Effect of Garlic Extract on the Erythrocyte as a Simple Model Cell
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Reagents and Equipment
4.2. Ethical Approval of Experiments with Blood Plasma
4.3. Erythrocytes and Erythrocyte Membranes
4.4. Preparation of the Garlic Extract
4.5. Observation of Heinz Bodies
4.6. Hemoglobin Oxidation
4.7. Hemoglobin Binding to the Membrane
4.8. Estimation of the Extent of Hemolysis
4.9. Measurement of Hemolysis in Isotonic Ammonium Chloride
4.10. Estimation of the Acid-Soluble Thiol Level
4.11. Estimation of Thiol Groups Content of the Membranes
4.12. Estimation of the Glutathione Level
4.13. Estimation of the Redox Potential of the Erythrocyte Glutathione System
4.14. Estimation of the Level of Intracellular Reactive Oxygen Species
4.15. Estimation of Lipid Peroxidation
4.16. Carbonylation of Membrane Proteins
4.17. Estimation of Membrane Fluidity with Pyrene
4.18. Estimation of Membrane Fluidity with Spin Probes
4.19. Determination of Acetylcholinesterase Activity
4.20. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ansary, J.; Forbes-Hernández, T.Y.; Gil, E.; Cianciosi, D.; Zhang, J.; Elexpuru-Zabaleta, M.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Potential health benefit of garlic based on human intervention studies: A brief overview. Antioxidants 2020, 9, 619. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, T.; Valencia, E.; Albarrán, M.; Díaz, D.; Quevedo, R.A.; Díaz, O.; Bastías, J. Garlic (Allium sativum L.) and its beneficial properties for health: A review. Agroind. Sci. 2020, 10, 103–115. [Google Scholar] [CrossRef]
- Netzel, M.E. Garlic: Much more than a common spice. Foods 2020, 9, 1544. [Google Scholar] [CrossRef]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial properties of organosulfur compounds of garlic (Allium sativum). Front. Microbiol. 2021, 12, 1869. [Google Scholar] [CrossRef]
- Rouf, R.; Uddin, S.J.; Sarker, D.K.; Islam, M.T.; Ali, E.S.; Shilpi, J.A.; Nahar, L.; Tiralongo, E.; Sarker, S.D. Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci. Technol. 2020, 104, 219–234. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Alshammari, N.; Saeed, A.; Aqil, F.; Saeed, M. Updates on the anticancer potential of garlic organosulfur compounds and their nanoformulations: Plant therapeutics in cancer management. Front. Pharmacol. 2023, 14, 1154034. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Roberts, L.A.; Ma, S.; Suzuki, K. Organosulfur compounds: A review of their anti-inflammatory effects in human health. Front. Nutr. 2020, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Goran, A.; Igic, R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem. 2008, 111, 925–929. [Google Scholar] [CrossRef]
- Škrovánková, S.; Mlček, J.; Snopek, L.; Planetová, T. Polyphenols and antioxidant capacity in different types of garlic. Potravin. Slovak J. Food Sci. 2018, 12, 267–272. [Google Scholar] [CrossRef]
- Kopec, A.; Piatkowska, E.; Leszczynska, T.; Sikora, E. Healthy properties of garlic. Curr. Nutr. Food Sci. 2013, 9, 59–64. [Google Scholar]
- Azzini, E.; Durazzo, A.; Foddai, M.S.; Temperini, O.; Venneria, E.; Valentini, S.; Maiani, G. Phytochemicals content in Italian garlic bulb (Allium sativum L.) varieties. J. Food Res. 2014, 3, 26. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef]
- DeLeon, E.R.; Gao, Y.; Huang, E.; Olson, K.R. Garlic oil polysulfides: H2S-and O2-independent prooxidants in buffer and antioxidants in cells. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2016, 310, R1212–R1225. [Google Scholar] [CrossRef]
- Kulikova, V.V.; Morozova, E.A.; Koval, V.S.; Solyev, P.N.; Demidkina, T.V.; Revtovich, S.V. Thiosulfinates: Cytotoxic and antitumor activity. Biochemistry (Mosc.) 2023, 88, 912–923. [Google Scholar] [CrossRef]
- Hamlaoui, S.; Mokni, M.; Limam, N.; Zouaoui, K.; Ben Rayana, M.C.; Carrier, A.; Limam, F.; Amri, M.; Marzouki, L.; Aouani, E. Protective effect of grape seed and skin extract on garlic-induced erythrocyte oxidative stress. J. Physiol. Pharmacol. 2012, 63, 381–388. [Google Scholar]
- Salami, H.A.; Tukur, M.A.; Bukar, A.; John, I.A.; Abubakar, A.; Jibrin, J. High reticulocyte count with abnormal red blood cell morphology in normal Wistar rats after garlic administration. Niger. J. Physiol. Sci. 2018, 33, 165–168. [Google Scholar] [PubMed]
- Oboh, G. Prevention of garlic-induced hemolytic anemia using some tropical green leafy vegetables. J. Med. Food 2004, 7, 498–501. [Google Scholar] [CrossRef]
- Hamlaoui-Gasmi, S.; Mokni, M.; Aouani, E.; Amri, M.; Marzouki, L. Modulation of hematological parameters by garlic based on route of administration in rat. J. Food Biochem. 2011, 35, 442–453. [Google Scholar] [CrossRef]
- Lee, K.W.; Yamato, O.; Tajima, M.; Kuraoka, M.; Omae, S.; Maede, Y. Hematologic changes associated with the appearance of eccentrocytes after intragastric administration of garlic extract to dogs. Am. J. Vet. Res. 2000, 61, 1446–1450. [Google Scholar] [CrossRef]
- Kempaiah, R.K.; Srinivasan, K. Influence of dietary curcumin, capsaicin and garlic on the antioxidant status of red blood cells and the liver in high-fat-fed rats. Ann. Nutr. Metab. 2004, 48, 314–320. [Google Scholar] [CrossRef]
- Kempaiah, R.K.; Srinivasan, K. Beneficial influence of dietary curcumin, capsaicin and garlic on erythrocyte integrity in high-fat fed rats. J. Nutr. Biochem. 2006, 17, 471–478. [Google Scholar] [CrossRef]
- Zalejska-Fiolka, J.; Kasperczyk, A.; Kasperczyk, S.; Błaszczyk, U.; Birkner, E. Effect of garlic supplementation on erythrocytes antioxidant parameters, lipid peroxidation, and atherosclerotic plaque formation process in oxidized oil-fed rabbits. Biol. Trace Elem. Res. 2006, 120, 195–204. [Google Scholar] [CrossRef]
- Sarkar, A.; Sengupta, D.; Mandal, S.; Sen, G.; Dutta Chowdhury, K.; Chandra Sadhukhan, G. Treatment with garlic restores membrane thiol content and ameliorates lead induced early death of erythrocytes in mice. Env. Toxicol. 2015, 30, 396–410. [Google Scholar] [CrossRef]
- Tikare, S.N.; Saeed, Y.; Amrita, D.G.; Salim, A.D.; Kusal, K.D. Effect of garlic (Allium sativum) on hematology and erythrocyte antioxidant defense system of albino rats exposed to heavy metals (nickel ii & chromium vi). J. Physiol. Pharmacol. 2012, 56, 137–146. [Google Scholar]
- Avcı, A.; Atlı, T.; Ergüder, İ.B.; Varlı, M.; Devrim, E.; Aras, S.; Durak, İ. Effects of garlic consumption on plasma and erythrocyte antioxidant parameters in elderly subjects. Gerontology 2008, 54, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Takasu, J.; Uykimpang, R.; Sunga, M.A.; Amagase, H.; Niihara, Y. Aged garlic extract is a potential therapy for sickle-cell anemia. J. Nutr. 2006, 136, 803S–805S. [Google Scholar] [CrossRef] [PubMed]
- Salami, H.A.; John, A.I.; Ekanem, A.U. The effect of aqueous preparation of Allium Cepa (onion) and Allium Sativa (garlic) on erythrocyte osmotic fragility in Wistar rats: In vivo and in vitro studies. Niger. J. Physiol. Sci. 2012, 27, 29–34. [Google Scholar]
- Furdak, P.; Pieńkowska, N.; Kapusta, I.; Bartosz, G.; Sadowska-Bartosz, I. Comparison of antioxidant and antiproliferative effects of various forms of garlic and ramsons. Molecules 2023, 28, 6512. [Google Scholar] [CrossRef] [PubMed]
- Miron, T.; Rabinkov, A.; Mirelman, D.; Wilchek, M.; Weiner, L. The mode of action of allicin: Its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim. Biophys. Acta Biomembranes 2000, 1463, 20–30. [Google Scholar] [CrossRef]
- Rabinkov, A.; Miron, T.; Konstantinovski, L.; Wilchek, M.; Mirelman, D.; Weiner, L. The mode of action of allicin: Trapping of radicals and interaction with thiol containing proteins. Biochim. Biophys. Acta Gen. Sub. 1998, 1379, 233–244. [Google Scholar] [CrossRef]
- Miron, T.; Shin, I.; Feigenblat, G.; Weiner, L.; Mirelman, D.; Wilchek, M.; Rabinkov, A. A spectrophotometric assay for allicin, alliin, and alliinase (alliin lyase) with a chromogenic thiol: Reaction of 4-mercaptopyridine with thiosulfinates. Anal. Biochem. 2002, 307, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, J.; Rodriguez, E.N.; Beyley, V.; Vega, I.E. Allylation of intraerythrocytic hemoglobin by raw garlic extracts. J. Med. Food 2010, 13, 943–949. [Google Scholar] [CrossRef]
- Hu, Q.; Yang, Q.; Yamato, O.; Yamasaki, M.; Maede, Y.; Yoshihara, T. Isolation and identification of organosulfur compounds oxidizing canine erythrocytes from garlic (Allium sativum). J. Agric. Food Chem. 2002, 50, 1059–1062. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Pieńkowska, N.; Bartosz, G.; Sadowska-Bartosz, I. Effect of 6-hydroxydopamine increase the glutathione level in SH-SY5Y human neuroblastoma cells. Acta Biochim. Pol. 2023, 70, 457–464. [Google Scholar] [CrossRef]
- Aubert, L.; Motais, R. Molecular features of organic anion permeability in ox red blood cell. J. Physiol. 1975, 246, 159–179. [Google Scholar] [CrossRef]
- Jennings, M.L. Cell physiology and molecular mechanism of anion transport by erythrocyte band 3/AE1. Am. J. Physiol.-Cell Physiol. 2021, 321, C1028–C1059. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Nagayama, M. Garlic allyl derivatives interact with membrane lipids to modify the membrane fluidity. J. Biomed. Sci. 2008, 15, 653–660. [Google Scholar] [CrossRef]
- Ezer, N.; Sahin, I.; Kazanci, N. Alliin interacts with DMPC model membranes to modify the membrane dynamics: FTIR and DSC Studies. Vibrat. Spectrosc. 2017, 89, 1–8. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. The cellular and organismal effects of nitroxides and nitroxide-containing nanoparticles. Int. J. Mol. Sci. 2024, 25, 1446. [Google Scholar] [CrossRef]
- Deslauriers, R.; Butler, K.; Smith, I.C. Oxidant stress in malaria as probed by stable nitroxide radicals in erythrocytes infected with Plasmodium berghei. The effects of primaquine and chloroquine. Biochim. Biophys. Acta Mol. Cell Res. 1987, 931, 267–275. [Google Scholar] [CrossRef]
- Banerjee, S.K.; Mukherjee, P.K.; Maulik, S.K. Garlic as an antioxidant: The good, the bad and the ugly. Phytother. Res. 2003, 17, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.; Ingold, K.U.; Pratt, D.A. Garlic: Source of the ultimate antioxidants–sulfenic acids. Angew. Chem. Int. Ed. Engl. 2009, 48, 157–160. [Google Scholar] [CrossRef]
- Amorati, R.; Lynett, P.T.; Valgimigli, L.; Pratt, D.A. The reaction of sulfenic acids with peroxyl radicals: Insights into the radical-trapping antioxidant activity of plant-derived thiosulfinates. Chemistry 2012, 18, 6370–6379. [Google Scholar] [CrossRef]
- Moriguchi, T.; Takasugi, N.; Itakura, Y. The effects of aged garlic extract on lipid peroxidation and the deformability of erythrocytes. J. Nutr. 2001, 131, 1016S–1019S. [Google Scholar] [CrossRef]
- Ohnishi, S.T.; Ohnishi, T. In vitro effects of aged garlic extract and other nutritional supplements on sickle erythrocytes. J. Nutr. 2001, 131, 1085S–1092S. [Google Scholar] [CrossRef]
- Kumar, S. Dual inhibition of acetylcholinesterase and butyrylcholinesterase enzymes by allicin. Indian J. Pharmacol. 2015, 47, 444–446. [Google Scholar] [CrossRef]
- Akinyemi, A.J.; Faboya, L.; Awonegan, A.; Olayide, I.; Anadozie, S.; Oluwasola, T. Antioxidant and anti-acetylcholinesterase activities of essential oils from garlic (Allium sativum) bulbs. Int. J. Plant Res. 2018, 31, 1000397. [Google Scholar]
- Mukherjee, D.; Banerjee, S. Learning and memory promoting effects of crude garlic extract. Indian J. Exp. Biol. 2013, 51, 1094–1100. [Google Scholar] [PubMed]
- Dodge, J.T.; Mitchell, C.; Hanahan, D.J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch. Biochem. Biophys. 1963, 100, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Soszynski, M.; Filipiak, A.; Bartosz, G.; Gebicki, J.M. Effect of amino acid peroxides on the erythrocyte. Free Radic. Biol. Med. 1996, 20, 45–51. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Protection by ascorbate against acetylphenylhydrazine-induced Heinz body formation in glucose-6-phosphate dehydrogenase deficient erythrocytes. Br. J. Haematol. 1979, 41, 245–252. [Google Scholar] [CrossRef]
- Eyer, P.; Worek, F.; Kiderlen, D.; Sinko, G.; Stuglin, A.; Simeon-Rudolf, V.; Reiner, E. Molar absorption coefficients for the reduced Ellman reagent: Reassessment. Anal. Biochem. 2003, 312, 224–227. [Google Scholar] [CrossRef]
- Senft, A.P.; Dalton, T.P.; Shertzer, H.G. Determining glutathione and glutathione disulfide using the fluorescence probe o-phthalaldehyde. Anal. Biochem. 2000, 280, 80–86. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar]
- Ježek, P.; Freisleben, H.J. Fatty acid binding site of the mitochondrial uncoupling protein: Demonstration of its existence by EPR spectroscopy of 5-DOXYL-stearic acid. FEBS Lett. 1994, 343, 22–26. [Google Scholar] [CrossRef]
- Hubbell, W.L.; McConnell, H.M. Molecular motion in spin-labeled phospholipids and membranes. J. Am. Chem. Soc. 1971, 93, 314–326. [Google Scholar]
- Schreier, S.; Polnaszek, C.F.; Smith, I.C. Spin labels in membranes. Problems in practice. Biochim. Biophys. Acta 1978, 515, 395–436. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]

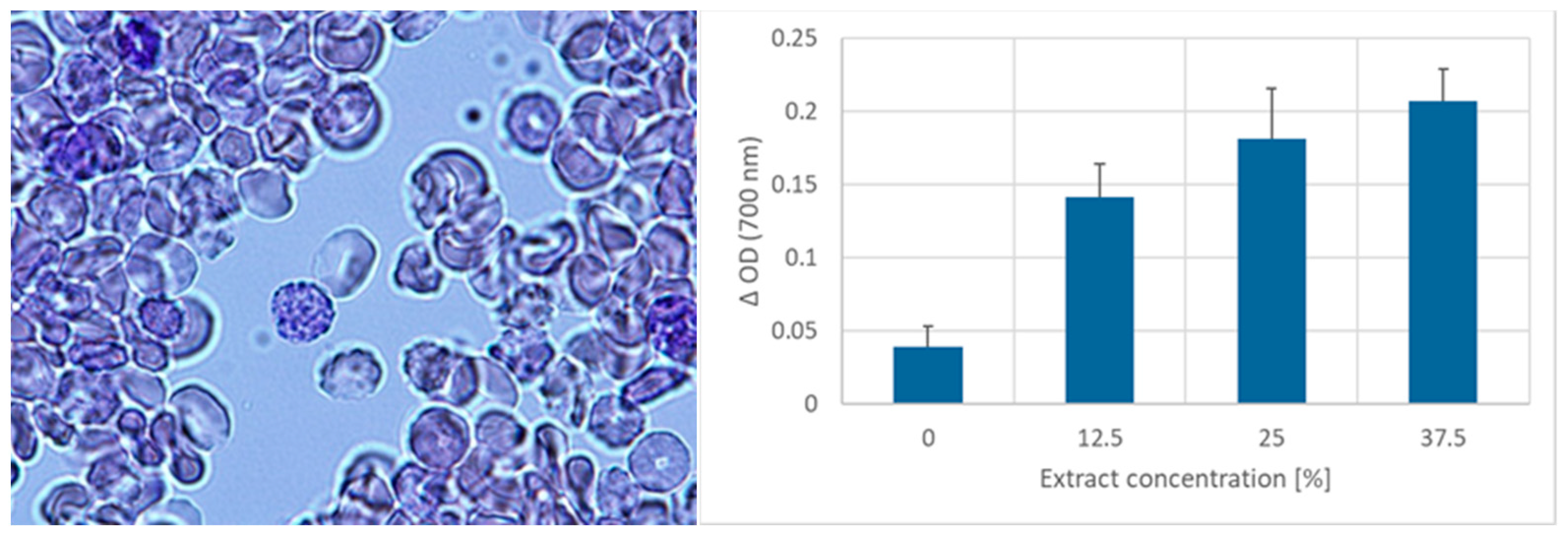
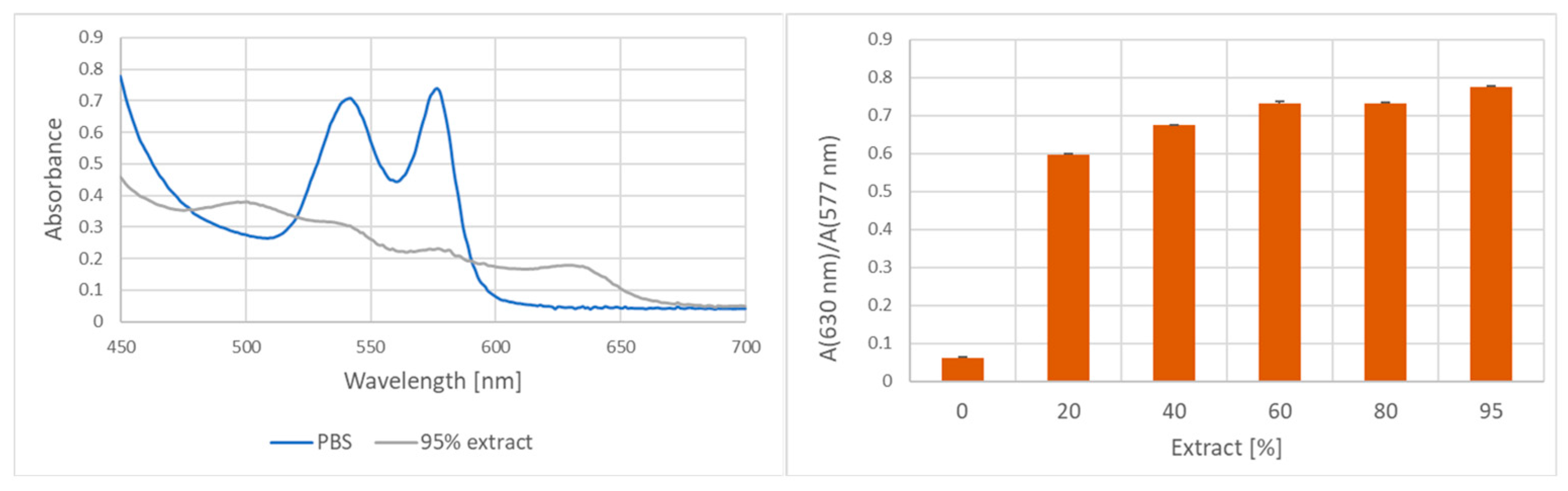
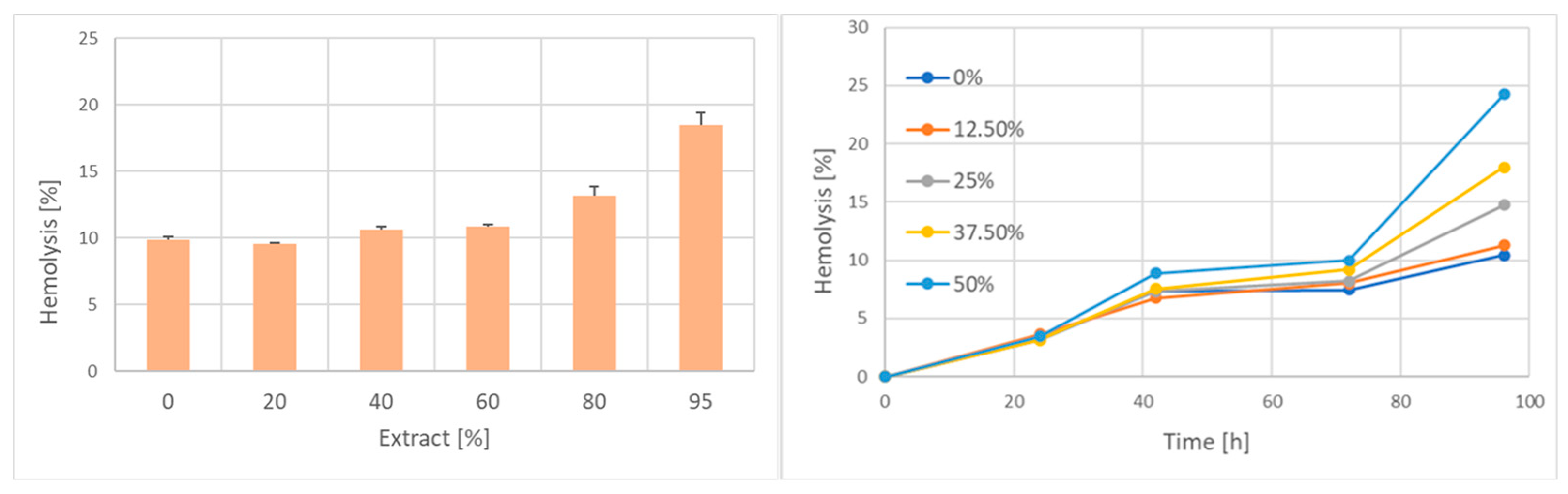
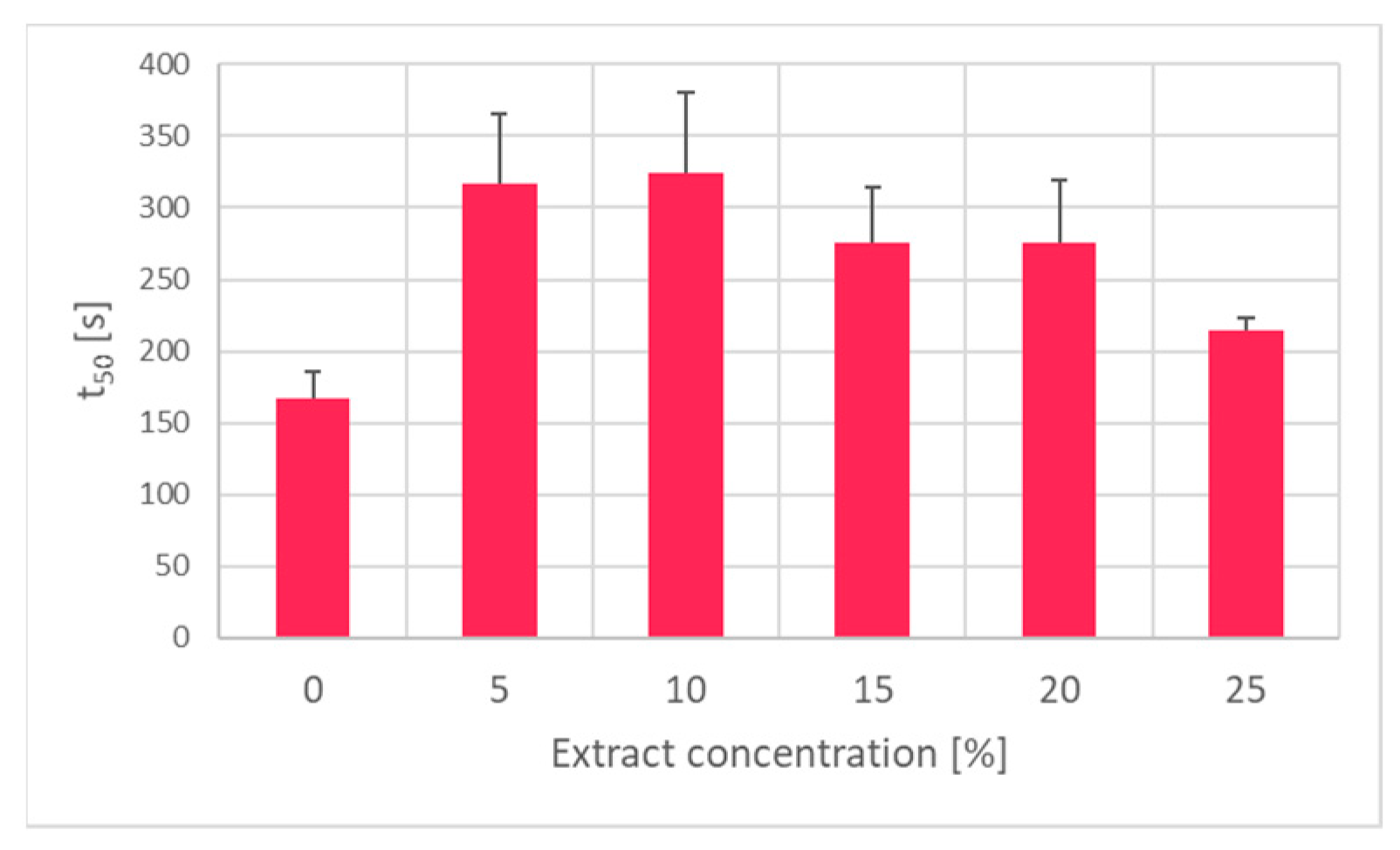

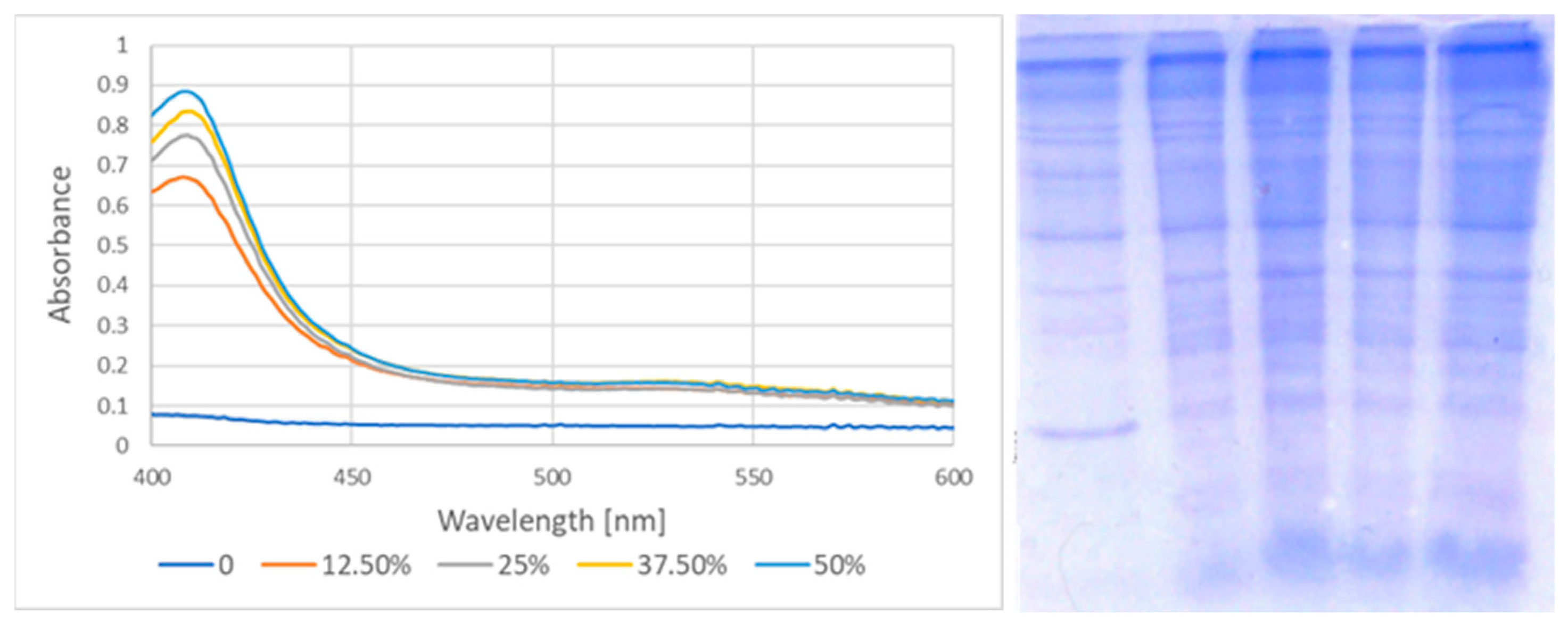
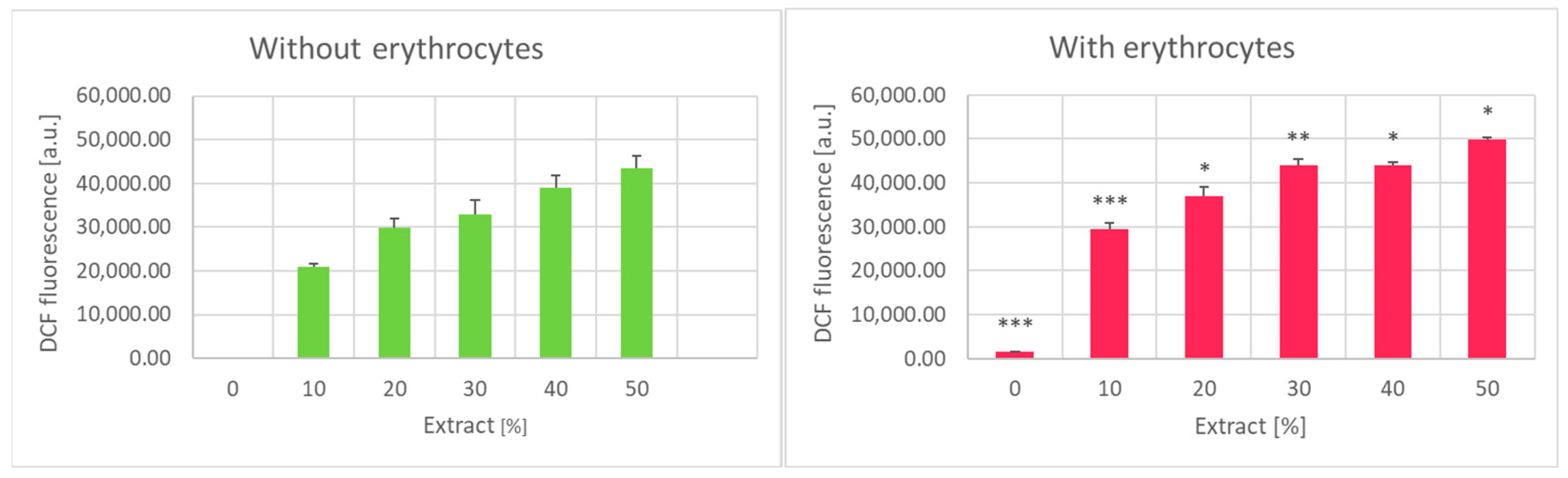
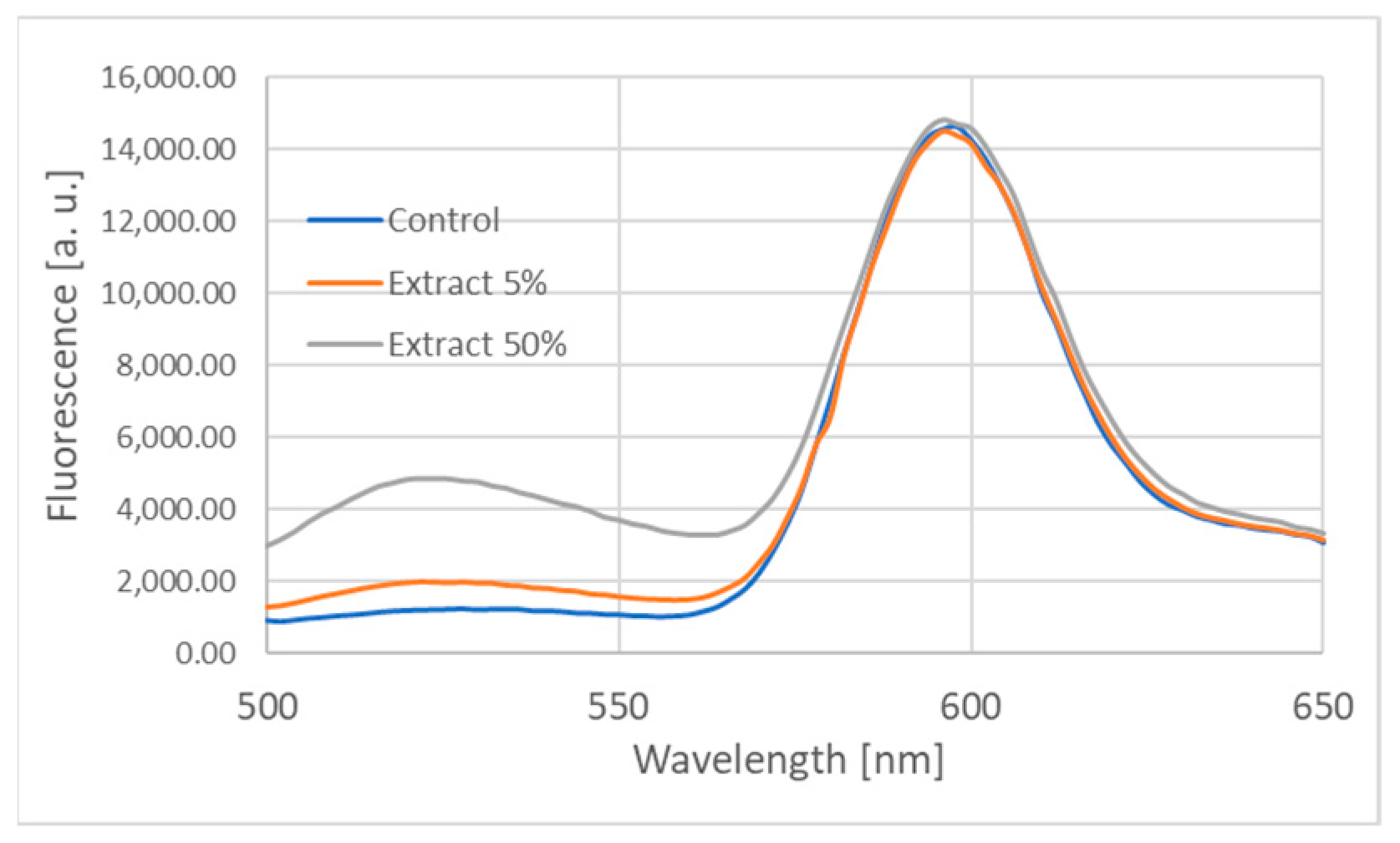

| Extract Concentration [%] | GSH [μM] | GSSG [μM] | GSH/GSSG | Eh of the GSH/GSSG Redox Couple [mV] |
|---|---|---|---|---|
| 0 | 1880.7 ± 53.3 | 112.5 ± 14.5 | 16.7 | −220 |
| 2.5 | 68.4 ± 3.2 | 20.7 ± 2.2 | 3.3 | −156 |
| 5 | 31.6 ± 5.2 | 19.7 ± 2.3 | 1.6 | −137 |
| 10 | 21.7 ± 2.9 | 16.5 ± 3.2 | 1.3 | −130 |
| 20 | 19.3 ± 4.1 | 10.7 ± 2.8 | 1.8 | −132 |
| 50 | 17.8 ± 1.8 | 9.7 ± 1.5 | 1.8 | −131 |
| Garlic Extract [%] | Absorbance at 410 nm | Hemoglobin as % of Total Membrane Protein |
|---|---|---|
| 0 | 0.075 ± 0.011 | 0.5 ± 0.3 |
| 12.5 | 0.663 ± 0.042 | 5.9 ± 0.9 |
| 25 | 0.769 ± 0.038 | 6.6 ± 0.8 |
| 37.5 | 0.834 ± 0.045 | 8.5 ± 1.1 |
| 50 | 0.880 ± 0.061 | 10.1 ± 1.7 |
| Extract Concentration [%] | F(526 nm)/F(598 nm) | |
|---|---|---|
| Without Erythrocyte Membranes | With Erythrocyte Membranes | |
| 0 | 0.062 ± 0.002 | 0.085 ± 0.009 |
| 5 | 0.121 ± 0.005 | 0.102 ± 0.011 |
| 12.5 | 0.130 ± 0.007 | 0.131 ± 0.017 |
| 25 | 0.140 ± 0.005 | 0.139 ± 0.009 |
| 50 | 0.211 ± 0.012 | 0.330 ± 0.012 |
| Extract Concentration [%] | Pyrene Excimer to Monomer Fluorescence Ratio |
|---|---|
| 0 | 0.411 ± 0.004 |
| 5 | 0.469 ± 0.006 |
| 12.5 | 0.453 ± 0.009 |
| 25 | 0.462 ± 0.004 |
| 50 | 0.436 ± 0.003 |
| Extract Concentration [%] | Order Parameter S of 5-DS | Rotational Correlation Time τc of 16-DS [ns] |
|---|---|---|
| 0 | 0.640 ± 0.009 | 1.56 ± 0.25 |
| 10 | 0.632 ± 0.017 | 1.37 ± 0.15 |
| 25 | 0.628 ± 0.05 | 1.24 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furdak, P.; Bartosz, G.; Stefaniuk, I.; Cieniek, B.; Bieszczad-Bedrejczuk, E.; Soszyński, M.; Sadowska-Bartosz, I. Effect of Garlic Extract on the Erythrocyte as a Simple Model Cell. Int. J. Mol. Sci. 2024, 25, 5115. https://doi.org/10.3390/ijms25105115
Furdak P, Bartosz G, Stefaniuk I, Cieniek B, Bieszczad-Bedrejczuk E, Soszyński M, Sadowska-Bartosz I. Effect of Garlic Extract on the Erythrocyte as a Simple Model Cell. International Journal of Molecular Sciences. 2024; 25(10):5115. https://doi.org/10.3390/ijms25105115
Chicago/Turabian StyleFurdak, Paulina, Grzegorz Bartosz, Ireneusz Stefaniuk, Bogumił Cieniek, Edyta Bieszczad-Bedrejczuk, Mirosław Soszyński, and Izabela Sadowska-Bartosz. 2024. "Effect of Garlic Extract on the Erythrocyte as a Simple Model Cell" International Journal of Molecular Sciences 25, no. 10: 5115. https://doi.org/10.3390/ijms25105115





