Preparation and Optical Study of 1-Formamido-5-Isocyanonaphthalene, the Hydrolysis Product of the Potent Antifungal 1,5-Diisocyanonaphthalene
Abstract
:1. Introduction
2. Results and Discussion
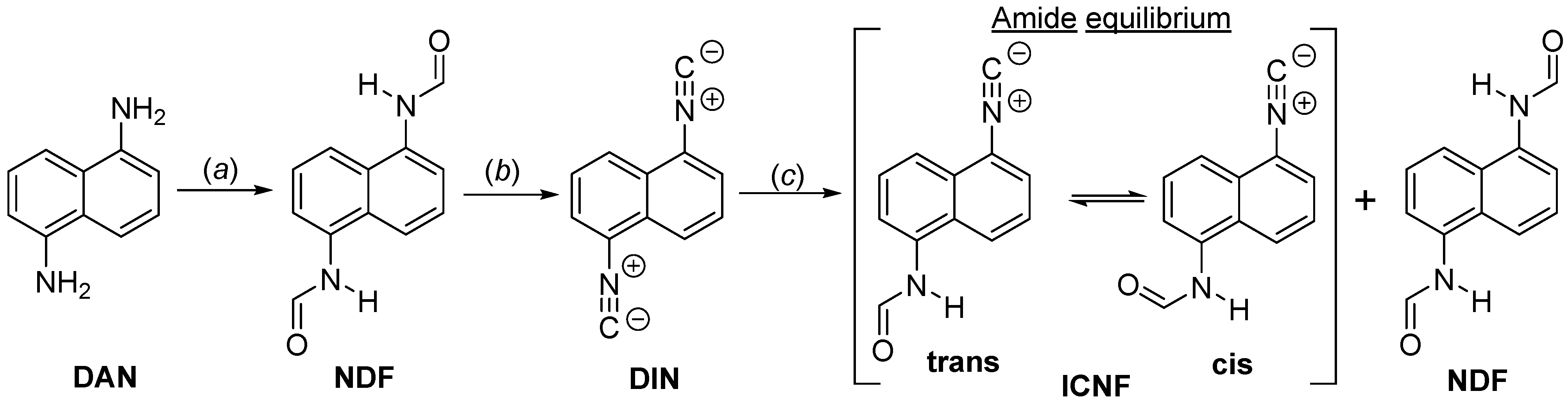
2.1. Synthesis of 1,5-Diisocyanonaphthalene
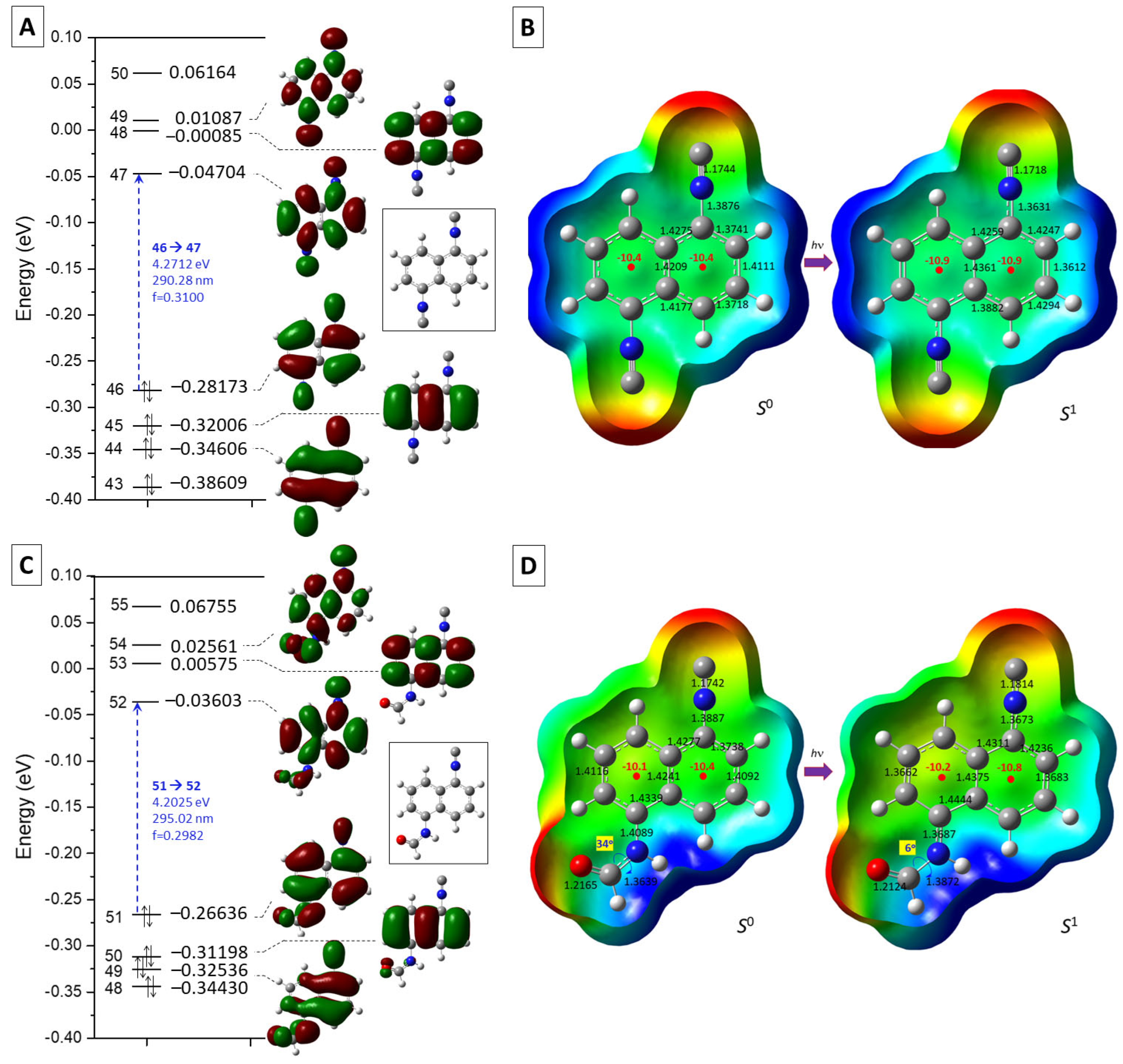
2.2. Optical Characterization of 1,5-Diisocyanonaphthalene (DIN) and Related Computational Studies
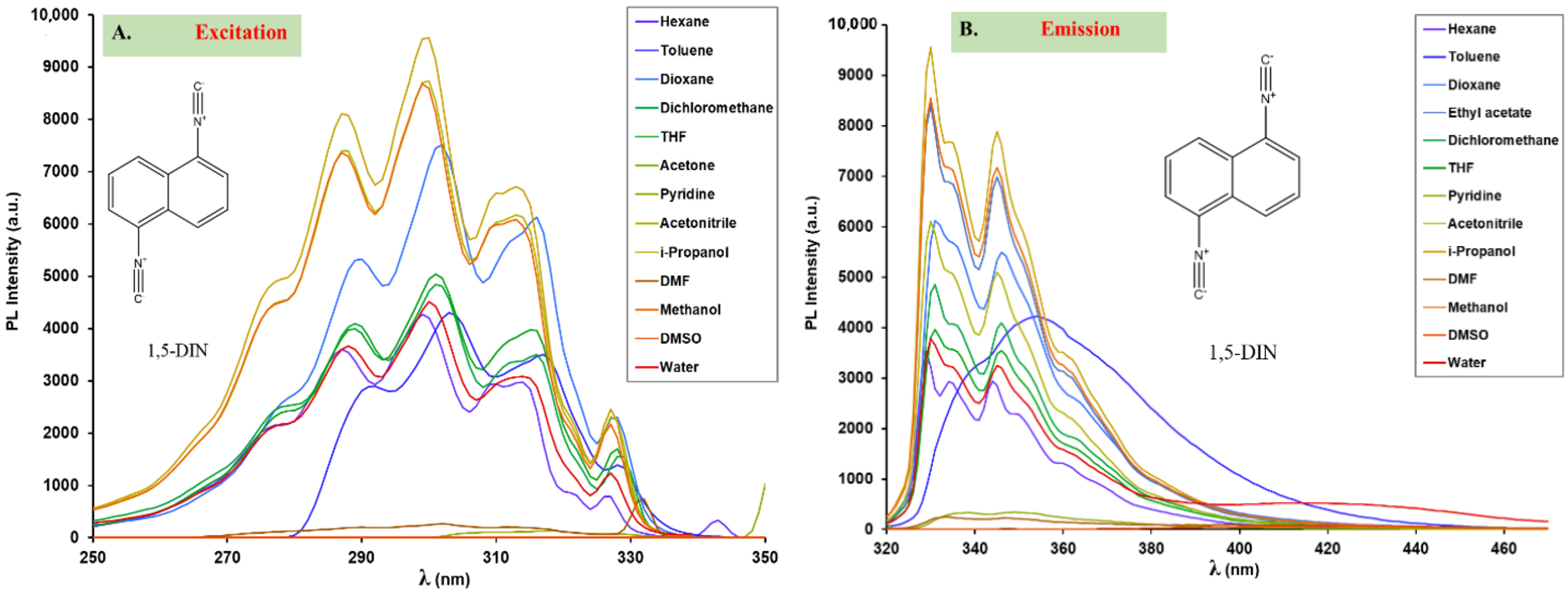
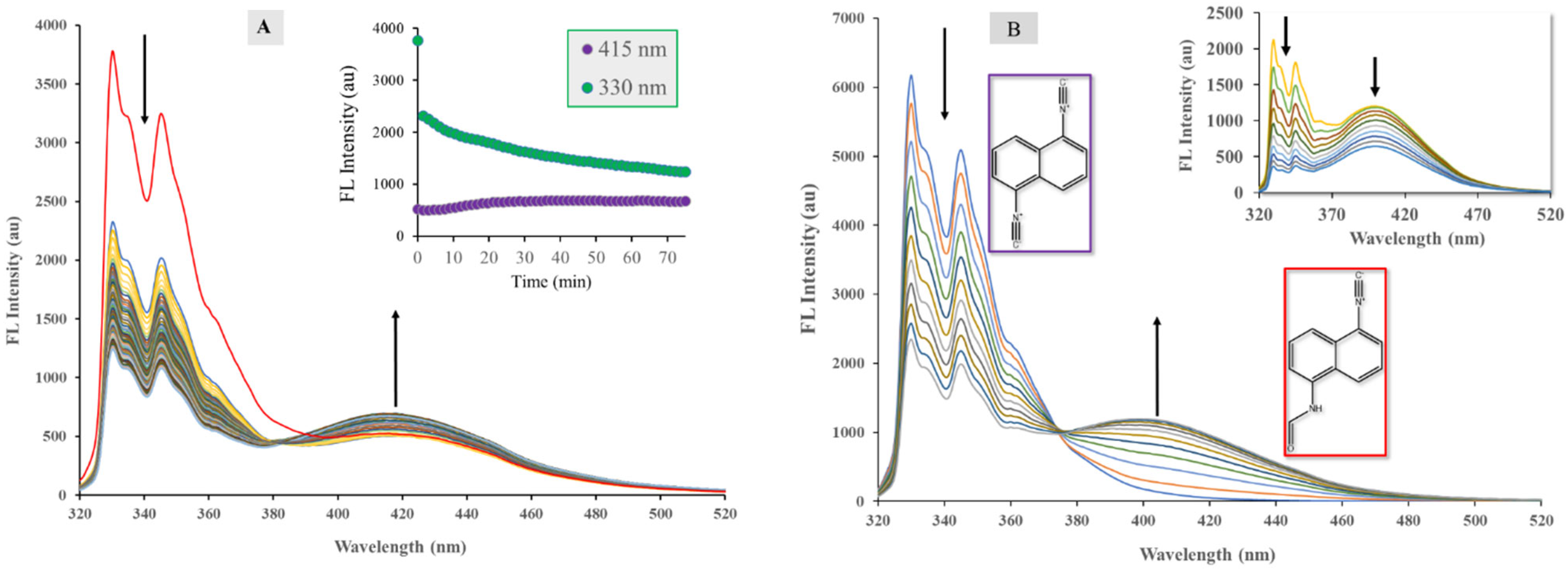
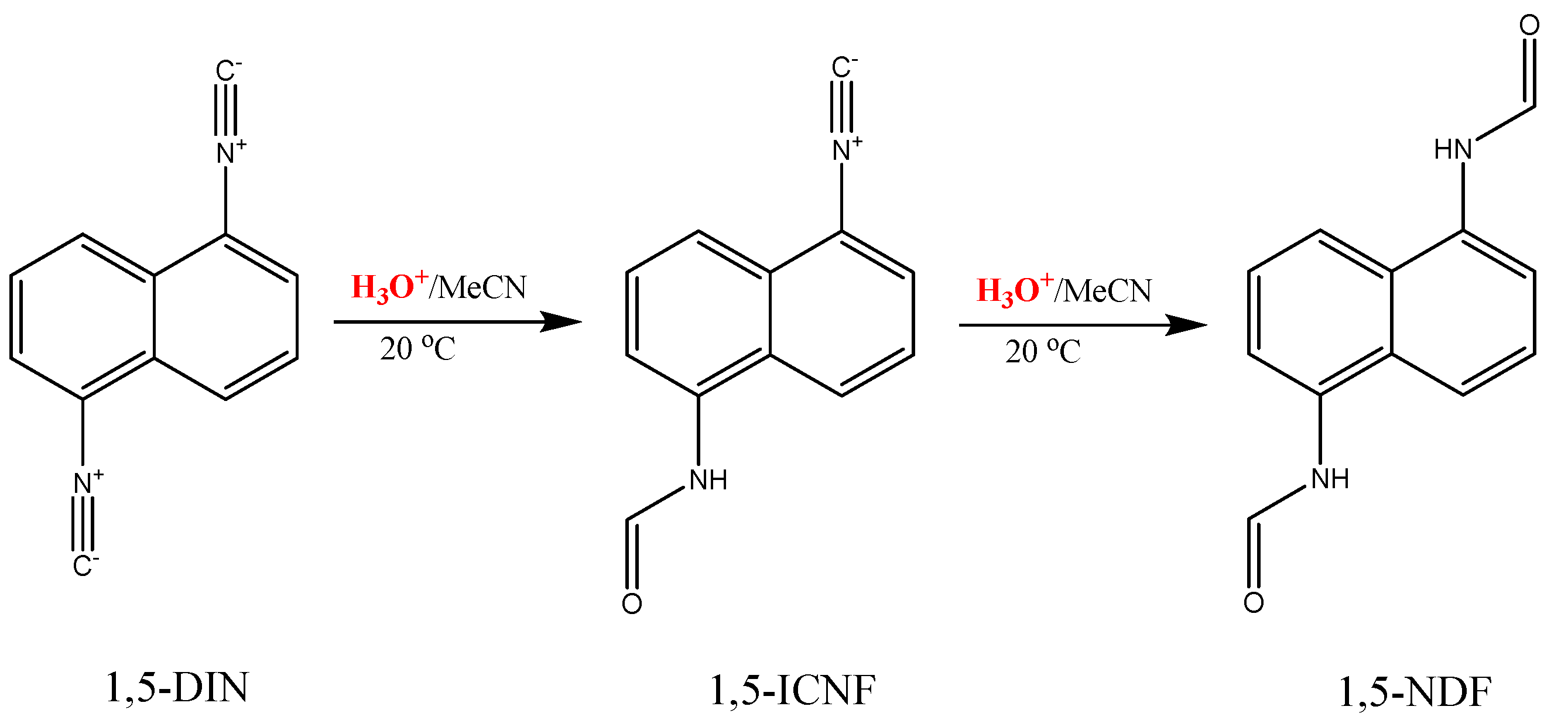
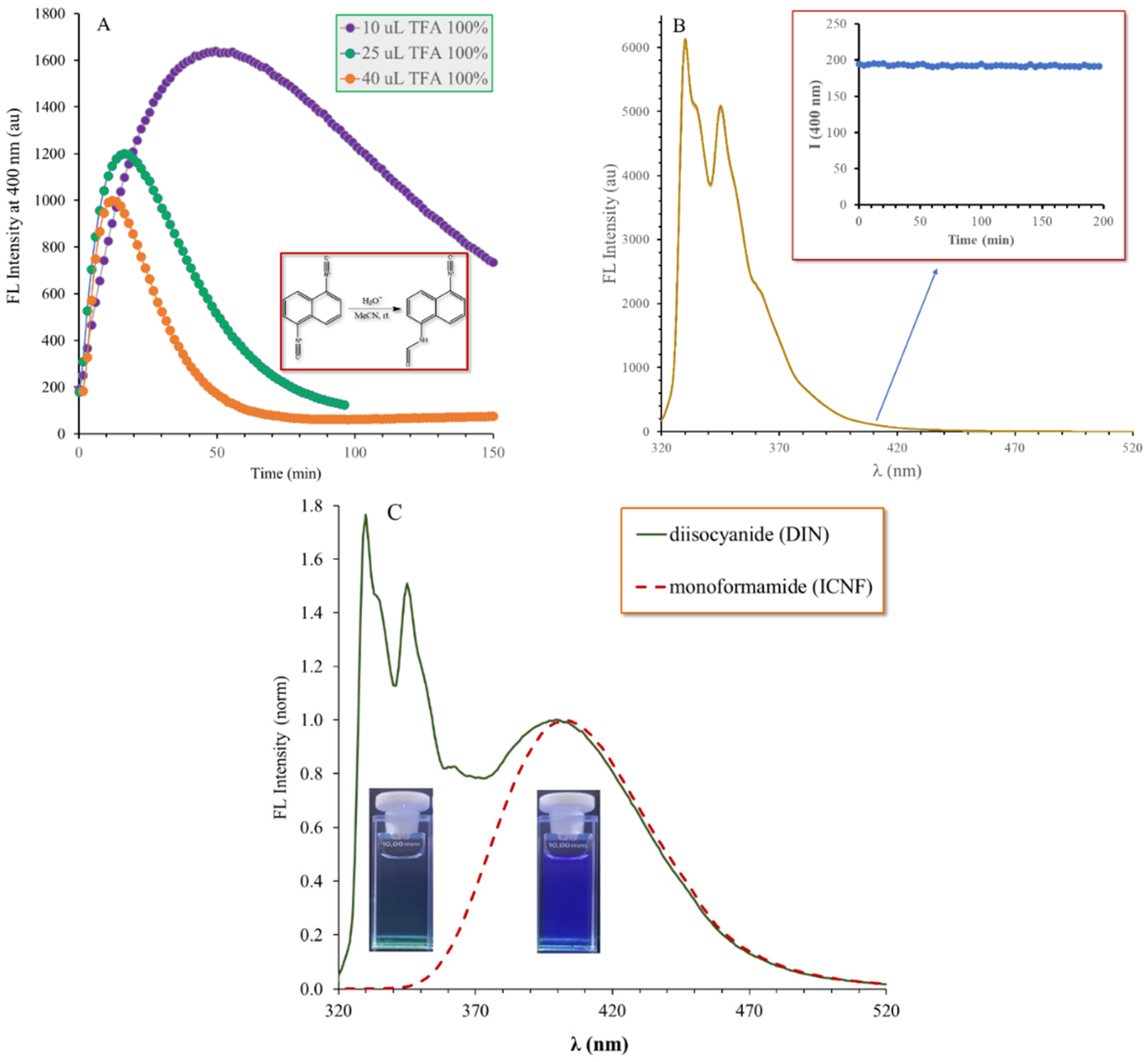
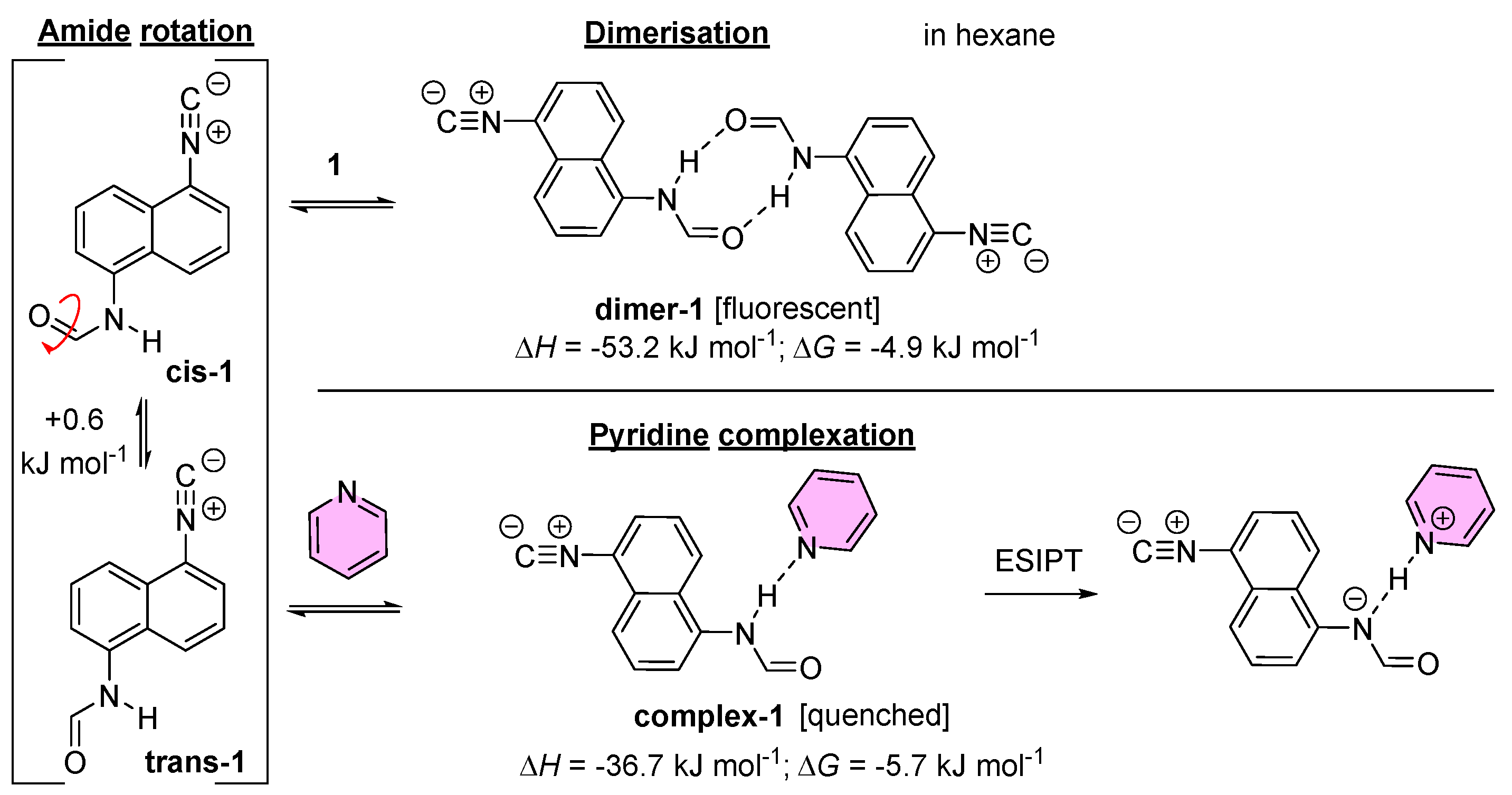
2.3. The Hydrolysis of 1,5-Diisocyanonaphthalene and the Formation of 1-Formamido-5-Isocyanonaphthalene (ICAF)
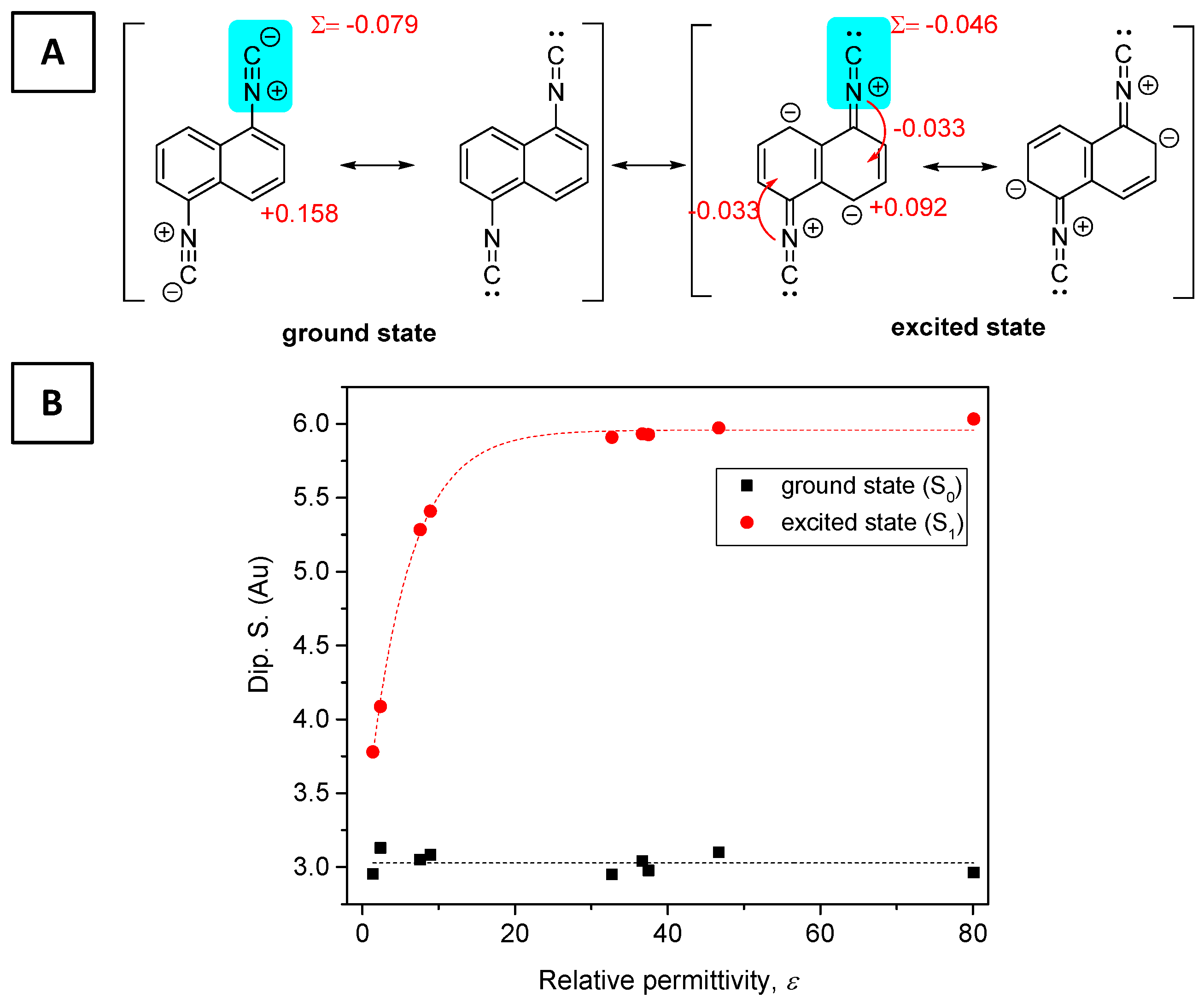
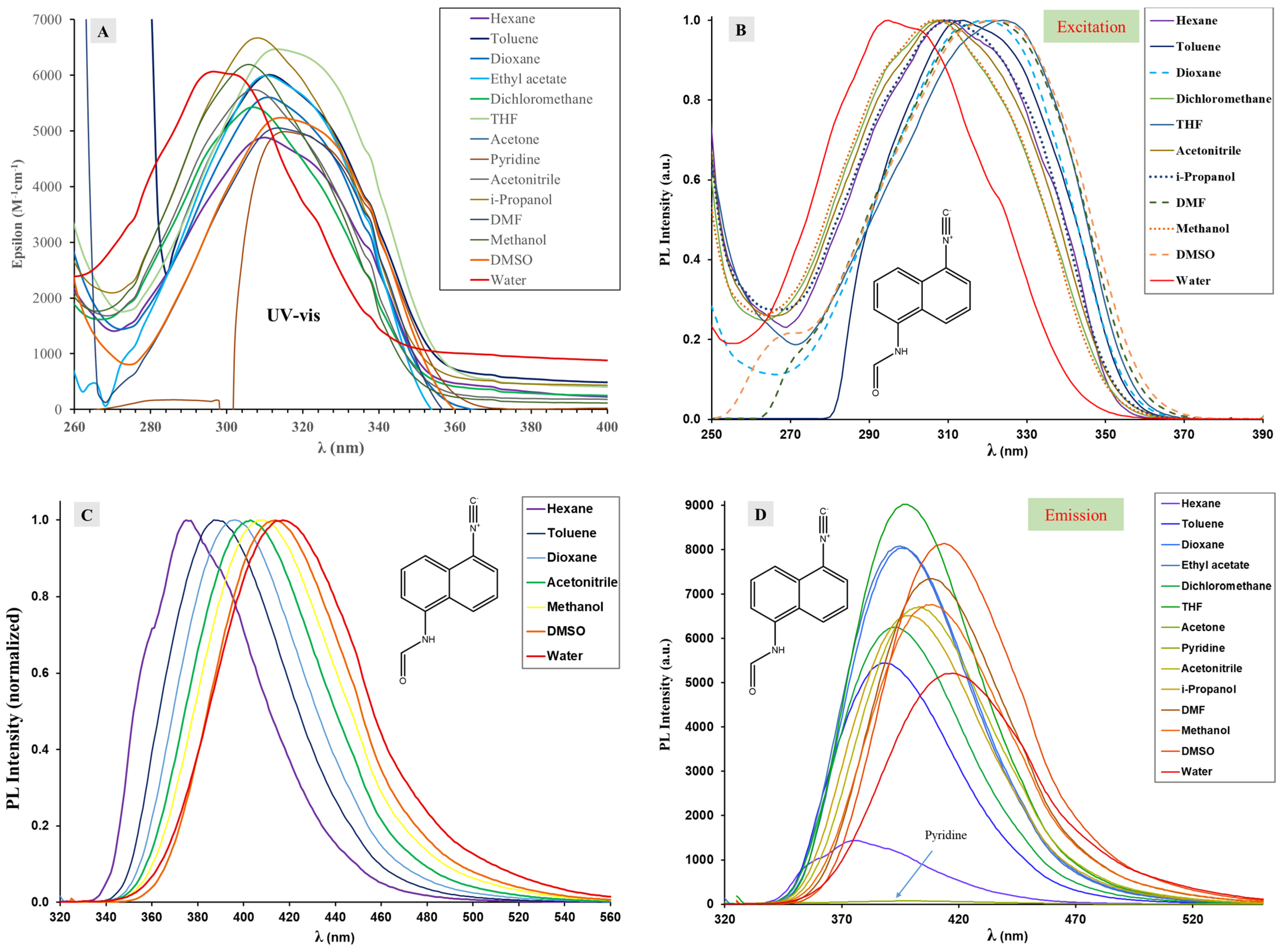
2.4. Optophysical Properties of 1-Formamido-5-Isocyanonaphthalene (ICNF) and the Related Computational Study
2.5. Analysis of ICNF Fluorescence Quenching by Pyridine
3. Materials and Methods
3.1. Synthesis
- 1H NMR (400 MHz, DMSO-d6) δ = 10.68 (s, 1H, H9), 10.51 (s, 1H, H7), 8.64 (d, J = 5.6 Hz, 1H, H10), 8.51 (d, J = 1.6 Hz, 1H, H8), 8.30 (dd, J = 8.8, 3.9 Hz, 2H, H4), 8.16 (d, J = 7.6 Hz, 1H, H3), 7.92 (dd, J = 12.9, 8.1 Hz, 4H, H1, H6), 7.80–7.69 (m, 2H, H2), 7.69–7.59 (m, 3H, H5, H11) ppm (Figure S5).
- 13C NMR (101 MHz, DMSO-d6) δ = 160.5, 133.6, 128.5, 127.9, 125.7, 125.6, 125.4, 124.4, 120.9, 119.3, 118.8 ppm (Figure S6).
- HRMS (ESI/Q-TOF) m/z: [M+H]+ Calc. for C12H9N2O 197.0709; found 197.0652.
3.2. Density Functional Theory (DFT) Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nenajdenko, V.G. Isocyanide Chemistry; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar] [CrossRef]
- Massarotti, A.; Brunelli, F.; Aprile, S.; Giustiniano, M.; Tron, G.C. Medicinal Chemistry of Isocyanides. Chem. Rev. 2021, 121, 10742–10788. [Google Scholar] [CrossRef]
- Singleton, E.; Oosthuizen, H.E. Metal Isocyanide Complexes. In Advances in Organometallic Chemistry; Elsevier Inc.: Amsterdam, The Netherlands, 1983; pp. 209–310. [Google Scholar] [CrossRef]
- Cankařová, N.; Krchňák, V. Isocyanide Multicomponent Reactions on Solid Phase: State of the Art and Future Application. Int. J. Mol. Sci. 2020, 21, 9160. [Google Scholar] [CrossRef]
- Sung, K.; Chen, C.-C. Kinetics and mechanism of acid-catalyzed hydrolysis of cyclohexyl isocyanide and pKa determination of N-cyclohexylnitrilium ion. Tetrahedron Lett. 2001, 42, 4845–4848. [Google Scholar] [CrossRef]
- Lu, T.; Yang, X.; Wang, X.-Y.; Li, Z.; Yin, J.; Liu, S.H. Dithienylethene-bridged gold(I) isocyanide complexes: Synthesis, photochromism and “turn-on” fluorescent switching behavior. Dye. Pigment. 2021, 185, 108933. [Google Scholar] [CrossRef]
- Mikherdov, A.S.; Popov, R.A.; Smirnov, A.S.; Eliseeva, A.A.; Novikov, A.S.; Boyarskiy, V.P.; Gomila, R.M.; Frontera, A.; Kukushkin, V.Y.; Bokach, N.A. Isocyanide and Cyanide Entities Form Isostructural Halogen Bond-Based Supramolecular Networks Featuring Five-Center Tetrafurcated Halogen···C/N Bonding. Cryst. Growth Des. 2022, 22, 6079–6087. [Google Scholar] [CrossRef]
- Zhou, N.; Zhang, Y.; Chen, Z.; Zhu, X.; He, X. Developing luminescent ratiometric thermometers based on copolymers containing Platinum(II) isocyanide complex. Dye. Pigment. 2021, 184, 108815. [Google Scholar] [CrossRef]
- Praveen, P.L. Estimation of absorption spectral shifts of cyano biphenyl liquid crystals: An impact of different solvents and oxygen substitution. J. Mol. Liq. 2021, 343, 117620. [Google Scholar] [CrossRef]
- Nayak, S.K.; Praveen, P.L. Mesophase behaviour of a cyanobiphenyl molecule in polar aprotic solvent: Rigidity effect. J. Phys. Sci. 2020, 31, 33–45. [Google Scholar] [CrossRef]
- Rácz, D.; Nagy, M.; Mándi, A.; Zsuga, M.; Kéki, S. Solvatochromic properties of a new isocyanonaphthalene based fluorophore. J. Photochem. Photobiol. A Chem. 2013, 270, 19–27. [Google Scholar] [CrossRef]
- Kovács, S.L.; Nagy, M.; Fehér, P.P.; Zsuga, M.; Kéki, S. Effect of the Substitution Position on the Electronic and Solvatochromic Properties of Isocyanoaminonaphthalene (ICAN) Fluorophores. Molecules 2019, 24, 2434. [Google Scholar] [CrossRef]
- Nagy, M.; Rácz, D.; Lázár, L.; Purgel, M.; Ditrói, T.; Zsuga, M. Solvatochromic Study of Highly Fluorescent Alkylated Isocyanonaphthalenes, Their π-Stacking, Hydrogen-Bonding Complexation, and Quenching with Pyridine. Chemphyschem 2014, 15, 3614–3625. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.; Rácz, D.; Nagy, Z.L.; Nagy, T.; Fehér, P.P.; Purgel, M.; Zsuga, M.; Kéki, S. An acrylated isocyanonaphthalene based solvatochromic click reagent: Optical and biolabeling properties and quantum chemical modeling. Dye. Pigm. 2016, 133, 445–457. [Google Scholar] [CrossRef]
- Nagy, M.; Rácz, D.; Nagy, Z.L.; Fehér, P.P.; Kalmár, J.; Fábián, I.; Kiss, A.; Zsuga, M.; Kéki, S. Solvatochromic isocyanonaphthalene dyes as ligands for silver(I) complexes, their applicability in silver(I) detection and background reduction in biolabelling. Sens. Actuators B Chem. 2018, 255, 2555–2567. [Google Scholar] [CrossRef]
- Nagy, M.; Kéki, S.; Rácz, D.; Mathur, J.; Vereb, G.; Garda, T.; M-Hamvas, M.; Chaumont, F.; Bóka, K.; Böddi, B.; et al. Novel fluorochromes label tonoplast in living plant cells and reveal changes in vacuolar organization after treatment with protein phosphatase inhibitors. Protoplasma 2017, 255, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.; Kovács, S.L.; Nagy, T.; Rácz, D.; Zsuga, M.; Kéki, S. Isocyanonaphthalenes as extremely low molecular weight, selective, ratiometric fluorescent probes for Mercury(II). Talanta 2019, 201, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.; Szemán-Nagy, G.; Kiss, A.; Nagy, Z.L.; Tálas, L.; Rácz, D.; Majoros, L.; Tóth, Z.; Szigeti, Z.M.; Pócsi, I.; et al. Antifungal Activity of an Original Amino-Isocyanonaphthalene (ICAN) Compound Family: Promising Broad Spectrum Antifungals. Molecules 2020, 25, 903. [Google Scholar] [CrossRef]
- Nagy, M.; Rácz, D.; Nagy, Z.L.; Fehér, P.P.; Kovács, S.L.; Bankó, C.; Bacsó, Z.; Kiss, A.; Zsuga, M.; Kéki, S. Amino-isocyanoacridines: Novel, Tunable Solvatochromic Fluorophores as Physiological pH Probes. Sci. Rep. 2019, 9, 8250. [Google Scholar] [CrossRef]
- Bankó, C.; Nagy, Z.L.; Nagy, M.; Szemán-Nagy, G.G.; Rebenku, I.; Imre, L.; Tiba, A.; Hajdu, A.; Szöllősi, J.; Kéki, S.; et al. Isocyanide Substitution in Acridine Orange Shifts DNA Damage-Mediated Phototoxicity to Permeabilization of the Lysosomal Membrane in Cancer Cells. Cancers 2021, 13, 5652. [Google Scholar] [CrossRef]
- Nagy, M.; Fiser, B.; Szőri, M.; Vanyorek, L.; Viskolcz, B. Optical Study of Solvatochromic Isocyanoaminoanthracene Dyes and 1,5-Diaminoanthracene. Int. J. Mol. Sci. 2022, 23, 1315. [Google Scholar] [CrossRef]
- Szigeti, Z.M.; Talas, L.; Szeles, A.; Hargitai, Z.; Nagy, Z.L.; Nagy, M.; Kiss, A.; Keki, S.; Szeman-Nagy, G. Potential Original Drug for Aspergillosis: In Vitro and In Vivo Effects of 1-N,N-Dimethylamino-5-Isocyanonaphthalene (DIMICAN) on Aspergillus fumigatus. J. Fungi 2022, 8, 985. [Google Scholar] [CrossRef]
- Steenwyk, J.L.; Mead, M.E.; de Castro, P.A.; Valero, C.; Damasio, A.; dos Santos, R.A.C.; Labella, A.L.; Li, Y.; Knowles, S.L.; Raja, H.A.; et al. Genomic and Phenotypic Analysis of COVID-19-Associated Pulmonary Aspergillosis Isolates of Aspergillus fumigatus. Microbiol. Spectr. 2021, 9, e0001021. [Google Scholar] [CrossRef] [PubMed]
- Scheuer, P.J. Isocyanides and cyanides as natural products. Acc. Chem. Res. 2002, 25, 433–439. [Google Scholar] [CrossRef]
- Zhang, X.; Evanno, L.; Poupon, E. Biosynthetic Routes to Natural Isocyanides. Eur. J. Org. Chem. 2020, 2020, 1919–1929. [Google Scholar] [CrossRef]
- Edenborough, M.S.; Herbert, R.B. Naturally occurring isocyanides. Nat. Prod. Rep. 1988, 5, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Galli, U.; Tron, G.C.; Purghe, B.; Grosa, G.; Aprile, S. Metabolic Fate of the Isocyanide Moiety: Are Isocyanides Pharmacophore Groups Neglected by Medicinal Chemists? Chem. Res. Toxicol. 2020, 33, 955–966. [Google Scholar] [CrossRef]
- Marcelli, T.; Himo, F. Reaction of Carboxylic Acids with Isocyanides: A Mechanistic DFT Study. Eur. J. Org. Chem. 2008, 2008, 4751–4754. [Google Scholar] [CrossRef]
- Bruffaerts, J.; von Wolff, N.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. Formamides as Isocyanate Surrogates: A Mechanistically Driven Approach to the Development of Atom-Efficient, Selective Catalytic Syntheses of Ureas, Carbamates, and Heterocycles. J. Am. Chem. Soc. 2019, 141, 16486–16493. [Google Scholar] [CrossRef]
- Salami, S.A.; Siwe-Noundou, X.; Krause, R.W.M. A More Sustainable Isocyanide Synthesis from N-Substituted Formamides Using Phosphorus Oxychloride in the Presence of Triethylamine as Solvent. Molecules 2022, 27, 6850. [Google Scholar] [CrossRef]
- Berlman, I.B. Handbook of Fluorescence Spectra of Aromatic Molecules; Academic Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Schleyer, P.V.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; Hommes, N.J.R.V.E. Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef]
- Mucsi, Z.; Viskolcz, B.; Csizmadia, I.G. A Quantitative Scale for the Degree of Aromaticity and Antiaromaticity: A Comparison of Theoretical and Experimental Enthalpies of Hydrogenation. J. Phys. Chem. A 2007, 111, 1123–1132. [Google Scholar] [CrossRef]
- Jancsó, A.; Kovács, E.; Cseri, L.; Rózsa, B.J.; Galbács, G.; Csizmadia, I.G.; Mucsi, Z. Synthesis and spectroscopic characterization of novel GFP chromophore analogues based on aminoimidazolone derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 218, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Pálfi, D.; Chiovini, B.; Szalay, G.; Kaszás, A.; Turi, G.F.; Katona, G.; Ábrányi-Balogh, P.; Szőri, M.; Potor, A.; Frigyesi, O.; et al. High efficiency two-photon uncaging coupled by the correction of spontaneous hydrolysis. Org. Biomol. Chem. 2018, 16, 1958–1970. [Google Scholar] [CrossRef]
- Zhao, G.-J.; Han, K.-L. Hydrogen Bonding in the Electronic Excited State. Acc. Chem. Res. 2011, 45, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Catalan, J.; Hopf, H. Empirical treatment of inductive and dispersive components of solute-solvent interactions: The polarizability solvent scale. Eur. J. Org. Chem. 2004, 2004, 4694–4702. [Google Scholar] [CrossRef]
- Catalan, J. Toward a generalized treatment of the solvent effect on flow empirical scales: Dipolarity (SdP, a new scale), polarizability (SP), acidity (SA) and basicity (SB) of the medium. J. Phys. Chem. B 2009, 113, 5951–5960. [Google Scholar] [CrossRef] [PubMed]
- Menges, F. Spectragryph Software v1.2. Available online: https://www.effemm2.de/spectragryph/index.html (accessed on 26 March 2023).
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Raghavachari, K.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. 20. Basis set for correlated wave-functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. (Creators); GaussView, version 5.0; Revision; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
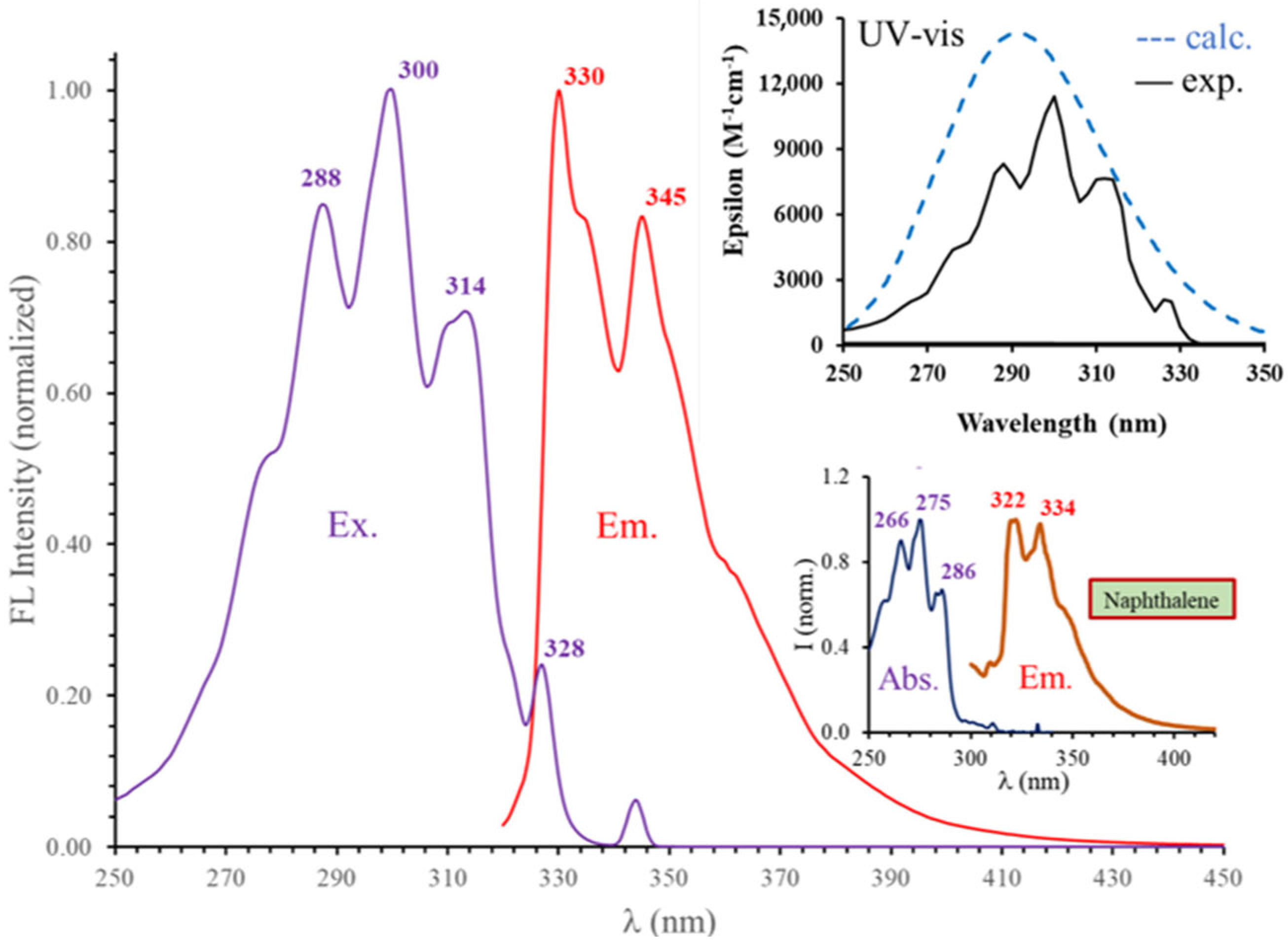


| Solvent (εr) | Experimental | Computed | ||||
|---|---|---|---|---|---|---|
| λAbs (nm) | ε × 10−3 (M−1cm−1) | λEm (nm) | Φf (%) | λAbs (nm) | λEm (nm) | |
| n-Hexane (1.89) | 300 | 10.3 | 329, 344 | 13.2 | 290 | 349 |
| Dioxane (2.25) | 302 | 14.2 | 331, 346 | 17.7 | nc | nc |
| Toluene (2.38) | 302 | 11.0 | 352 | 21.4 | 291 | 352 |
| EtOAc (6.02) | 300 | 14.9 | 330, 345 | 15.7 | nc | nc |
| THF (7.58) | 300 | 11.6 | 331, 346 | 14.8 | 291 | 360 |
| DCM (8.93) | 302 | 14.6 | 331, 346 | 10.0 | 291 | 361 |
| Pyridine (12.4) | 304 | 9.8 | 337, 352 | 0.7 | nc | nc |
| 2-Propanol (17.9) | 300 | 13.3 | 330, 345 | 19.4 | nc | nc |
| Methanol (32.7) | 300 | 12.0 | 330, 345 | 18.3 | 290 | 365 |
| DMF (36.7) | 302 | 13.3 | 333, 347 | 1.1 | 291 | 365 |
| Acetonitrile (37.5) | 300 | 11.4 | 330, 345 | 20.6 | 290 | 365 |
| DMSO (46.7) | 303 | 11.3 | - | 0.5 | 291 | 365 |
| Water (80.1) | 300 | 4.0 | 330, 345 | - | 290 | 366 |
| Solvent (εr) | 1,5-ICNF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λAbs (nm) | ε × 10−3 (M−1cm−1) | λEx (nm) | λEm (nm) | Φf | ΔνSS (cm−1) | λAbs (nm) Implicit Calc. | λEm (nm) Implicit Calc. | λAbs (nm) Explicit Calc. | λEm (nm) Explicit Calc. | |
| n-Hexane (1.89) | 310 | 4.9 | 310 | 375 | 0.11 | 5591 | 308 | 373 | na | na |
| dimer in hexane | 297 | 367 | ||||||||
| Dioxane (2.25) | 310 | 5.6 | 318 | 396 | 0.55 | 6070 | nc | nc | nc | nc |
| Toluene (2.38) | 313 | 6.0 | 314 | 388 | 0.42 | 6176 | 305 | 375 | nc | nc |
| EtOAc (6.02) | 313 | 6.0 | 313 | 395 | 0.52 | 6600 | nc | nc | nc | nc |
| THF (7.58) | 314 | 6.5 | 324 | 397 | 0.55 | 6658 | 303 | 383 | nc | nc |
| DCM (8.93) | 308 | 5.4 | 307 | 393 | 0.52 | 7022 | 303 | 384 | nc | nc |
| Pyridine (12.4) | 316 | 4.9 | 316 | 397 | 0.005 | 6457 | 305 | 375 | 303 | 378 |
| 2-Propanol (17.9) | 308 | 6.7 | 309 | 399 | 0.41 | 7405 | na | nc | nc | nc |
| Methanol (32.7) | 307 | 6.2 | 306 | 407 | 0.44 | 8081 | 302 | 387 | 295 | 388 |
| DMF (36.7) | 314 | 5.1 | 321 | 408 | 0.61 | 7343 | 302 | 387 | 301 | 392 |
| Acetonitrile (37.5) | 308 | 5.7 | 308 | 403 | 0.44 | 7591 | 302 | 387 | nc | nc |
| DMSO (46.7) | 314 | 5.2 | 322 | 414 | 0.72 | 7669 | 302 | 387 | 336 | 414 |
| Water (80.1) | 296 | 6.1 | 295 | 418 | 0.42 | 9843 | 302 | 388 | 292 | 385 |
| y0 (cm−1) | aSA | bSB | cSP | dSdP | R2 | n | |
|---|---|---|---|---|---|---|---|
| νem,max | 28,943 | −1519 | −982 | −3729 | −744 | 0.93 | 12 |
| Δνss | 3416 | 3025 | 448 | 3722 | 561 | 0.93 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopcsik, E.; Mucsi, Z.; Kontra, B.; Vanyorek, L.; Váradi, C.; Viskolcz, B.; Nagy, M. Preparation and Optical Study of 1-Formamido-5-Isocyanonaphthalene, the Hydrolysis Product of the Potent Antifungal 1,5-Diisocyanonaphthalene. Int. J. Mol. Sci. 2023, 24, 7780. https://doi.org/10.3390/ijms24097780
Kopcsik E, Mucsi Z, Kontra B, Vanyorek L, Váradi C, Viskolcz B, Nagy M. Preparation and Optical Study of 1-Formamido-5-Isocyanonaphthalene, the Hydrolysis Product of the Potent Antifungal 1,5-Diisocyanonaphthalene. International Journal of Molecular Sciences. 2023; 24(9):7780. https://doi.org/10.3390/ijms24097780
Chicago/Turabian StyleKopcsik, Erika, Zoltán Mucsi, Bence Kontra, László Vanyorek, Csaba Váradi, Béla Viskolcz, and Miklós Nagy. 2023. "Preparation and Optical Study of 1-Formamido-5-Isocyanonaphthalene, the Hydrolysis Product of the Potent Antifungal 1,5-Diisocyanonaphthalene" International Journal of Molecular Sciences 24, no. 9: 7780. https://doi.org/10.3390/ijms24097780
APA StyleKopcsik, E., Mucsi, Z., Kontra, B., Vanyorek, L., Váradi, C., Viskolcz, B., & Nagy, M. (2023). Preparation and Optical Study of 1-Formamido-5-Isocyanonaphthalene, the Hydrolysis Product of the Potent Antifungal 1,5-Diisocyanonaphthalene. International Journal of Molecular Sciences, 24(9), 7780. https://doi.org/10.3390/ijms24097780








