Heating Capacity and Biocompatibility of Hybrid Nanoparticles for Magnetic Hyperthermia Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of the Optimal Synthesis Conditions to Obtain the Smallest Particle Sizes
Analysis of the Factorial Design
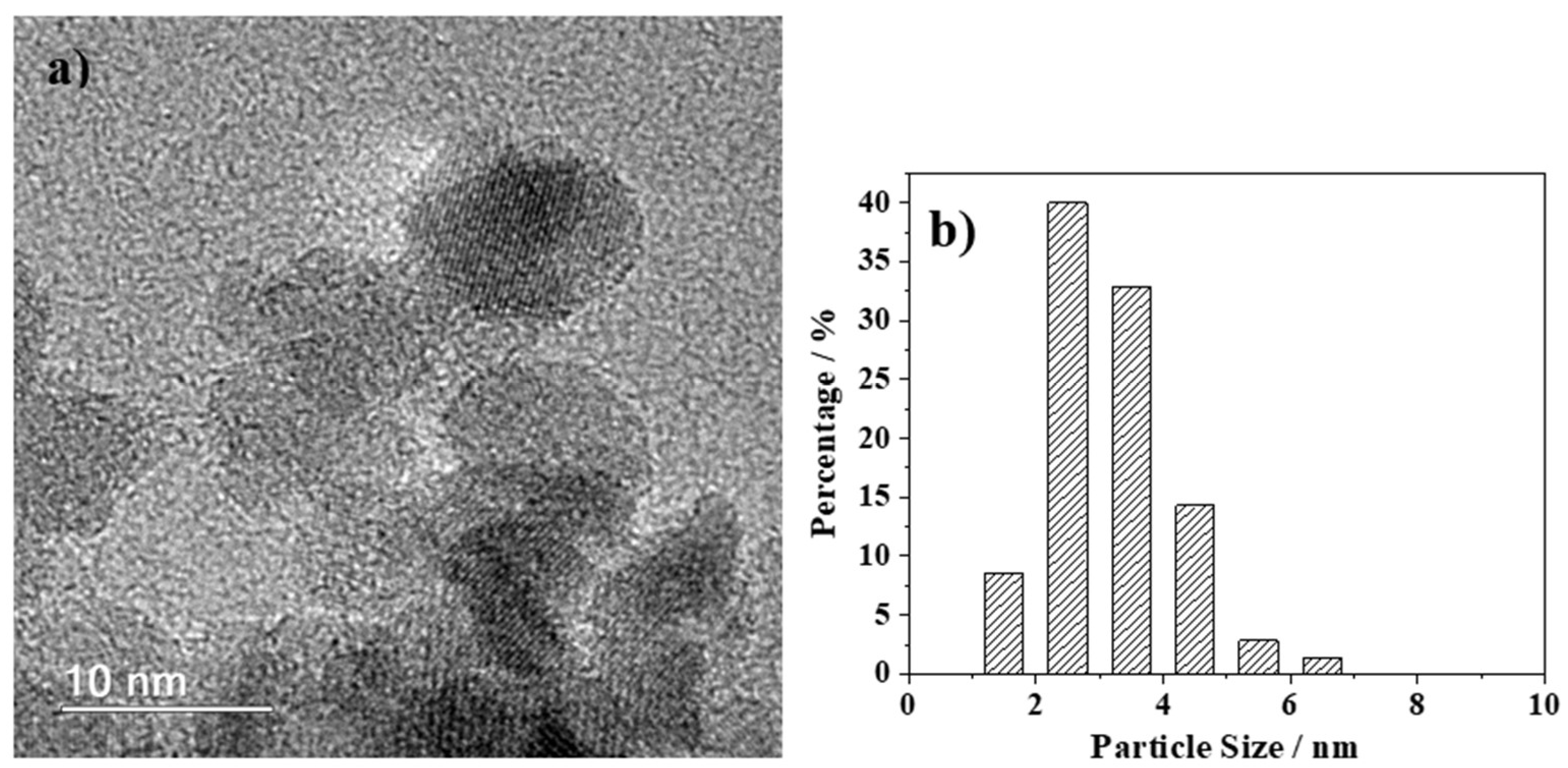
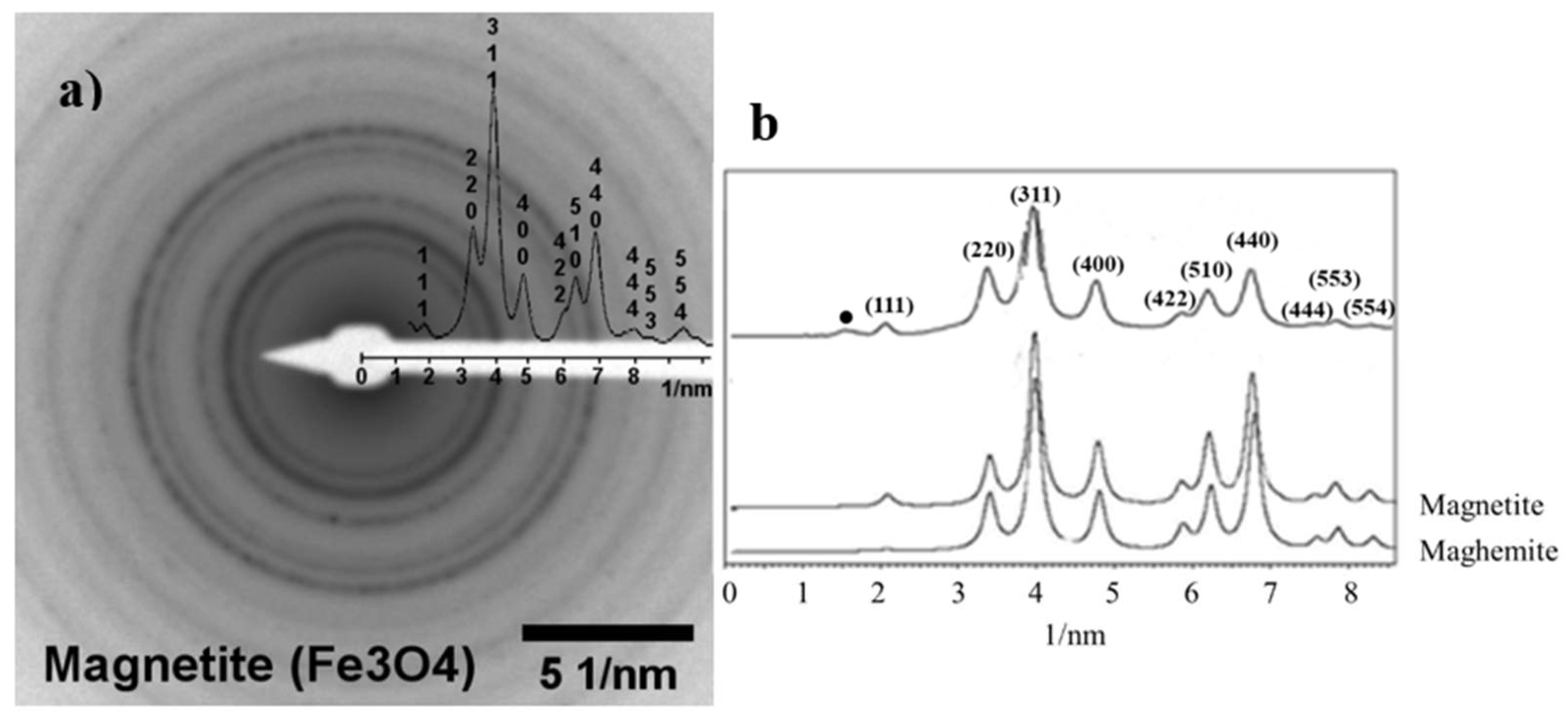
2.2. Characterization of the Synthesized MNPs
2.3. Stability
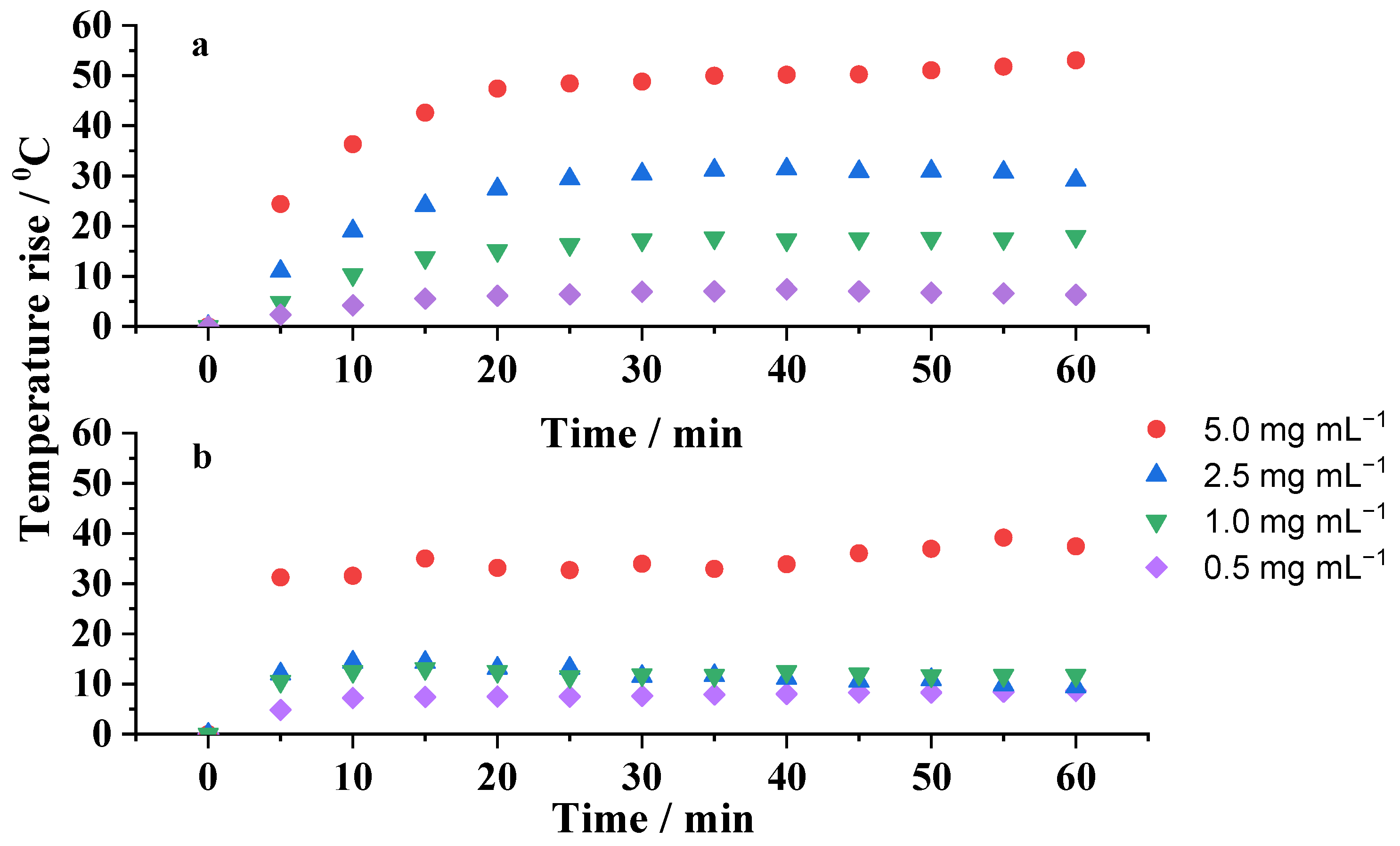
2.4. Heating Capability Studies
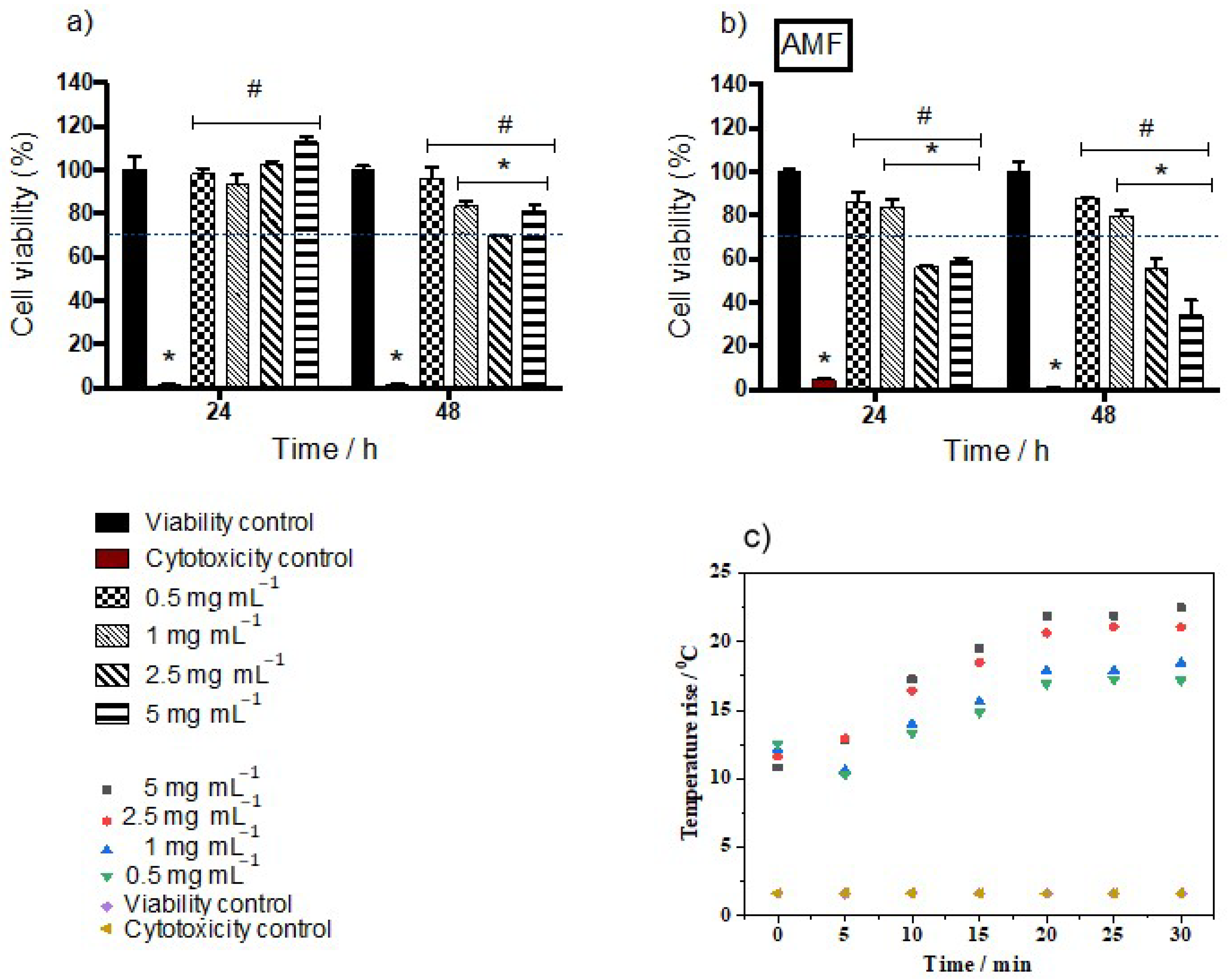
2.5. Effect of Mag1@poly on Cellular Growth and Hemolysis with or without Magnetic Field Exposure
3. Materials and Methods
3.1. Reagents
3.2. Methods and Techniques
3.2.1. Analysis of the Factorial Design
3.2.2. Design of Mag Synthesis Experiments
3.2.3. Coating of MNPs with PLDLA-co-TMC and PEO–PPO–PEO by SDM
3.2.4. Characterization
3.2.5. Heating Ability Studies
3.3. Biological Assay
3.3.1. Culture of HUVEC
3.3.2. Biocompatibility/Cell Cytotoxicity Assay with or without an AC Magnetic Field
3.3.3. Hemolysis Assay with or without an AC Magnetic Field
3.3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machado, V.O.; Andrade, A.L.; Simon, A.; Rodríguez-Fernández, D.E.; Fabris, J.D.; Domingues, R.Z.; da Silva, R.F.; Silva, T.C.E.; Peixoto, T.L.; dos Santos, C.T.; et al. Development of a novel nano-biomaterial for biomedical applications. Mater. Res. Express 2018, 5, 125014. [Google Scholar] [CrossRef]
- Bourgeout-Lami, E.; Lansalot, M. Organic/inorganic composite latexes: The marriage of emulsion polymerization and inorganic chemistry. In Hybrid Latex Particles. Advances in Polymer Science; van Herk, A., Landfester, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; p. 233. [Google Scholar]
- Romio, A.P.; Rodrigues, H.H.; Peres, A.; Viegas, A.D.C.; Kobitskaya, E.; Ziener, U.; Landfester, K.; Sayer, C.; Araújo, P.H.H. Encapsulation of magnetic nickel nanoparticles via inverse miniemulsion polymerization. J. Appl. Polym. Sci. 2013, 129, 1426–1433. [Google Scholar] [CrossRef]
- Staudt, T.; Machado, T.O.; Vogel, N.; Weiss, C.K.; Araujo, P.H.H.; Sayer, C.; Landfester, K. Magnetic polymer/nickel hybrid nanoparticles via miniemulsion polymerization. Macromol. Chem. Phys. 2013, 214, 2213–2222. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Badria, W.; Miladi, K.; Nazari, Q.A.; Fessi, H.; Elaissari, A. Effect of process and formulation parameters on polycaprolactone nanoparticles prepared by solvent displacement. Colloids Surf. A. 2017, 516, 238–244. [Google Scholar] [CrossRef]
- Lepock, J.R.; Frey, H.E.; Bayne, H.; Markus, J. Relationship of hyperthermia-induced hemolysis of human erythrocytes to the thermal denaturation of membrane proteins. Biochim. Biophys. Acta 1989, 980, 191–201. [Google Scholar] [CrossRef]
- Komatsu, D.; Hausen, M.A.; Eri, R.Y.; Leal, V.; Pedrini, F.; Yaksic, C.; Alves, T.F.R.; Chaud, M.V.; Fanelli, C.; Noronha, I.; et al. Alternative cutaneous substitutes based on poly(L-co-D,L-lactic acid-co-trimethylene carbonate) with Schinus terebinthifolius raddi extract designed for skin healing. ACS Omega 2019, 4, 18317–18326. [Google Scholar] [CrossRef]
- Duek, J.R.; Riquetto, M.L.; Jesus, D.C.; Sabongi Neto, J.J.; Barbo, M.L.P.; Duek, E.A.R.; Motta, A.C. Membrana de PLDLA-TMC como protetor na regeneração do tendão calcâneo. Estudo in vivo em Coelhos. Polim. Cienc. Tecnol. 2014, 24, 360–366. [Google Scholar] [CrossRef]
- Motta, A.C.; Fedrizzi, V.M.; Barbo, M.L.P.; Duek, E.A.R. In vitro and in vivo studies on devices of poly(l-co-d,l lactic acid)-co-TMC for bone repair. Polym. Bull. 2018, 75, 4515–4529. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Johnston, T.P.; Palmer, W.K. Mechanism of poloxamer 407-induced hypertriglyceridemia in the rat. Biochem. Pharmacol. 1993, 46, 1037–1042. [Google Scholar] [CrossRef]
- Machado, V.O.; Andrade, A.L.; Cavalcante, L.C.D.; Fabris, J.D.; Domingues, R.Z.; Ardisson, J.D.; Fernandez-Outon, L.E.; Pizarro, C.; Elias, C.N. A novel hybrid nanoparticle based on Fe3O4/TMAOH/poly(L-co-D,L lactic acid-co-trimethylene carbonate) prepared through the solvent displacement method. Hyperfine Interac. 2019, 240, 21. [Google Scholar] [CrossRef]
- Machado, V.O.; Andrade, A.L.; Fabris, J.D.; Freitas, E.T.F.; Ferreira, J.M.F.; Simon, A.; Domingues, R.Z.; Fernandez-Outon, L.E.; do Carmo, F.A.; Souza, A.C.S.; et al. Preparation of hybrid nanocomposite particles for medical practices. Colloids Surf. A. 2021, 624, 126706. [Google Scholar] [CrossRef]
- Hu, X.; He, J.; Yong, X.; Lu, J.; Xiao, J.; Liao, Y.; Li, Q.; Xiong, C. Biodegradable poly (lactic acid-co-trimethylene carbonate)/chitosan microsphere scaffold with shape-memory effect for bone tissue engineering. Colloids Surf. B. 2020, 195, 111218. [Google Scholar] [CrossRef]
- Polte, J. Fundamental growth principles of colloidal metal nanoparticles—A new perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Namvar, F.; Nadi, B.; Rahman, M.Z.A.; Amin, J. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef]
- Nadi, B.; Ahmad, M.B.; Haron, M.J.; Rahman, M.Z.A.A.; Fatehi, A. Optimized conditions for graft copolymerization of poly(acrylamide) onto rubberwood fibre. BioResources 2011, 6, 5110–5120. [Google Scholar] [CrossRef]
- Saragi, T.; Depi, B.L.; Butarbutar, S.; Permana, B.; Risdiana. The impact of synthesis temperature on magnetite nanoparticles size synthesized by co-precipitation method. J. Phys. Conf. Ser. 2018, 1013, 012190. [Google Scholar] [CrossRef]
- Gnanaprakash, G.; Philip, J.; Jayakumar, T.; Raj, B. Effect of digestion time and alkali addition rate on physical properties of magnetite nanoparticles. J. Phys. Chem. B 2007, 111, 7978–7986. [Google Scholar] [CrossRef]
- Yeap, S.P.; Lim, J.K.; Ngang, H.P.; Ooi, B.S.; Ahmad, A.L. Role of particle–particle interaction towards effective interpretation of z-average and particle size distributions from dynamic light scattering (DLS) Analysis. J. Nanosci. Nanotechnol. 2018, 18, 6957–6964. [Google Scholar] [CrossRef]
- Fissan, H.; Ristig, S.; Kaminski, H.; Asbach, C.; Epple, M. Comparison of different characterization methods for nanoparticle dispersions before and after aerosolization. Anal. Methods 2014, 6, 7324–7334. [Google Scholar] [CrossRef]
- Du, Z.; Munye, M.M.; Tagalakis, A.D.; Manunta, M.D.I.; Hart, S.L. The role of the helper lipid on the DNA transfection efficiency of lipopolyplex formulations. Sci. Rep. 2014, 4, 7107. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.L.; Cavalcante, L.C.D.; Fabris, J.D.; Pereira, M.C.; Fernandez-Outong, L.E.; Pedersoli, D.C.; Ardisson, J.D.; Domingues, R.Z.; Ferreira, J.M.F. Magnetically induced heating by iron oxide nanoparticles dispersed in liquids of different viscosities. Ceram. Int. 2020, 46, 21496–21504. [Google Scholar] [CrossRef]
- Pineiro-Redondo, Y.; Banobre-Lopez, M.; Pardinas-Blanco, I.; Goya, G.; Lopez-Quintela, M.A.; Rivas, J. The influence of colloidal parameters on the specific power absorption of PAA-coated magnetite nanoparticles. Nanoscale Res. Lett. 2011, 6, 383. [Google Scholar] [CrossRef] [PubMed]
- Barrioni, B.R.; de Laia, A.G.S.; Valverde, T.M.; Martins, T.M.M.; Caliari, M.V.; de Sa, M.A.; de Goes, A.M.; Pereira, M.M. Evaluation of in vitro and in vivo biocompatibility and structure of cobalt-releasing sol-gel bioactive glass. Ceram. Int. 2018, 44, 20337–20347. [Google Scholar] [CrossRef]
- Repar, N.; Jovicic, E.J.; Kump, A.; Birarda, G.; Vaccari, L.; Erman, A.; Kralj, S.; Nemec, S.; Petan, T.; Drobne, D. Oleic acid protects endothelial cells from silica-coated superparamagnetic iron oxide nanoparticles (spions)-induced oxidative stress and cell death. Int. J. Mol. Sci. 2022, 23, 6972. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Choi, W.I.; Kim, Y.H.; Tae, G.; Lee, S.-Y.; Kim, K.; Kwon, I.C. In-vivo tumor targeting of pluronic-based nano-carriers. J. Control. Release 2010, 147, 109–117. [Google Scholar] [CrossRef]
- Morales, M.A.; Jain, T.K.; Labhasetwar, V.; Leslie-Pelecky, D.L. Magnetic studies of iron oxide nanoparticles coated with oleic acid and Pluronic block copolymer. J. Appl. Phys. 2005, 97, 10Q905. [Google Scholar] [CrossRef]
- Jain, T.K.; Richey, J.; Strand, M.; Leslie-Pelecky, D.L.; Flask, C.; Labhasetwar, V. Magnetic nanoparticles with dual functional properties: Drug delivery and magnetic resonance imaging. Biomaterials 2008, 29, 4012–4021. [Google Scholar] [CrossRef]
- Gong, P.; Li, H.; He, X.; Wang, K.; Hu, J.; Tan, W.; Zhang, S.; Yang, X. Preparation and antibacterial activity of Fe3O4@Ag nanoparticles. Nanotechnology 2007, 18, 285604. [Google Scholar] [CrossRef]
- Wu, X.; Tan, Y.; Mao, H.; Zhang, M. Toxic effects of iron oxide nanoparticles on human umbilical vein endothelial cells. Int. J. Nanomed. 2010, 5, 385–399. [Google Scholar] [CrossRef]
- Berry, C.C.; Wells, S.; Charles, S.; Curtis, A.S.G. Dextran and albumin derivatised iron oxide nanoparticles: Influence on fibroblasts in vitro. Biomaterials 2003, 24, 4551–4557. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.; Yang, L.; Wang, X.; Long, J.; Liu, J. Superparamagnetic iron oxide nanoparticles exacerbate the risks of reactive oxygen species-mediated external stresses. Arch. Toxicol. 2015, 89, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Mirrahimi, M.; Abed, Z.; Beik, J.; Shiri, I.; Dezfuli, A.S.; Mahabadi, V.P.; Kamrava, S.K.; Ghaznavi, H.; Shakeri-Zadeh, A. A thermoresponsive alginate nanogel platform co-loaded with gold nanoparticles and cisplatin for combined cancer chemo-photothermal therapy. Pharmacol. Res. 2019, 143, 178–185. [Google Scholar] [CrossRef]
- Cherukuri, P.; Glazer, E.S.; Curley, S.A. Targeted hyperthermia using metal nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 339–345. [Google Scholar] [CrossRef]
- Cabrera, D.; Lak, A.; Yoshida, T.; Materia, M.E.; Ortega, D.; Ludwig, F.; Guardia, P.; Sathya, A.; Pellegrino, T.; Teran, F.J. Unraveling viscosity effects on the hysteresis losses of magnetic nanocubes. Nanoscale 2017, 9, 5094–5101. [Google Scholar] [CrossRef] [PubMed]
- ASTM F-756; Standard Practice for Assessment of Hemolytic Properties of Materials; Annual Book of ASTM Standards. Committee F04 Medical and Surgical Materials and Devices, Subcommittee F04.16 Biocompatibility Test Methods. ASTM: West Conshohocken, PA, USA, 2009.
- Motta, A.C.; Duek, E.A.R. Synthesis and characterization of a novel terpolymer based on L-lactide, D,L-lactide and trimethylene carbonate. Mater. Res. 2014, 17, 619–626. [Google Scholar] [CrossRef]
- Vogel, A.I. A Text Book of Quantitative Inorganic Analysis; Longman: London, UK, 1962. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, N.L.; Tavarez, S.; Gonzalez-Sanchez, Z.I. In vitro toxicity assessment of zinc and nickel ferrite nanoparticles in human erythrocytes and peripheral blood mononuclear cell. Toxicol. In Vitro 2019, 57, 54–61. [Google Scholar] [CrossRef]

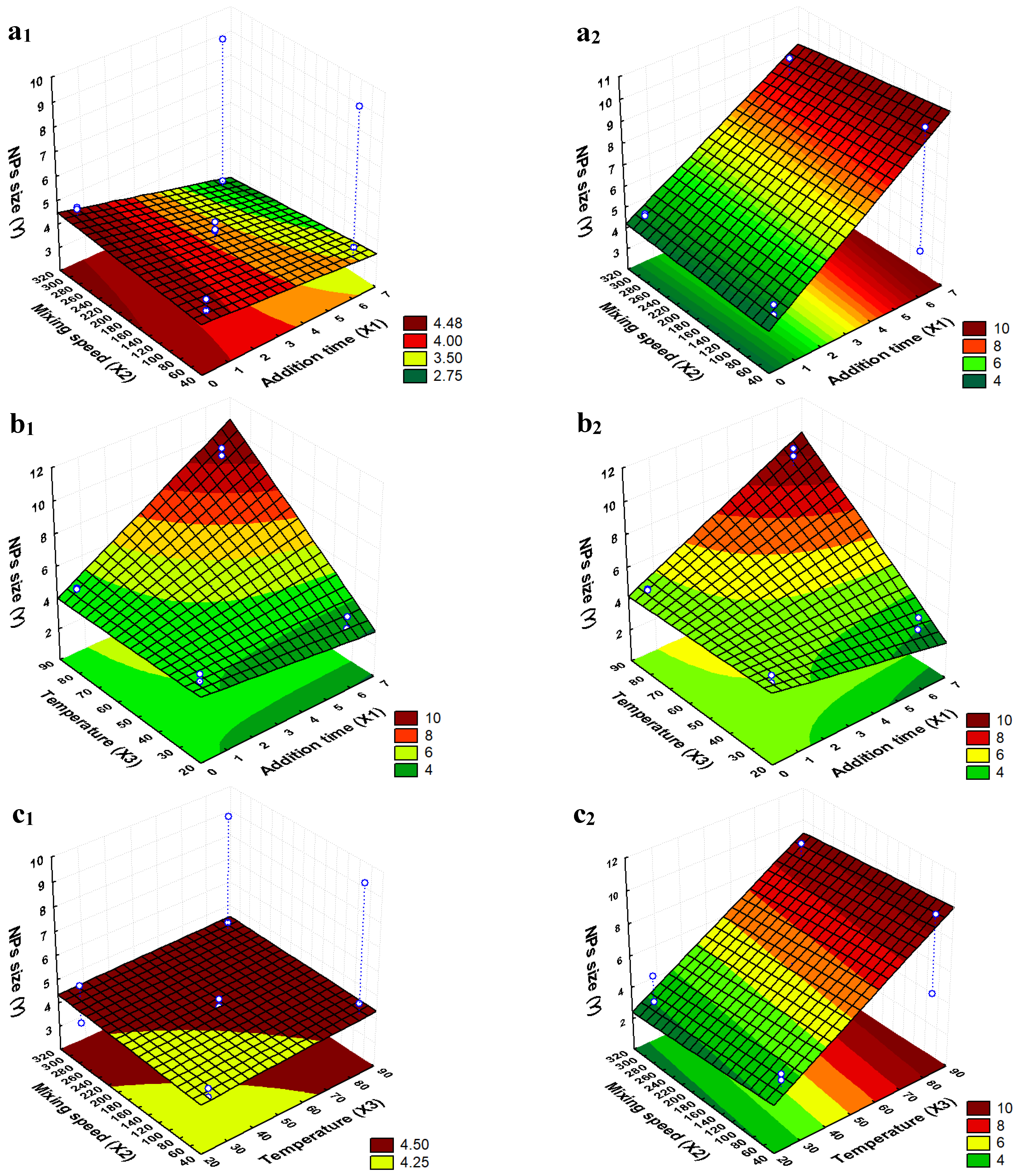


| Independent Factor | Level | ||
|---|---|---|---|
| Low Level (−1) | Middle Level (0) | High Level (+1) | |
| X1 (time) | 40 s | 3 min 32 s | 6 min 24 s |
| X2 (rpm) | 50 | 175 | 300 |
| X3 (°C) | 25 | 55 | 85 |
| Run | X1 | X2 | X3 | X1X2 | X1X3 | X2X3 | Y (nm) |
|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 1 | 1 | 1 | 4.3 |
| 2 | 1 | −1 | −1 | −1 | −1 | 1 | 3.9 |
| 3 | −1 | 1 | −1 | −1 | 1 | −1 | 4.8 |
| 4 | 1 | 1 | −1 | 1 | −1 | −1 | 3.2 |
| 5 | −1 | −1 | 1 | 1 | −1 | −1 | 4.8 |
| 6 | 1 | −1 | 1 | −1 | 1 | −1 | 9.6 |
| 7 | −1 | 1 | 1 | −1 | −1 | 1 | 4.7 |
| 8 | 1 | 1 | 1 | 1 | 1 | 1 | 9.2 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 4.2 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 4.6 |
| Factor and Interaction | Effect Value | p-Value |
|---|---|---|
| X1 | 1.84 | 0.006 |
| X2 | −0.195 | 0.314 |
| X3 | 3.03 | 0.002 |
| X1X2 | −0.405 | 0.110 |
| X1X3 | 2.84 | 0.003 |
| X2X3 | −0.065 | 0.701 |
| Sample | Z-Ave/nm | PDI | ZP/mV |
|---|---|---|---|
| Blank nanoparticles * | 243 ± 6 | 0.130 | −18 ± 6 |
| mag1 | 78 ± 1 | 0.194 | −22 ± 4 |
| mag1@poly | 193 ± 5 | 0.219 | −15 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, A.A.; Valverde, T.M.; Machado, V.d.O.; do Nascimento da Silva, E.; Fagundes, D.A.; Oliveira, F.d.P.; Freitas, E.T.F.; Ardisson, J.D.; Ferreira, J.M.d.F.; Oliveira, J.A.d.C.; et al. Heating Capacity and Biocompatibility of Hybrid Nanoparticles for Magnetic Hyperthermia Treatment. Int. J. Mol. Sci. 2024, 25, 493. https://doi.org/10.3390/ijms25010493
Gomes AA, Valverde TM, Machado VdO, do Nascimento da Silva E, Fagundes DA, Oliveira FdP, Freitas ETF, Ardisson JD, Ferreira JMdF, Oliveira JAdC, et al. Heating Capacity and Biocompatibility of Hybrid Nanoparticles for Magnetic Hyperthermia Treatment. International Journal of Molecular Sciences. 2024; 25(1):493. https://doi.org/10.3390/ijms25010493
Chicago/Turabian StyleGomes, Aline Alexandrina, Thalita Marcolan Valverde, Vagner de Oliveira Machado, Emanueli do Nascimento da Silva, Daniele Alves Fagundes, Fernanda de Paula Oliveira, Erico Tadeu Fraga Freitas, José Domingos Ardisson, José Maria da Fonte Ferreira, Junnia Alvarenga de Carvalho Oliveira, and et al. 2024. "Heating Capacity and Biocompatibility of Hybrid Nanoparticles for Magnetic Hyperthermia Treatment" International Journal of Molecular Sciences 25, no. 1: 493. https://doi.org/10.3390/ijms25010493





