C1q/TNF-Related Proteins 1, 6 and 8 Are Involved in Corneal Epithelial Wound Closure by Targeting Relaxin Receptor RXFP1 In Vitro
Abstract
:1. Introduction
2. Results
2.1. Gene Expression of CTRP1, CTRP6, and CTRP8 in Tissues of the Lacrimal Apparatus and Ocular Surface
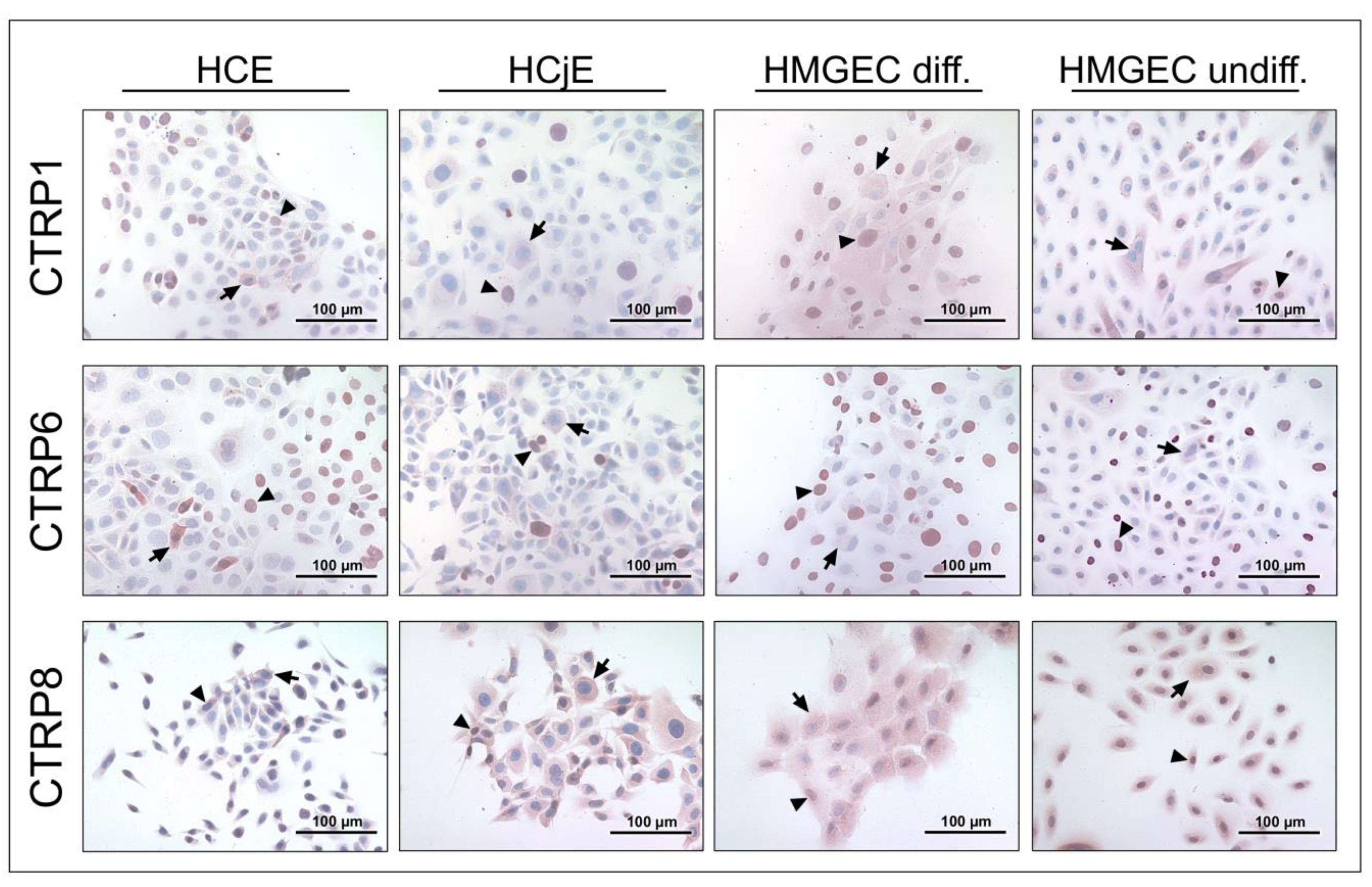
2.2. CTRP1, CTRP6, and CTRP8 Protein Expression in Tissues of the Lacrimal Apparatus and Ocular Surface
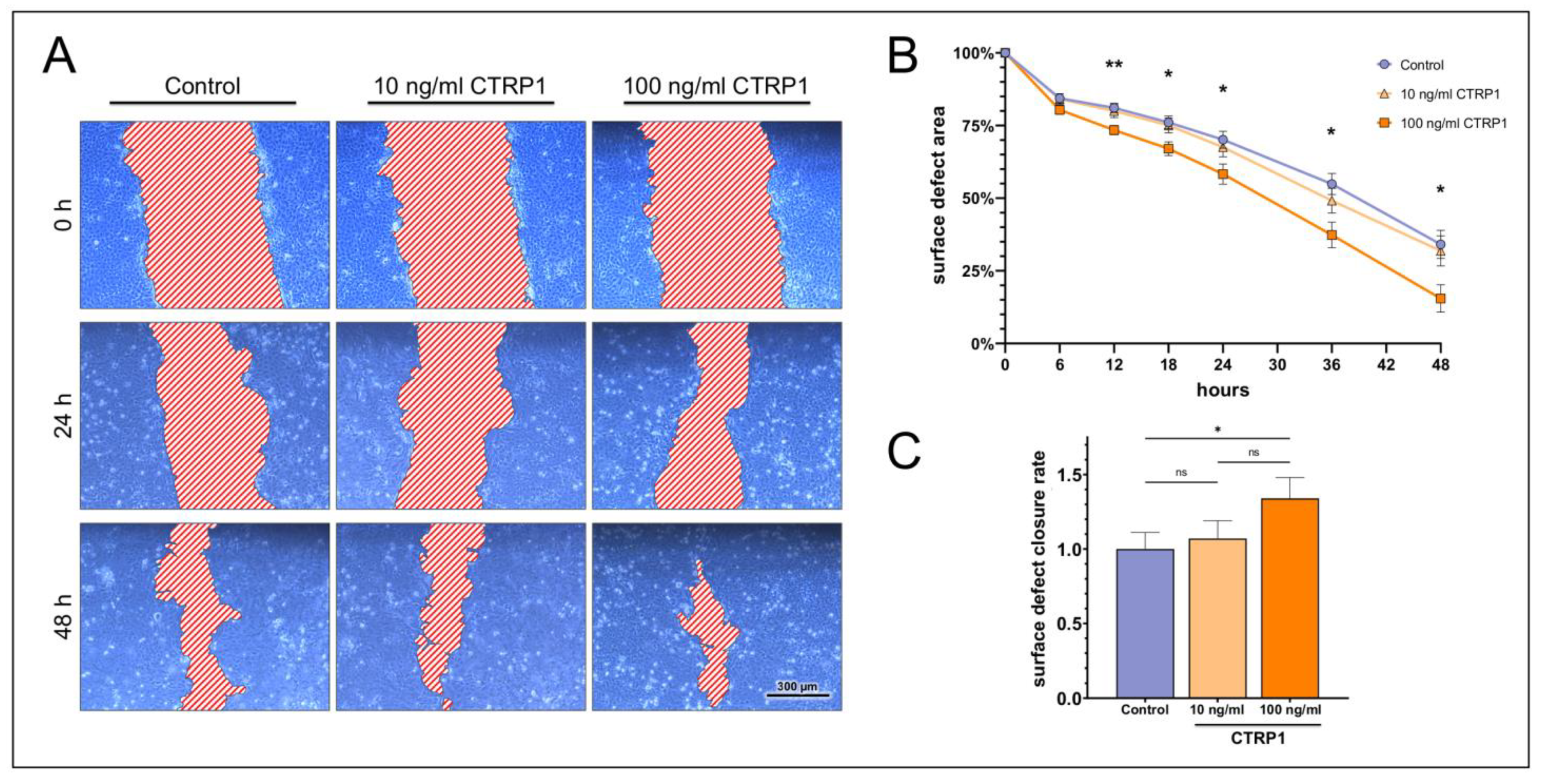
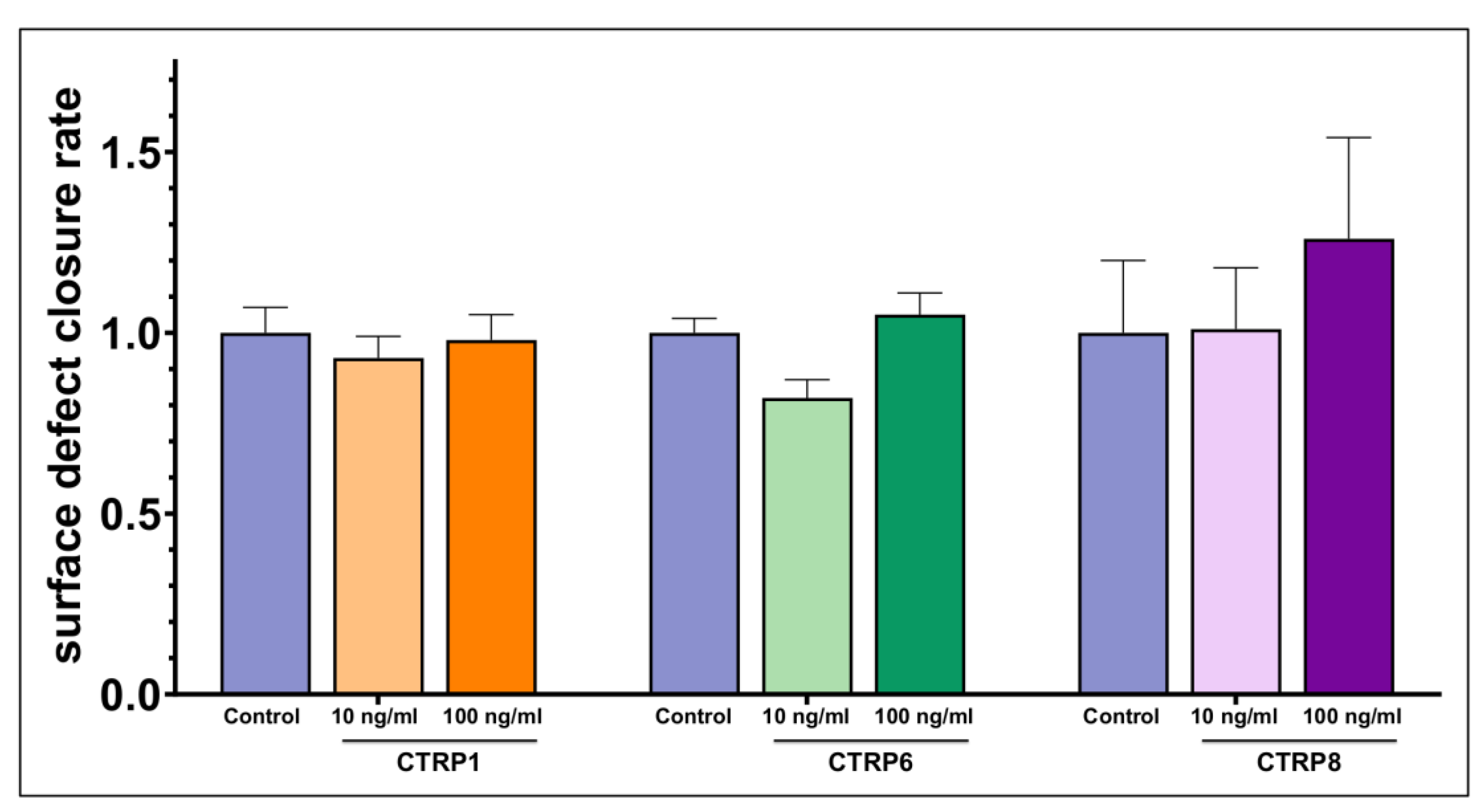
2.3. Dose-Dependent Impact on Ocular Surface Epithelial Wound Closure
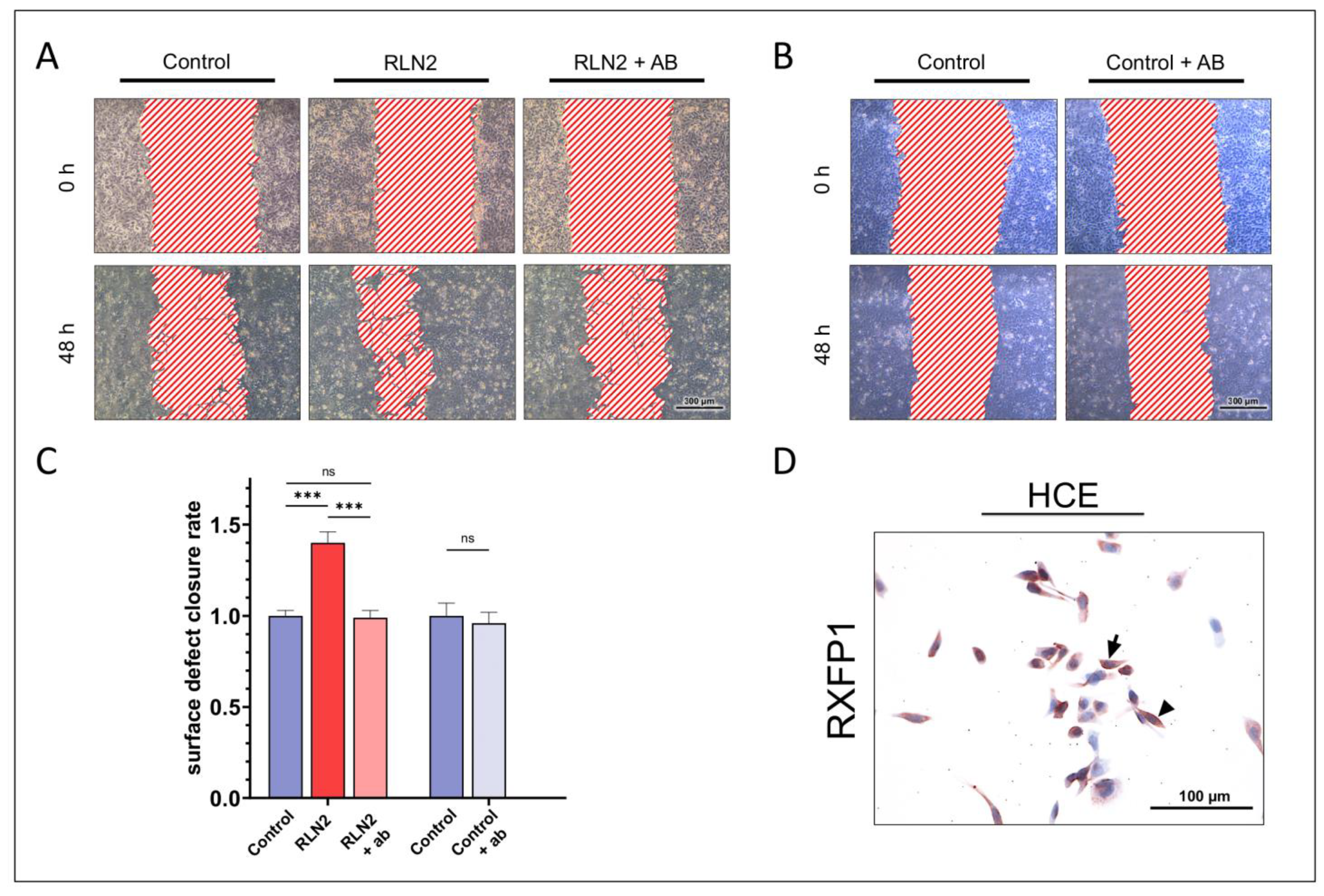
2.4. Dependence on RXFP1 Receptor Pathway
3. Materials and Methods
3.1. Subjects and Tissues
3.2. Cells and Cell Culture
3.3. RNA Preparation and Complementary DNA (cDNA) Synthesis
3.4. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
3.5. Immunohistochemistry
3.6. Wound Closure Assay (Scratch Assay)
3.7. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [Green Version]
- Brockmann, T.; Walckling, M.; Brockmann, C.; Fuchsluger, T.M.A.; Pleyer, U. Corneal wound healing-Pathophysiology and principles. Ophthalmologe 2021, 118, 1167–1177. [Google Scholar] [CrossRef]
- Hampel, U.; Klonisch, T.; Makrantonaki, E.; Sel, S.; Schulze, U.; Garreis, F.; Seltmann, H.; Zouboulis, C.C.; Paulsen, F.P. Relaxin 2 is functional at the ocular surface and promotes corneal wound healing. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7780–7790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampel, U.; Klonisch, T.; Sel, S.; Schulze, U.; Garreis, F.; Seitmann, H.; Zouboulis, C.C.; Paulsen, F.P. Insulin-like factor 3 promotes wound healing at the ocular surface. Endocrinology 2013, 154, 2034–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glogowska, A.; Kunanuvat, U.; Stetefeld, J.; Patel, T.R.; Thanasupawat, T.; Krcek, J.; Weber, E.; Wong, G.W.; Del Bigio, M.R.; Hoang-Vu, C.; et al. C1q-tumour necrosis factor-related protein 8 (CTRP8) is a novel interaction partner of relaxin receptor RXFP1 in human brain cancer cells. J. Pathol. 2013, 231, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.W.; Krawczyk, S.A.; Kitidis-Mitrokostas, C.; Revett, T.; Gimeno, R.; Lodish, H.F. Molecular, biochemical and functional characterizations of C1q/TNF family members: Adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem. J. 2008, 416, 161–177. [Google Scholar] [CrossRef] [Green Version]
- Thanasupawat, T.; Glogowska, A.; Burg, M.; Wong, G.W.; Hoang-Vu, C.; Hombach-Klonisch, S.; Klonisch, T. RXFP1 is Targeted by Complement C1q Tumor Necrosis Factor-Related Factor 8 in Brain Cancer. Front. Endocrinol. 2015, 6, 127. [Google Scholar] [CrossRef] [Green Version]
- Schäffler, A.; Buechler, C. CTRP family: Linking immunity to metabolism. Trends Endocrinol. Metab. 2012, 23, 194–204. [Google Scholar] [CrossRef]
- Peterson, J.M.; Wei, Z.; Wong, G.W. CTRP8 and CTRP9B are novel proteins that hetero-oligomerize with C1q/TNF family members. Biochem. Biophys. Res. Commun. 2009, 388, 360–365. [Google Scholar] [CrossRef]
- Klonisch, T.; Glogowska, A.; Thanasupawat, T.; Burg, M.; Krcek, J.; Pitz, M.; Jaggupilli, A.; Chelikani, P.; Wong, G.W.; Hombach-Klonisch, S. Structural commonality of C1q TNF-related proteins and their potential to activate relaxin/insulin-like family peptide receptor 1 signalling pathways in cancer cells. Br. J. Pharmacol. 2017, 174, 1025–1033. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, S.; Lei, X.; Petersen, P.S.; Tan, S.Y.; Little, H.C.; Wong, G.W. Loss of CTRP1 disrupts glucose and lipid homeostasis. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E678–E697. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Gao, L.; Zhang, D.; Yao, R.; Huang, Z.; Du, B.; Wang, Z.; Xiao, L.; Li, P.; Li, Y.; et al. C1QTNF1 attenuates angiotensin II-induced cardiac hypertrophy via activation of the AMPKa pathway. Free Radic. Biol. Med. 2018, 121, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, D.; Ohashi, K.; Shibata, R.; Mizutani, N.; Kataoka, Y.; Kambara, T.; Uemura, Y.; Matsuo, K.; Kanemura, N.; Hayakawa, S.; et al. C1q/TNF-related protein-1 functions to protect against acute ischemic injury in the heart. FASEB J. 2016, 30, 1065–1075. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Q.; Liu, Z.H.; Xue, L.; Lu, L.; Gao, J.; Shen, Y.; Yang, K.; Chen, Q.J.; Zhang, R.Y.; Shen, W.F. C1q/TNF-related protein 1 links macrophage lipid metabolism to inflammation and atherosclerosis. Atherosclerosis 2016, 250, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, S.Y. C1q and TNF related protein 1 regulates expression of inflammatory genes in vascular smooth muscle cells. Genes Genom. 2019, 41, 397–406. [Google Scholar] [CrossRef]
- Tang, J.N.; Shen, D.L.; Liu, C.L.; Wang, X.F.; Zhang, L.; Xuan, X.X.; Cui, L.L.; Zhang, J.Y. Plasma levels of C1q/TNF-related protein 1 and interleukin 6 in patients with acute coronary syndrome or stable angina pectoris. Am. J. Med. Sci. 2015, 349, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Su, Y.; Luo, L.; Jing, F.; Yi, Q. Serum levels of C1q/tumor necrosis factor-related protein-1 in children with Kawasaki disease. Pediatr. Res. 2018, 83, 999–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.Y.; Kim, H.Y.; Kim, J.H.; Lee, C.H.; Kim, D.H.; Lee, Y.H.; Han, S.H.; Lim, J.S.; Cho, D.H.; Lee, M.S.; et al. Tumor necrosis factor-alpha and interleukin-1beta increases CTRP1 expression in adipose tissue. FEBS Lett. 2006, 580, 3953–3960. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, H.; Otani, M.; Sato, S.; Toyosawa, S.; Furukawa, S.; Wakisaka, S.; Maeda, T. A novel adipokine C1q/TNF-related protein 1 (CTRP1) regulates chondrocyte proliferation and maturation through the ERK1/2 signaling pathway. Mol. Cell Endocrinol. 2013, 369, 63–71. [Google Scholar] [CrossRef]
- Park, R.; Jang, M.; Park, Y.I.; Park, Y.; Namkoong, S.; Lee, J.I.; Park, J. Elevated Levels of CTRP1 in Obesity Contribute to Tumor Progression in a p53-Dependent Manner. Cancers 2021, 13, 3619. [Google Scholar] [CrossRef]
- Chen, L.; Su, G. Identification of CTRP1 as a Prognostic Biomarker and Oncogene in Human Glioblastoma. Biomed. Res. Int. 2019, 2019, 2582416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Tang, X.; Li, L.; Liu, D.; Liu, H.; Zheng, H.; Deng, W.; Zhao, X.; Yang, G. C1q/TNF-related protein-6 is associated with insulin resistance and the development of diabetes in Chinese population. Acta Diabetol. 2018, 55, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Shemirani, F.; Djafarian, K.; Fotouhi, A.; Azadbakht, L.; Rezaei, N.; Chamari, M.; Shabani, S.; Mahmoudi, M. Effect of Paleolithic-based low-carbohydrate vs. moderate-carbohydrate diets with portion-control and calorie-counting on CTRP6, asprosin and metabolic markers in adults with metabolic syndrome: A randomized clinical trial. Clin. Nutr. ESPEN 2022, 48, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Murayama, M.A.; Kakuta, S.; Inoue, A.; Umeda, N.; Yonezawa, T.; Maruhashi, T.; Tateishi, K.; Ishigame, H.; Yabe, R.; Ikeda, S.; et al. CTRP6 is an endogenous complement regulator that can effectively treat induced arthritis. Nat. Commun. 2015, 6, 8483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Lee, W.; Park, E.J.; Park, S.Y. C1qTNF-related protein-6 increases the expression of interleukin-10 in macrophages. Mol. Cells 2010, 30, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.H.; Xiao, Y.; Huang, Q.; Hu, X.F.; Gong, Z.C.; Du, J. Role of the CTRP6/AMPK pathway in kidney fibrosis through the promotion of fatty acid oxidation. Eur. J. Pharmacol. 2021, 892, 173755. [Google Scholar] [CrossRef]
- Lei, H.; Wu, D.; Wang, J.Y.; Li, L.; Zhang, C.L.; Feng, H.; Fu, F.Y.; Wu, L.L. C1q/tumor necrosis factor-related protein-6 attenuates post-infarct cardiac fibrosis by targeting RhoA/MRTF-A pathway and inhibiting myofibroblast differentiation. Basic Res. Cardiol. 2015, 110, 35. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Z.; Yang, S.; Chen, B.; Shi, J. CTRP6 inhibits cell proliferation and ECM expression in rat mesangial cells cultured under TGF-β1. Biomed. Pharmacother. 2018, 97, 280–285. [Google Scholar] [CrossRef]
- Fan, R.H.; Zhu, X.M.; Sun, Y.W.; Peng, H.Z.; Wu, H.L.; Gao, W.J. CTRP6 inhibits fibrogenesis in TGF-β1-stimulated human dermal fibroblasts. Biochem. Biophys. Res. Commun. 2016, 475, 356–360. [Google Scholar] [CrossRef]
- Wan, X.; Zheng, C.; Dong, L. Inhibition of CTRP6 prevented survival and migration in hepatocellular carcinoma through inactivating the AKT signaling pathway. J. Cell Biochem. 2019, 120, 17059–17066. [Google Scholar] [CrossRef]
- Song, X.; Li, L.; Shi, L.; Liu, X.; Qu, X.; Wei, F.; Wang, K. C1QTNF6 promotes oral squamous cell carcinoma by enhancing proliferation and inhibiting apoptosis. Cancer Cell Int. 2021, 21, 666. [Google Scholar] [CrossRef]
- Hano, K.; Hatano, K.; Saigo, C.; Kito, Y.; Shibata, T.; Takeuchi, T. An adiponectin paralog protein, CTRP6 decreased the proliferation and invasion activity of oral squamous cell carcinoma cells: Possible interaction with laminin receptor pathway. Mol. Biol. Rep. 2019, 46, 4967–4973. [Google Scholar] [CrossRef]
- Glogowska, A.; Thanasupawat, T.; Beiko, J.; Pitz, M.; Hombach-Klonisch, S.; Klonisch, T. Novel CTRP8-RXFP1-JAK3-STAT3 axis promotes Cdc42-dependent actin remodeling for enhanced filopodia formation and motility in human glioblastoma cells. Mol. Oncol. 2022, 16, 368–387. [Google Scholar] [CrossRef] [PubMed]
- Araki-Sasaki, K.; Ohashi, Y.; Sasabe, T.; Hayashi, K.; Watanabe, H.; Tano, Y.; Handa, H. An SV40-immortalized human corneal epithelial cell line and its characterization. Investig. Ophthalmol. Vis. Sci. 1995, 36, 614–621. [Google Scholar]
- Gipson, I.K.; Spurr-Michaud, S.; Argüeso, P.; Tisdale, A.; Ng, T.F.; Russo, C.L. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2496–2506. [Google Scholar] [CrossRef] [Green Version]
- Robertson, D.M.; Li, L.; Fisher, S.; Pearce, V.P.; Shay, J.W.; Wright, W.E.; Cavanagh, H.D.; Jester, J.V. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Investig. Ophthalmol. Vis. Sci. 2005, 46, 470–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diebold, Y.; Calonge, M.; Enríquez de Salamanca, A.; Callejo, S.; Corrales, R.M.; Sáez, V.; Siemasko, K.F.; Stern, M.E. Characterization of a spontaneously immortalized cell line (IOBA-NHC) from normal human conjunctiva. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4263–4274. [Google Scholar] [CrossRef] [Green Version]
- Hampel, U.; Garreis, F.; Burgemeister, F.; Eßel, N.; Paulsen, F. Effect of intermittent shear stress on corneal epithelial cells using an in vitro flow culture model. Ocul. Surf. 2018, 16, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Garreis, F.; Schlorf, T.; Worlitzsch, D.; Steven, P.; Bräuer, L.; Jäger, K.; Paulsen, F.P. Roles of human beta-defensins in innate immune defense at the ocular surface: Arming and alarming corneal and conjunctival epithelial cells. Histochem. Cell Biol. 2010, 134, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Hampel, U.; Garreis, F. The human meibomian gland epithelial cell line as a model to study meibomian gland dysfunction. Exp. Eye Res. 2017, 163, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Garreis, F.; Jahn, J.; Wild, K.; Abrar, D.B.; Schicht, M.; Schröder, J.M.; Paulsen, F. Expression and Regulation of S100 Fused-Type Protein Hornerin at the Ocular Surface and Lacrimal Apparatus. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5968–5977. [Google Scholar] [CrossRef] [Green Version]
- Ferlin, A.; Menegazzo, M.; Gianesello, L.; Selice, R.; Foresta, C. Effect of relaxin on human sperm functions. J. Androl. 2012, 33, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.H.; Hong, X.; Zhang, Y.; Cong, X.; Xiang, R.L.; Mei, M.; Su, J.Z.; Wu, L.L.; Yu, G.Y. C1q/tumor necrosis factor-related protein-6 attenuates TNF-α-induced apoptosis in salivary acinar cells via AMPK/SIRT1-modulated miR-34a-5p expression. J. Cell Physiol. 2021, 236, 5785–5800. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Liu, S.; Tang, X.; Yang, D.; Liu, H.; Gao, L.; Yang, G. Circulating CTRP6 Levels are Increased in Overweight or Obese Chinese Individuals and Associated with Insulin Resistance Parameters: A Pilot Study. Exp. Clin. Endocrinol. Diabetes 2021, 129, 535–541. [Google Scholar] [CrossRef]
- Muendlein, A.; Leiherer, A.; Saely, C.; Ebner, J.; Geiger, K.; Brandtner, E.M.; Vonbank, A.; Fraunberger, P.; Drexel, H. The novel adipokine CTRP1 is significantly associated with the incidence of major adverse cardiovascular events. Atherosclerosis 2019, 286, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Majidi, Z.; Emamgholipour, S.; Omidifar, A.; Rahmani Fard, S.; Poustchi, H.; Shanaki, M. The circulating levels of CTRP1 and CTRP5 are associated with obesity indices and carotid intima-media thickness (cIMT) value in patients with type 2 diabetes: A preliminary study. Diabetol. Metab. Syndr. 2021, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Yagmur, E.; Buergerhausen, D.; Koek, G.H.; Weiskirchen, R.; Trautwein, C.; Koch, A.; Tacke, F. Elevated CTRP1 Plasma Concentration Is Associated with Sepsis and Pre-Existing Type 2 Diabetes Mellitus in Critically Ill Patients. J. Clin. Med. 2019, 8, 661. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Kim, J.D.; Lee, S.; Jeong, A.L.; Park, J.S.; Yong, H.J.; Boldbaatar, A.; Ka, H.I.; Rhee, E.J.; Lee, W.Y.; et al. Circulating CTRP1 Levels in Type 2 Diabetes and Their Association with FGF21. Int. J. Endocrinol. 2016, 2016, 5479627. [Google Scholar] [CrossRef] [Green Version]
- Xin, Y.; Lyu, X.; Wang, C.; Fu, Y.; Zhang, S.; Tian, C.; Li, Q.; Zhang, D. Elevated circulating levels of CTRP1, a novel adipokine, in diabetic patients. Endocr. J. 2014, 61, 841–847. [Google Scholar] [CrossRef] [Green Version]
- Shabani, P.; Naeimi Khaledi, H.; Beigy, M.; Emamgholipour, S.; Parvaz, E.; Poustchi, H.; Doosti, M. Circulating level of CTRP1 in patients with nonalcoholic fatty liver disease (NAFLD): Is it through insulin resistance? PLoS ONE 2015, 10, e0118650. [Google Scholar] [CrossRef]
- Pan, X.; Lu, T.; Wu, F.; Jin, L.; Zhang, Y.; Shi, L.; Li, X.; Lin, Z. Circulating complement-C1q TNF-related protein 1 levels are increased in patients with type 2 diabetes and are associated with insulin sensitivity in Chinese subjects. PLoS ONE 2014, 9, e94478. [Google Scholar] [CrossRef]
- Su, Z.; Tian, S.; Liang, W. Circulating CTRP1 Levels Are Increased and Associated with the STOD in Essential Hypertension in Chinese Patients. Cardiovasc. Ther. 2019, 2019, 4183781. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, R.; Du, D.; Li, F.; Li, Y. Serum levels of C1q/TNF-related protein-1 (CTRP-1) are closely associated with coronary artery disease. BMC Cardiovasc. Disord. 2016, 16, 92. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Wang, S.; Ling, Y.; Liang, W. Association of C1q/TNF-related protein-1 (CTRP1) serum levels with coronary artery disease. J. Int. Med. Res. 2019, 47, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Maury, E.; Brichard, S.M. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell Endocrinol. 2010, 314, 1–16. [Google Scholar] [CrossRef]
- Rasouli, N.; Kern, P.A. Adipocytokines and the metabolic complications of obesity. J. Clin. Endocrinol. Metab. 2008, 93, S64–S73. [Google Scholar] [CrossRef] [Green Version]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Liu, Q.; Zhang, X. C1q/tumor necrosis factor-related protein-1 attenuates microglia autophagy and inflammatory response by regulating the Akt/mTOR pathway. Life Sci. 2020, 256, 117992. [Google Scholar] [CrossRef]
- Massingale, M.L.; Li, X.; Vallabhajosyula, M.; Chen, D.; Wei, Y.; Asbell, P.A. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea 2009, 28, 1023–1027. [Google Scholar] [CrossRef]
- Wilson, S.E. Corneal wound healing. Exp. Eye Res. 2020, 197, 108089. [Google Scholar] [CrossRef]
- Wilson, S.E.; Schultz, G.S.; Chegini, N.; Weng, J.; He, Y.G. Epidermal growth factor, transforming growth factor alpha, transforming growth factor beta, acidic fibroblast growth factor, basic fibroblast growth factor, and interleukin-1 proteins in the cornea. Exp. Eye Res. 1994, 59, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Mohan, R.R.; Li, Q.; Wilson, S.E. IL-1 upregulates keratinocyte growth factor and hepatocyte growth factor mRNA and protein production by cultured stromal fibroblast cells: Interleukin-1 beta expression in the cornea. Cornea 1997, 16, 465–471. [Google Scholar] [CrossRef]
- Park, R.; Park, Y.I.; Park, Y.; Lee, S.; So, J.; Park, J. CTRP1 Knockout Attenuates Tumor Progression in A549 and HCT116 Cancer Cells. Cancers 2022, 14, 4495. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Kawakita, T.; Tsubota, K. IL-6 induction in desiccated corneal epithelium in vitro and in vivo. Mol. Vis. 2011, 17, 2400–2406. [Google Scholar] [PubMed]
- Cole, N.; Bao, S.; Willcox, M.; Husband, A.J. Expression of interleukin-6 in the cornea in response to infection with different strains of Pseudomonas aeruginosa. Infect. Immun. 1999, 67, 2497–2502. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.L.; Zhu, H.; Willcox, M.; Wakefield, D.; Lloyd, A.; Thakur, A. The role of IL-1beta in the regulation of IL-8 and IL-6 in human corneal epithelial cells during Pseudomonas aeruginosa colonization. Curr. Eye Res. 2001, 23, 406–414. [Google Scholar] [CrossRef]
- Arranz-Valsero, I.; Soriano-Romaní, L.; García-Posadas, L.; López-García, A.; Diebold, Y. IL-6 as a corneal wound healing mediator in an in vitro scratch assay. Exp. Eye Res. 2014, 125, 183–192. [Google Scholar] [CrossRef]
- Ebihara, N.; Matsuda, A.; Nakamura, S.; Matsuda, H.; Murakami, A. Role of the IL-6 classic- and trans-signaling pathways in corneal sterile inflammation and wound healing. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8549–8557. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Nishida, T. Differential effects of epidermal growth factor and interleukin 6 on corneal epithelial cells and vascular endothelial cells. Cornea 1999, 18, 452–458. [Google Scholar] [CrossRef]
- Rieck, P.W.; Pleyer, U. Corneal wound healing. II. Treatment of disorders of wound healing. Ophthalmologe 2003, 100, 1109–1130. [Google Scholar] [CrossRef]
- Pini, A.; Shemesh, R.; Samuel, C.S.; Bathgate, R.A.; Zauberman, A.; Hermesh, C.; Wool, A.; Bani, D.; Rotman, G. Prevention of bleomycin-induced pulmonary fibrosis by a novel antifibrotic peptide with relaxin-like activity. J. Pharmacol. Exp. Ther. 2010, 335, 589–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Cheng, F.; Songyang, Y.Y.; Yang, S.Y.; Wei, J.; Ruan, Y. CTRP1 Attenuates UUO-induced Renal Fibrosis via AMPK/NOX4 Pathway in Mice. Curr. Med. Sci. 2020, 40, 48–54. [Google Scholar] [CrossRef]
- Chow, B.S.M.; Kocan, M.; Shen, M.; Wang, Y.; Han, L.; Chew, J.Y.; Wang, C.; Bosnyak, S.; Mirabito-Colafella, K.M.; Barsha, G.; et al. AT1R-AT2R-RXFP1 Functional Crosstalk in Myofibroblasts: Impact on the Therapeutic Targeting of Renal and Cardiac Fibrosis. J. Am. Soc. Nephrol. 2019, 30, 2191–2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unemori, E.N.; Amento, E.P. Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. J. Biol. Chem. 1990, 265, 10681–10685. [Google Scholar] [CrossRef] [PubMed]
- Heeg, M.H.; Koziolek, M.J.; Vasko, R.; Schaefer, L.; Sharma, K.; Müller, G.A.; Strutz, F. The antifibrotic effects of relaxin in human renal fibroblasts are mediated in part by inhibition of the Smad2 pathway. Kidney Int. 2005, 68, 96–109. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhang, Y.; Han, X.; Li, Y.; Zhao, X.; Sheng, L. Relaxin alleviates TGFβ1-induced cardiac fibrosis via inhibition of Stat3-dependent autophagy. Biochem. Biophys. Res. Commun. 2017, 493, 1601–1607. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolaus, H.F.; Klonisch, T.; Paulsen, F.; Garreis, F. C1q/TNF-Related Proteins 1, 6 and 8 Are Involved in Corneal Epithelial Wound Closure by Targeting Relaxin Receptor RXFP1 In Vitro. Int. J. Mol. Sci. 2023, 24, 6839. https://doi.org/10.3390/ijms24076839
Nicolaus HF, Klonisch T, Paulsen F, Garreis F. C1q/TNF-Related Proteins 1, 6 and 8 Are Involved in Corneal Epithelial Wound Closure by Targeting Relaxin Receptor RXFP1 In Vitro. International Journal of Molecular Sciences. 2023; 24(7):6839. https://doi.org/10.3390/ijms24076839
Chicago/Turabian StyleNicolaus, Hagen Fabian, Thomas Klonisch, Friedrich Paulsen, and Fabian Garreis. 2023. "C1q/TNF-Related Proteins 1, 6 and 8 Are Involved in Corneal Epithelial Wound Closure by Targeting Relaxin Receptor RXFP1 In Vitro" International Journal of Molecular Sciences 24, no. 7: 6839. https://doi.org/10.3390/ijms24076839





