.; Steluti, J.; Fisberg, R.M.; Marchioni, D.M. Association between Coffee Consumption and Its Polyphenols with Cardiovascular Risk Factors: A Population-Based Study. Nutrients 2017, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, T.F.F.; Meinhart, A.D.; de Souza, T.C.L.; Cunha, E.C.E.; de Moraes, M.R.; Filho, J.T.; Godoy, H.T. Optimization of the Preparation Conditions of Yerba Mate tea Beverage to Maximize Chlorogenic Acids Extraction. Plant Foods Hum. Nutr. 2017, 72, 219–223. [Google Scholar] [CrossRef] [PubMed]
- de Mejía, E.G.; Song, Y.S.; Heck, C.I.; Ramírez-Mares, M. Yerba mate tea (Ilex paraguariensis): Phenolics, antioxidant capacity and in vitro inhibition of colon cancer cell proliferation. J. Funct. Foods 2010, 2, 23–34. [Google Scholar] [CrossRef]
- Macedo, J.A.; Battestin, V.; Ribeiro, M.L.; Macedo, G.A. Increasing the antioxidant power of tea extracts by biotransformation of polyphenols. Food Chem. 2011, 126, 491–497. [Google Scholar] [CrossRef]
- Boaventura, B.C.B.; Di Pietro, P.F.; Klein, G.A.; Stefanuto, A.; de Morais, E.C.; de Andrade, F.; Wazlawik, E.; da Silva, E.L. Antioxidant potential of mate tea (Ilex paraguariensis) in type 2 diabetic mellitus and pre-diabetic individuals. J. Funct. Foods 2013, 5, 1057–1064. [Google Scholar] [CrossRef]
- Arçari, D.P.; Porto, V.B.; Rodrigues, E.R.V.; Martins, F.; Lima, R.J.d.; Sawaya, A.C.H.F.; Ribeiro, M.L.; Carvalho, P.d.O. Effect of mate tea (Ilex paraguariensis) supplementation on oxidative stress biomarkers and LDL oxidisability in normo- and hyperlipidaemic humans. J. Funct. Foods 2011, 3, 190–197. [Google Scholar] [CrossRef]
- Gastélum-Estrada, A.; Rabadán-Chávez, G.; Reza-Zaldívar, E.E.; de la Cruz-López, J.L.; Fuentes-Palma, S.A.; Mojica, L.; Díaz de la Garza, R.I.; Jacobo-Velázquez, D.A. Biofortified Beverage with Chlorogenic Acid from Stressed Carrots: Anti-Obesogenic, Antioxidant, and Anti-Inflammatory Properties. Foods 2023, 12, 3959. [Google Scholar] [CrossRef] [PubMed]
- Viacava, F.; Santana-Gálvez, J.; Heredia-Olea, E.; Pérez-Carrillo, E.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Sequential application of postharvest wounding stress and extrusion as an innovative tool to increase the concentration of free and bound phenolics in carrots. Food Chem. 2020, 307, 125551. [Google Scholar] [CrossRef]
- Szczepańska, J.; Barba, F.J.; Skąpska, S.; Marszałek, K. High pressure processing of carrot juice: Effect of static and multi-pulsed pressure on the polyphenolic profile, oxidoreductases activity and colour. Food Chem. 2020, 307, 125549. [Google Scholar] [CrossRef]
- Viacava, F.; Ramos-Parra, P.A.; Welti-Chanes, J.; Jacobo-Velázquez, D.A. High Hydrostatic Pressure Processing of Whole Carrots: Effect of Static and Multi-Pulsed Mild Intensity Hydrostatic Pressure Treatments on Bioactive Compounds. Foods 2021, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velázquez, D.A. Transformation of carrots into novel food ingredients and innovative healthy foods. Appl. Food Res. 2023, 3, 100303. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Typek, R. Thermal stability of 5-o-caffeoylquinic acid in aqueous solutions at different heating conditions. J. Agric. Food Chem. 2010, 58, 12578–12584. [Google Scholar] [CrossRef] [PubMed]
- Pollini, L.; Ianni, F.; Verducci, G.; Blasi, F.; Cossignani, L. Is the household microwave recommended to obtain antioxidant-rich extracts from Lycium barbarum leaves? Processes 2021, 9, 656. [Google Scholar] [CrossRef]
- Ianni, F.; Barola, C.; Blasi, F.; Moretti, S.; Galarini, R.; Cossignani, L. Investigation on chlorogenic acid stability in aqueous solution after microwave treatment. Food Chem. 2022, 374, 131820. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Typek, R. Transformation of 5-O-caffeoylquinic acid in blueberries during high-temperature processing. J. Agric. Food Chem. 2014, 62, 10889–10895. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Typek, R. Transformation of chlorogenic acids during the coffee beans roasting process. Eur. Food Res. Technol. 2017, 243, 379–390. [Google Scholar] [CrossRef]
- Mangiapelo, L.; Blasi, F.; Ianni, F.; Barola, C.; Galarini, R.; Abualzulof, G.W.; Sardella, R.; Volpi, C.; Cossignani, L. Optimization of Ultrasound-Assisted Extraction of Chlorogenic Acid from Potato Sprout Waste and Enhancement of the In Vitro Total Antioxidant Capacity. Antioxidants 2023, 12, 348. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Su, J.Y.; Yang, C.Y. Ultrasound-Assisted Aqueous Extraction of Chlorogenic Acid and Cynarin with the Impact of Inulin from Burdock (Arctium lappa L.) Roots. Antioxidants 2022, 11, 1219. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Arab. Book 2011, 9, e0152. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Escamilla-Treviño, L.L.; Shen, H.; Hernandez, T.; Yin, Y.; Xu, Y.; Dixon, R.A. Early lignin pathway enzymes and routes to chlorogenic acid in switchgrass (Panicum virgatum L.). Plant Mol. Biol. 2014, 84, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Valiñas, M.A.; Lanteri, M.L.; ten Have, A.; Andreu, A.B. Chlorogenic Acid Biosynthesis Appears Linked with Suberin Production in Potato Tuber (Solanum tuberosum). J. Agric. Food Chem. 2015, 63, 4902–4913. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Abdullah, Y.; Benner, J.; Eberle, D.; Gehlen, K.; Hücherig, S.; Janiak, V.; Kim, K.H.; Sander, M.; Weitzel, C.; et al. Evolution of rosmarinic acid biosynthesis. Phytochemistry 2009, 70, 1663–1679. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lu, S. Biosynthesis and regulation of phenylpropanoids in plants. Crit. Rev. Plant Sci. 2017, 36, 257–290. [Google Scholar] [CrossRef]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Trugo, L.C. Chlorogenic Acid Isomers from Coffee Are Differentially Uptaken by HEPG2 Human Hepatocite Cell Line. In Proceedings of the 21st International Conference on Coffee Science, Montpellier, France, 11–15 September 2006; pp. 101–104. [Google Scholar]
- de Oliveira, D.M.; Sampaio, G.R.; Pinto, C.B.; Catharino, R.R.; Bastos, D.H.M. Bioavailability of chlorogenic acids in rats after acute ingestion of maté tea (Ilex paraguariensis) or 5-caffeoylquinic acid. Eur. J. Nutr. 2017, 56, 2541–2556. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.H.; Mullen, W.; Stalmach, A.; Auger, C.; Rouanet, J.M.; Teissedre, P.L.; Caldwell, S.T.; Hartley, R.C.; Crozier, A. Absorption, disposition, metabolism, and excretion of [3-(14)C]caffeic acid in rats. J. Agric. Food Chem. 2012, 60, 5205–5214. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishna, K.; Sudeep, H.V.; Shyamprasad, K. Acute and sub-chronic toxicity evaluation of a standardized green coffee bean extract (CGA-7™) in Wistar albino rats. SAGE Open Med. 2021, 9, 2050312120984885. [Google Scholar]
- Pereira, E.D.M.; da Silva, J.; Carvalho, P.D.S.; Grivicich, I.; Picada, J.N.; Salgado Júnior, I.B.; Vasques, G.J.; Pereira, M.; Reginatto, F.H.; Ferraz, A.B.F. In vivo and in vitro toxicological evaluations of aqueous extract from Cecropia pachystachya leaves. J. Toxicol. Environ. Health Part A 2020, 83, 659–671. [Google Scholar] [CrossRef]
- Bagdas, D.; Etoz, B.C.; Gul, Z.; Ziyanok, S.; Inan, S.; Turacozen, O.; Gul, N.Y.; Topal, A.; Cinkilic, N.; Tas, S.; et al. In vivo systemic chlorogenic acid therapy under diabetic conditions: Wound healing effects and cytotoxicity/genotoxicity profile. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 81, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nagae, Y.; Li, J.; Sakaba, H.; Mozawa, K.; Takahashi, A.; Shimizu, H. The micronucleus test and erythropoiesis. Effects of erythropoietin and a mutagen on the ratio of polychromatic to normochromatic erythrocytes (P/N ratio). Mutagenesis 1989, 4, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Sudeep, H.V.; Shyam Prasad, K. Supplementation of green coffee bean extract in healthy overweight subjects increases lean mass/fat mass ratio: A randomized, double-blind clinical study. SAGE Open Med. 2021, 9, 20503121211002590. [Google Scholar] [CrossRef] [PubMed]
- Castellino, G.; Nikolic, D.; Magán-Fernández, A.; Malfa, G.A.; Chianetta, R.; Patti, A.M.; Amato, A.; Montalto, G.; Toth, P.P.; Banach, M.; et al. Altilix(®) Supplement Containing Chlorogenic Acid and Luteolin Improved Hepatic and Cardiometabolic Parameters in Subjects with Metabolic Syndrome: A 6 Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2580. [Google Scholar] [CrossRef] [PubMed]
- Gatti, L.; Zunino, F. Overview of tumor cell chemoresistance mechanisms. Methods Mol. Med. 2005, 111, 127–148. [Google Scholar] [PubMed]
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The challenge of drug resistance in cancer treatment: A current overview. Clin. Exp. Metastasis 2018, 35, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Alsayed, A.R.; Barakat, M.; Abu-Taha, M.I.; Mahmod, A.I. Targeting Drug Chemo-Resistance in Cancer Using Natural Products. Biomedicines 2021, 9, 1353. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef]
- Mazari, A.M.A.; Zhang, L.; Ye, Z.W.; Zhang, J.; Tew, K.D.; Townsend, D.M. The Multifaceted Role of Glutathione S-Transferases in Health and Disease. Biomolecules 2023, 13, 688. [Google Scholar] [CrossRef]
- Guillemette, C.; Lévesque, É.; Rouleau, M. Pharmacogenomics of human uridine diphospho-glucuronosyltransferases and clinical implications. Clin. Pharmacol. Ther. 2014, 96, 324–339. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Sadeghi, S.; Tabatabaeian, H. Battling Chemoresistance in Cancer: Root Causes and Strategies to Uproot Them. Int. J. Mol. Sci. 2021, 22, 9451. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Hannan, M.A.; Dash, R.; Rahman, M.H.; Islam, R.; Uddin, M.J.; Sohag, A.A.M.; Rahman, M.H.; Rhim, H. Phytochemicals as a Complement to Cancer Chemotherapy: Pharmacological Modulation of the Autophagy-Apoptosis Pathway. Front. Pharmacol. 2021, 12, 639628. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Santos, M. Estimativa 2018: Incidência de câncer no Brasil. Rev. Bras. Cancerol. 2018, 64, 119–120. [Google Scholar] [CrossRef]
- Yan, Y.; Li, J.; Han, J.; Hou, N.; Song, Y.; Dong, L. Chlorogenic acid enhances the effects of 5-fluorouracil in human hepatocellular carcinoma cells through the inhibition of extracellular signal-regulated kinases. Anti-Cancer Drugs 2015, 26, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Salzillo, A.; Ragone, A.; Spina, A.; Naviglio, S.; Sapio, L. Chlorogenic Acid Enhances Doxorubicin-Mediated Cytotoxic Effect in Osteosarcoma Cells. Int. J. Mol. Sci. 2021, 22, 8586. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.E. The extracellular signal-regulated kinase (ERK) pathway: A potential therapeutic target in hypertension. J. Exp. Pharmacol. 2012, 4, 77–83. [Google Scholar] [CrossRef]
- Abd Elrazik, N.A.; El-Mesery, M.; El-Karef, A.; Eissa, L.A.; El Gayar, A.M. Chlorogenic acid potentiates antitumor effect of doxorubicin through upregulation of death receptors in solid Ehrlich carcinoma model in mice. Egypt. J. Basic Appl. Sci. 2019, 6, 158–172. [Google Scholar] [CrossRef]
- Willms, A.; Schupp, H.; Poelker, M.; Adawy, A.; Debus, J.F.; Hartwig, T.; Krichel, T.; Fritsch, J.; Singh, S.; Walczak, H.; et al. TRAIL-receptor 2—A novel negative regulator of p53. Cell Death Dis. 2021, 12, 757. [Google Scholar] [CrossRef] [PubMed]
- Volpe, E.; Sambucci, M.; Battistini, L.; Borsellino, G. Fas-Fas Ligand: Checkpoint of T Cell Functions in Multiple Sclerosis. Front. Immunol. 2016, 7, 382. [Google Scholar] [CrossRef] [PubMed]
- Price, S.A.; Roff, S.R.; Schwartz, J.A.; Chilton, J.A. Chapter 22—Immunohistochemistry for the non-human primate. In Spontaneous Pathology of the Laboratory Non-Human Primate; Bradley, A.E., Chilton, J.A., Mahler, B.W., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 553–586. [Google Scholar]
- Shaltiel, G.; Chen, G.; Manji, H.K. Neurotrophic signaling cascades in the pathophysiology and treatment of bipolar disorder. Curr. Opin. Pharmacol. 2007, 7, 22–26. [Google Scholar] [CrossRef]
- Lukitasari, M.; Nugroho, D.A.; Widodo, N. Chlorogenic Acid: The Conceivable Chemosensitizer Leading to Cancer Growth Suppression. J. Evid. Based Integr. Med. 2018, 23, 2515690X18789628. [Google Scholar] [CrossRef]
- Refolo, M.G.; Lippolis, C.; Carella, N.; Cavallini, A.; Messa, C.; D’Alessandro, R. Chlorogenic Acid Improves the Regorafenib Effects in Human Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2018, 19, 1518. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Fondevila, F.; Méndez-Blanco, C.; Fernández-Palanca, P.; González-Gallego, J.; Mauriz, J.L. Anti-tumoral activity of single and combined regorafenib treatments in preclinical models of liver and gastrointestinal cancers. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Stefani, C.; Miricescu, D.; Stanescu, S., II; Nica, R.I.; Greabu, M.; Totan, A.R.; Jinga, M. Growth Factors, PI3K/AKT/mTOR and MAPK Signaling Pathways in Colorectal Cancer Pathogenesis: Where Are We Now? Int. J. Mol. Sci. 2021, 22, 10260. [Google Scholar] [CrossRef]
- Catanzaro, D.; Filippini, R.; Vianello, C.; Carrara, M.; Ragazzi, E.; Montopoli, M. Chlorogenic Acid Interaction with Cisplatin and Oxaliplatin: Studies in Cervical Carcinoma Cells. Nat. Prod. Commun. 2016, 11, 499–502. [Google Scholar] [CrossRef]
- Abraham, S.K.; Sarma, L.; Kesavan, P.C. Protective effects of chlorogenic acid, curcumin and beta-carotene against gamma-radiation-induced in vivo chromosomal damage. Mutat. Res. 1993, 303, 109–112. [Google Scholar] [CrossRef]
- Chlopcíková, S.; Psotová, J.; Miketová, P.; Sousek, J.; Lichnovský, V.; Simánek, V. Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes. Part II. caffeic, chlorogenic and rosmarinic acids. Phytother. Res. PTR 2004, 18, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Iyama, K.; Ikeda, K.; Ishikawa, S.; Hayashi, N.; Miyanari, N.; Honda, Y.; Sado, Y.; Ninomiya, Y.; Baba, H. Differential expression of basement membrane type IV collagen alpha chains in gastric intramucosal neoplastic lesions. J. Gastroenterol. 2007, 42, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Bhattacharjee, A.; Samanta, A.; Bhattacharya, S. Prevention of cyclophosphamide-induced hepatotoxicity and genotoxicity: Effect of an L-cysteine based oxovanadium(IV) complex on oxidative stress and DNA damage. Environ. Toxicol. Pharmacol. 2015, 40, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Fang, B.; Hao, K.; Wang, H.; Zhang, L.; Wang, M.; Hao, Y.; Wang, X.; Wang, Q.; Yang, W. Combined effects of exposure to polycyclic aromatic hydrocarbons and metals on oxidative stress among healthy adults in Caofeidian, China. Ecotoxicol. Environ. Saf. 2022, 230, 113168. [Google Scholar] [CrossRef] [PubMed]
- AlkiŞ, İ.; Suat, E.; Yildirim, S.; Bakir, A.; Gizem, E.; Firat, M. Evaluation of the protective effect of chlorogenic acid and Rhabdosciadium anatolyi against cyclophosphamide-induced ovarian toxicity in the rat with histopathological and immunohistochemical findings. Kafkas Üniversitesi Vet. Fakültesi Derg. 2020, 26, 757–763. [Google Scholar]
- Kazaz, I.O.; Demir, S.; Kerimoglu, G.; Colak, F.; Turkmen Alemdar, N.; Yilmaz Dogan, S.; Bostan, S.; Mentese, A. Chlorogenic acid ameliorates torsion/detorsion-induced testicular injury via decreasing endoplasmic reticulum stress. J. Pediatr. Urol. 2022, 18, 289.e1–289.e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, G.; Liu, H.; Hou, Y.L. Protective effect of bioactive compounds from Lonicera japonica Thunb. against H2O2-induced cytotoxicity using neonatal rat cardiomyocytes. Iran. J. Basic Med. Sci. 2016, 19, 97–105. [Google Scholar] [PubMed]
- Hatse, S.; De Clercq, E.; Balzarini, J. Role of antimetabolites of purine and pyrimidine nucleotide metabolism in tumor cell differentiation. Biochem. Pharmacol. 1999, 58, 539–555. [Google Scholar] [CrossRef]
- Chabner, B.A.; Amrein, P.C.; Michaelson, M.D.; Mitsiades, C.S.; Goss, P.E.; Ryan, D.P.; Ramachandra, S.; Richardson, P.G.; Supko, J.G. Antineoplastic agents. In The Pharmacological Basis of Therapeutics 9/e; McGRAW-HILL: New York, NY, USA, 2006; pp. 1315–1465. [Google Scholar]
- Gamelin, E.; Boisdron-Celle, M.; Guérin-Meyer, V.; Delva, R.; Lortholary, A.; Genevieve, F.; Larra, F.; Ifrah, N.; Robert, J. Correlation between uracil and dihydrouracil plasma ratio, fluorouracil (5-FU) pharmacokinetic parameters, and tolerance in patients with advanced colorectal cancer: A potential interest for predicting 5-FU toxicity and determining optimal 5-FU dosage. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999, 17, 1105. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, G.; Yang, G.Y.; Wang, D.; Luo, X.; Chen, C.; Zhang, Z.; Li, Q.; Xu, W.; Li, Z.; et al. Induction chemotherapy with nedaplatin with 5-FU followed by intensity-modulated radiotherapy concurrent with chemotherapy for locoregionally advanced nasopharyngeal carcinoma. Jpn. J. Clin. Oncol. 2010, 40, 425–431. [Google Scholar] [CrossRef]
- Safwat, M.A.; Soliman, G.M.; Sayed, D.; Attia, M.A. Gold nanoparticles enhance 5-fluorouracil anticancer efficacy against colorectal cancer cells. Int. J. Pharm. 2016, 513, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Mentese, A.; Alemdar, N.T.; Livaoglu, A.; Ayazoglu Demir, E.; Aliyazicioglu, Y.; Demir, S. Suppression of cisplatin-induced ovarian injury in rats by chrysin: An experimental study. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2022, 42, 3584–3590. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Ali, N.; Nafees, S.; Ahmad, S.T.; Hasan, S.K.; Sultana, S. Abrogation of 5-flourouracil induced renal toxicity by bee propolis via targeting oxidative stress and inflammation in Wistar rats. J. Pharm. Res. 2013, 7, 189–194. [Google Scholar] [CrossRef]
- Ayazoglu Demir, E.; Mentese, A.; Kucuk, H.; Turkmen Alemdar, N.; Demir, S. The therapeutic effect of silibinin against 5-fluorouracil-induced ovarian toxicity in rats. J. Biochem. Mol. Toxicol. 2023, 37, e23408. [Google Scholar] [CrossRef] [PubMed]
- Du, X.F.; Zhang, L.L.; Zhang, D.Z.; Yang, L.; Fan, Y.Y.; Dong, S.P. Clinical significance of serum total oxidant/antioxidant status in patients with operable and advanced gastric cancer. OncoTargets Ther. 2018, 11, 6767–6775. [Google Scholar] [CrossRef]
- Costi, M.P.; Ferrari, S. Update on antifolate drugs targets. Curr. Drug Targets 2001, 2, 135–166. [Google Scholar] [CrossRef]
- Ali, N.; Rashid, S.; Nafees, S.; Hasan, S.K.; Shahid, A.; Majed, F.; Sultana, S. Protective effect of Chlorogenic acid against methotrexate induced oxidative stress, inflammation and apoptosis in rat liver: An experimental approach. Chem. Biol. Interact. 2017, 272, 80–91. [Google Scholar] [CrossRef]
- Vardi, N.; Parlakpinar, H.; Ates, B. Beneficial effects of chlorogenic acid on methotrexate-induced cerebellar Purkinje cell damage in rats. J. Chem. Neuroanat. 2012, 43, 43–47. [Google Scholar] [CrossRef]
- Minhas, R.; Bansal, Y.; Bansal, G. Inducible nitric oxide synthase inhibitors: A comprehensive update. Med. Res. Rev. 2020, 40, 823–855. [Google Scholar] [CrossRef]
- An, H.K.; Chung, K.M.; Park, H.; Hong, J.; Gim, J.E.; Choi, H.; Lee, Y.W.; Choi, J.; Mun, J.Y.; Yu, S.W. CASP9 (caspase 9) is essential for autophagosome maturation through regulation of mitochondrial homeostasis. Autophagy 2020, 16, 1598–1617. [Google Scholar] [CrossRef]
- Volarevic, V.; Djokovic, B.; Jankovic, M.G.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N. Molecular mechanisms of cisplatin-induced nephrotoxicity: A balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 2019, 26, 25. [Google Scholar] [CrossRef] [PubMed]
- Chválová, K.; Brabec, V.; Kaspárková, J. Mechanism of the formation of DNA-protein cross-links by antitumor cisplatin. Nucleic Acids Res. 2007, 35, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Berndtsson, M.; Hägg, M.; Panaretakis, T.; Havelka, A.M.; Shoshan, M.C.; Linder, S. Acute apoptosis by cisplatin requires induction of reactive oxygen species but is not associated with damage to nuclear DNA. Int. J. Cancer 2007, 120, 175–180. [Google Scholar] [CrossRef]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Zatloukal, P.; Roubec, J.; Floor, K.; Musil, J.; Kuta, M.; van Klaveren, R.J.; Chaudhary, S.; Gunther, A.; Shamsili, S. Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 4481–4486. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.J.; Park, N.; Kwon, Y.J.; Ye, D.J.; Moon, A.; Chun, Y.J. Role of annexin A5 in cisplatin-induced toxicity in renal cells: Molecular mechanism of apoptosis. J. Biol. Chem. 2014, 289, 2469–2481. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Lopes, F.; Gourley, C.; Anderson, R.A.; Spears, N. Cisplatin and doxorubicin induce distinct mechanisms of ovarian follicle loss; imatinib provides selective protection only against cisplatin. PLoS ONE 2013, 8, e70117. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.M.; Lim, E.; Yoon, S.; Jeong, K.; Bae, S.; Lee, D.R.; Yoon, T.K.; Choi, Y.; Lee, W.S. Cisplatin Induces Overactivation of the Dormant Primordial Follicle through PTEN/AKT/FOXO3a Pathway which Leads to Loss of Ovarian Reserve in Mice. PLoS ONE 2015, 10, e0144245. [Google Scholar] [CrossRef] [PubMed]
- Ayazoglu Demir, E.; Mentese, A.; Livaoglu, A.; Turkmen Alemdar, N.; Aliyazicioglu, Y.; Demir, S. Chlorogenic acid attenuates cisplatin-induced ovarian injury in rats. Drug Chem. Toxicol. 2024, 47, 213–217. [Google Scholar] [CrossRef]
- Kusari, S.; Zühlke, S.; Spiteller, M. An endophytic fungus from Camptotheca acuminata that produces camptothecin and analogues. J. Nat. Prod. 2009, 72, 2–7. [Google Scholar] [CrossRef]
- Pommier, Y. Camptothecins and topoisomerase I: A foot in the door. Targeting the genome beyond topoisomerase I with camptothecins and novel anticancer drugs: Importance of DNA replication, repair and cell cycle checkpoints. Curr. Med. Chemistry. Anti-Cancer Agents 2004, 4, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Pizzolato, J.F.; Saltz, L.B. The camptothecins. Lancet 2003, 361, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- del Río, M.A.G.; González, J.B. Fármacos antineoplásicos (y II): Revisión. Farm. Prof. 2006, 20, 42–46. [Google Scholar]
- Noel, P.; Von Hoff, D.D.; Saluja, A.K.; Velagapudi, M.; Borazanci, E.; Han, H. Triptolide and its derivatives as cancer therapies. Trends Pharmacol. Sci. 2019, 40, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Chen, R.X.; Zhang, L.L.; Ding, N.N.; Liu, C.; Cui, Y.; Cheng, Y.X. In vivo protective effects of chlorogenic acid against triptolide-induced hepatotoxicity and its mechanism. Pharm. Biol. 2018, 56, 626–631. [Google Scholar] [CrossRef]
- Doyen, P.; Bigot, A.; Vasseur, P.; Rodius, F. Molecular cloning and expression study of pi-class glutathione S-transferase (pi-GST) and selenium-dependent glutathione peroxidase (Se-GPx) transcripts in the freshwater bivalve Dreissena polymorpha. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 147, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Ho, C.-T. Antioxidant Activities of Caffeic Acid and Its Related Hydroxycinnamic Acid Compounds. J. Agric. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Zang, L.Y.; Cosma, G.; Gardner, H.; Castranova, V.; Vallyathan, V. Effect of chlorogenic acid on hydroxyl radical. Mol. Cell. Biochem. 2003, 247, 205–210. [Google Scholar] [CrossRef]
- Kono, Y.; Shibata, H.; Kodama, Y.; Sawa, Y. The suppression of the N-nitrosating reaction by chlorogenic acid. Biochem. J. 1995, 312 Pt 3, 947–953. [Google Scholar] [CrossRef]
- Høyer-Hansen, M.; Jäättelä, M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007, 14, 1576–1582. [Google Scholar] [CrossRef]
- Zhong, J.T.; Yu, J.; Wang, H.J.; Shi, Y.; Zhao, T.S.; He, B.X.; Qiao, B.; Feng, Z.W. Effects of endoplasmic reticulum stress on the autophagy, apoptosis, and chemotherapy resistance of human breast cancer cells by regulating the PI3K/AKT/mTOR signaling pathway. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2017, 39, 1010428317697562. [Google Scholar] [CrossRef] [PubMed]
- Foufelle, F.; Fromenty, B. Role of endoplasmic reticulum stress in drug-induced toxicity. Pharmacol. Res. Perspect. 2016, 4, e00211. [Google Scholar] [CrossRef] [PubMed]
- Komeili-Movahhed, T.; Heidari, F.; Moslehi, A. Chlorogenic acid alleviated testicular inflammation and apoptosis in tunicamycin induced endoplasmic reticulum stress. Physiol. Int. 2023, 110, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Nowsheen, S.; Aziz, K.; Georgakilas, A.G. Toxicity and adverse effects of Tamoxifen and other anti-estrogen drugs. Pharmacol. Ther. 2013, 139, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Solomon, V.R.; Lee, H. Chloroquine and its analogs: A new promise of an old drug for effective and safe cancer therapies. Eur. J. Pharmacol. 2009, 625, 220–233. [Google Scholar] [CrossRef] [PubMed]
- E Owumi, S.; K Olusola, J.; O Arunsi, U.; K Oyelere, A. Chlorogenic acid abates oxido-inflammatory and apoptotic responses in the liver and kidney of Tamoxifen-treated rats. Toxicol. Res. 2021, 10, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Anaikor, R.A.; Arunsi, U.O.; Adaramoye, O.A.; Oyelere, A.K. Chlorogenic acid co-administration abates tamoxifen-mediated reproductive toxicities in male rats: An experimental approach. J. Food Biochem. 2021, 45, e13615. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; de Biase, A.; Deeva, I.B.; Degan, P.; Doronin, Y.K.; Iaccarino, M.; Oral, R.; Trieff, N.M.; Warnau, M.; Korkina, L.G. The role of oxidative stress in developmental and reproductive toxicity of tamoxifen. Life Sci. 2001, 68, 1735–1749. [Google Scholar] [CrossRef]
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef]
- Hall, E.J. Radiation, the two-edged sword: Cancer risks at high and low doses. Cancer J. 2000, 6, 343–350. [Google Scholar] [PubMed]
- Cinkilic, N.; Cetintas, S.K.; Zorlu, T.; Vatan, O.; Yilmaz, D.; Cavas, T.; Tunc, S.; Ozkan, L.; Bilaloglu, R. Radioprotection by two phenolic compounds: Chlorogenic and quinic acid, on X-ray induced DNA damage in human blood lymphocytes in vitro. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 53, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Abedpour, N.; Zeinali, A.; Karimipour, M.; Pourheidar, B.; Farjah, G.H.; Abak, A.; Shoorei, H. Protective effects of chlorogenic acid against ionizing radiation-induced testicular toxicity. Heliyon 2022, 8, e10798. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Herranz, M.; Rafail, S.; Beghi, S.; Gil-de-Gómez, L.; Verginadis, I.; Bittinger, K.; Pustylnikov, S.; Pierini, S.; Perales-Linares, R.; Blair, I.A.; et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J. Clin. Investig. 2020, 130, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Filippone, E.J.; Kraft, W.K.; Farber, J.L. The Nephrotoxicity of Vancomycin. Clin. Pharmacol. Ther. 2017, 102, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Dai, C.; Hao, Z.; Tang, Q.; Wang, H.; Wang, J.; Zhao, H. Chlorogenic acid prevents vancomycin-induced nephrotoxicity without compromising vancomycin antibacterial properties. Phytother. Res. PTR 2020, 34, 3189–3199. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, L.; Kou, F.; Zhao, J.; Lei, J.; He, J. Targeted therapeutic effects of oral magnetically driven pectin nanoparticles containing chlorogenic acid on colon cancer. Particuology 2024, 84, 53–59. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Luo, Z.; Ding, M.; Shi, S.; Zhang, T. Combined immunochemotherapy achieving targeted co-delivery of chlorogenic acid and doxorubicin by sialic acid-modified liposomes enhances anti-cancer efficacy. Drug Deliv. Transl. Res. 2024, 14, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Neelakandan, M.; Manoharan, S.; Muralinaidu, R.; Thara, J.M. Tumor preventive and antioxidant efficacy of chlorogenic acid-loaded chitosan nanoparticles in experimental skin carcinogenesis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 533–546. [Google Scholar] [CrossRef]
- Alzahrani, B.; Elderdery, A.Y.; Alsrhani, A.; Alzerwi, N.A.N.; Althobiti, M.M.; Rayzah, M.; Idrees, B.; Elkhalifa, A.M.E.; Subbiah, S.K.; Mok, P.L. Effects of Albumin-Chlorogenic Acid Nanoparticles on Apoptosis and PI3K/Akt/mTOR Pathway Inhibitory Activity in MDA-MB-435s Cells. Nanomaterials 2023, 13, 1438. [Google Scholar] [CrossRef]
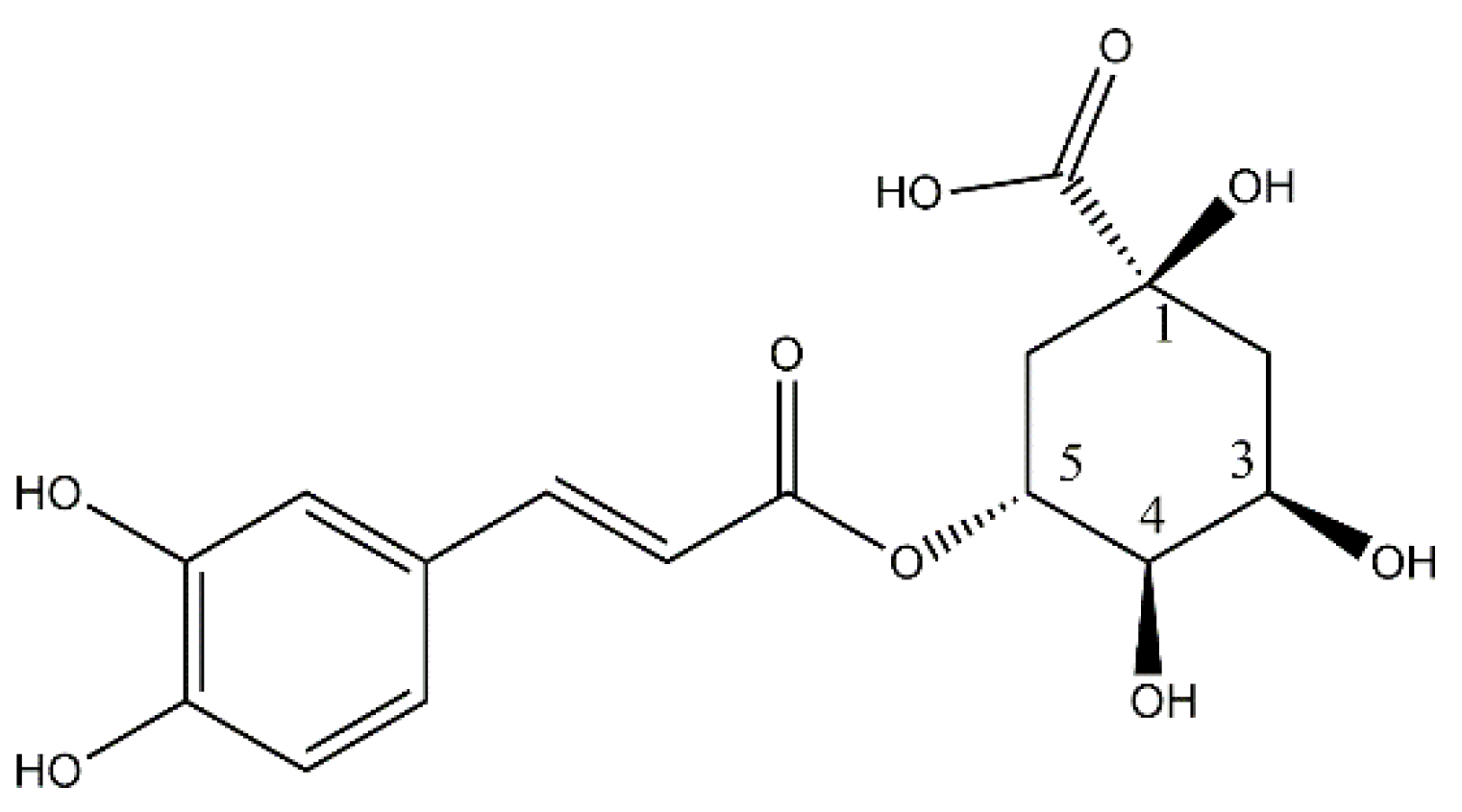


| Plant Source | CGA (mg/kg in dw) | References |
|---|---|---|
| Instant coffee | 2650–11,600 | [26] |
| Mate tea | 4800–24,900 | [27] |
| Sunflower seeds | 630–970 | [28] |
| Sweet potato leaves | 9600 | [29] |
| English potato 1 | 3.3–9 | [30] |
| Okra 1 | 3.9–21.6 | [30] |
| Eggplant | 4980–8050 | [31] |
| Carrot | 300–18,800 | [32] |
| Tomato | 200–400 | [33] |
| Aim | Treatment Conditions | Finding and Mechanisms of Action | References |
|---|---|---|---|
| To explore the influence of CGA on the effects of 5-fluorouracil (5-FU) on human hepatocellular carcinoma cells (HepG2 and Hep3B). | 250 μM CGA and 20 μM 5-FU for 4 h | CGA sensitizes hepatocellular carcinoma cells to 5-FU treatment by the suppression of ERK activation through the overproduction of ROS | [88] |
| To evaluate the antiproliferative effect of CGA in combination with cisplatin or oxaliplatin in cisplatin-sensitive (A431) human cervical carcinoma cell lines. | 10−6–10−4 M CGA and 1 μM cisplatin or 2 μM oxaliplatin for 24 h | Co-treatment with CGA at higher concentrations increased cisplatin activity compared to cisplatin alone. Conversely, lower concentrations of CGA enhanced the activity of oxaliplatin compared to the drug alone. | [101] |
| To examine the cooperating effects between CGA and doxorubicin (DOX) in U2OS and MG-63 human OS cells. | 200 μM CGA and 0.1 μM DOX for 48 h | Concomitant administration of CGA decreased cell viability and growth, promoting cell death potentially via apoptosis induction. CGA + doxorubicin caused a longer-lasting reduction in clonogenic potential. CGA increased inhibition of The ERK1/2 mitogen-activated protein kinase. | [89] |
| Evaluation of the effects of combined treatment using both low regorafenib concentrations and CGA as a natural compound in PLC/PRF/5 and HepG2 human (hepatocarcinoma cells). | 0.1 µM (PLC/PRF/5) or 1 µM (HepG2) and 100 µM of CGA | CGA enhanced regorafenib-mediated cell growth inhibition. CGA potentiated the apoptotic effect of regorafenib by the activation of the pro-apoptotic Annexin V, Bax and Caspase 3/7 and the inhibition of anti-apoptotic Bcl2 and Bcl-xL by inhibition of MAPK and PI3K/Akt/mTORC pathway. Combined treatments were also effective in inhibiting cell motility. | [97] |
| To evaluate the antitumor effect of CGA alone and in combination with doxorubicin in a solid Ehrlich carcinoma (SEC) model in mice. | CGA (60 mg/kg suspended in 0.5% CMC orally) and DOX (2 mg/kg/day, intraperitoneal) daily for 21 days. | CGA and/or DOX treatment showed a remarkable decrease in solid tumor volume and weight. CGA and/or DOX groups revealed upregulation in gene expressions of TRAIL/TRAILR2, FasL/Fas and caspase-3 genes and down-regulation of Bcl-2 gene expression. | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortez, N.; Villegas, C.; Burgos, V.; Ortiz, L.; Cabrera-Pardo, J.R.; Paz, C. Therapeutic Potential of Chlorogenic Acid in Chemoresistance and Chemoprotection in Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 5189. https://doi.org/10.3390/ijms25105189
Cortez N, Villegas C, Burgos V, Ortiz L, Cabrera-Pardo JR, Paz C. Therapeutic Potential of Chlorogenic Acid in Chemoresistance and Chemoprotection in Cancer Treatment. International Journal of Molecular Sciences. 2024; 25(10):5189. https://doi.org/10.3390/ijms25105189
Chicago/Turabian StyleCortez, Nicole, Cecilia Villegas, Viviana Burgos, Leandro Ortiz, Jaime R. Cabrera-Pardo, and Cristian Paz. 2024. "Therapeutic Potential of Chlorogenic Acid in Chemoresistance and Chemoprotection in Cancer Treatment" International Journal of Molecular Sciences 25, no. 10: 5189. https://doi.org/10.3390/ijms25105189






