The Geroprotective Drug Candidate CMS121 Alleviates Diabetes, Liver Inflammation, and Renal Damage in db/db Leptin Receptor Deficient Mice
Abstract
:1. Introduction
2. Results
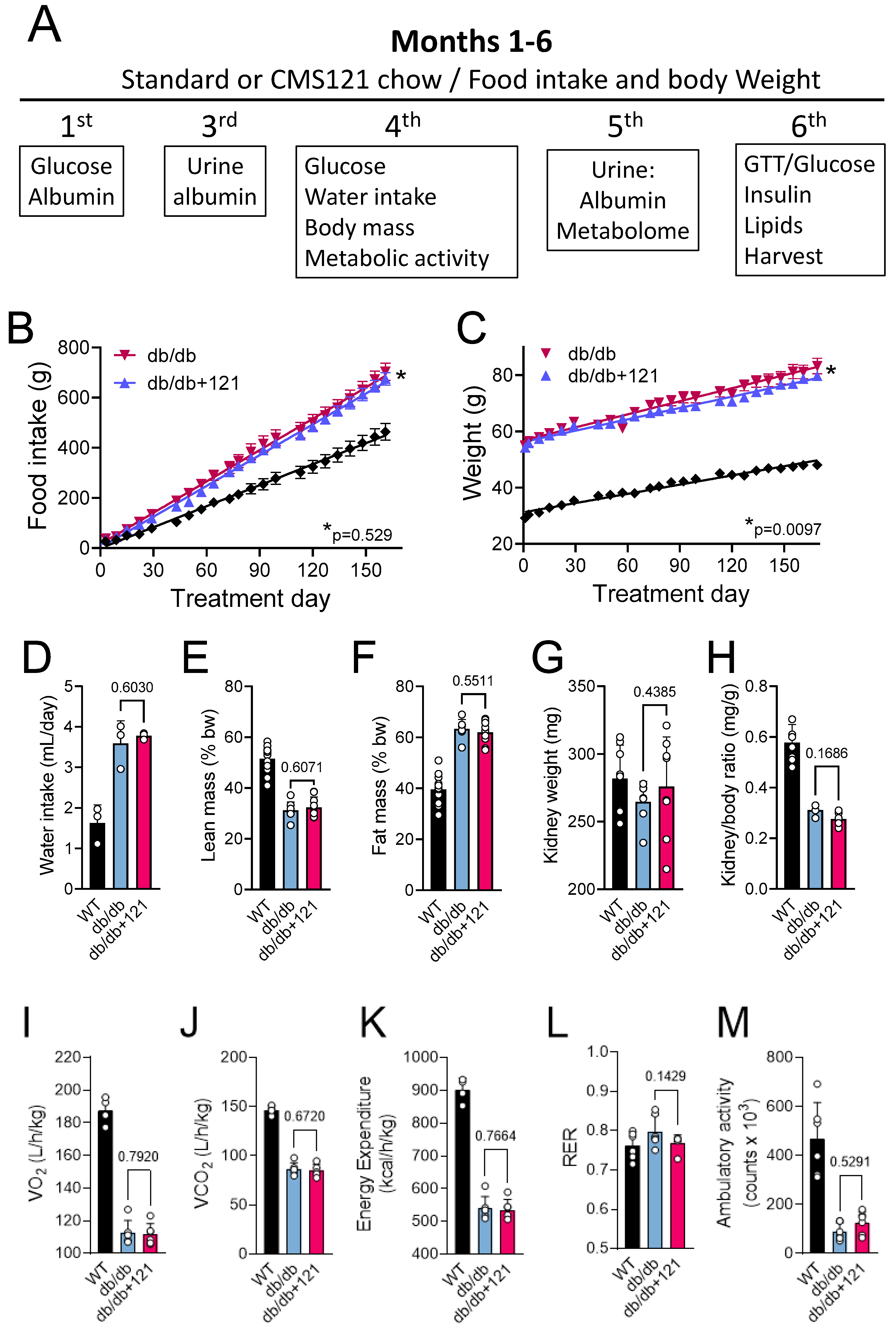
2.1. Food Intake, Weight Gain, Body Mass, Locomotion, Oxygen Consumption, and Memory
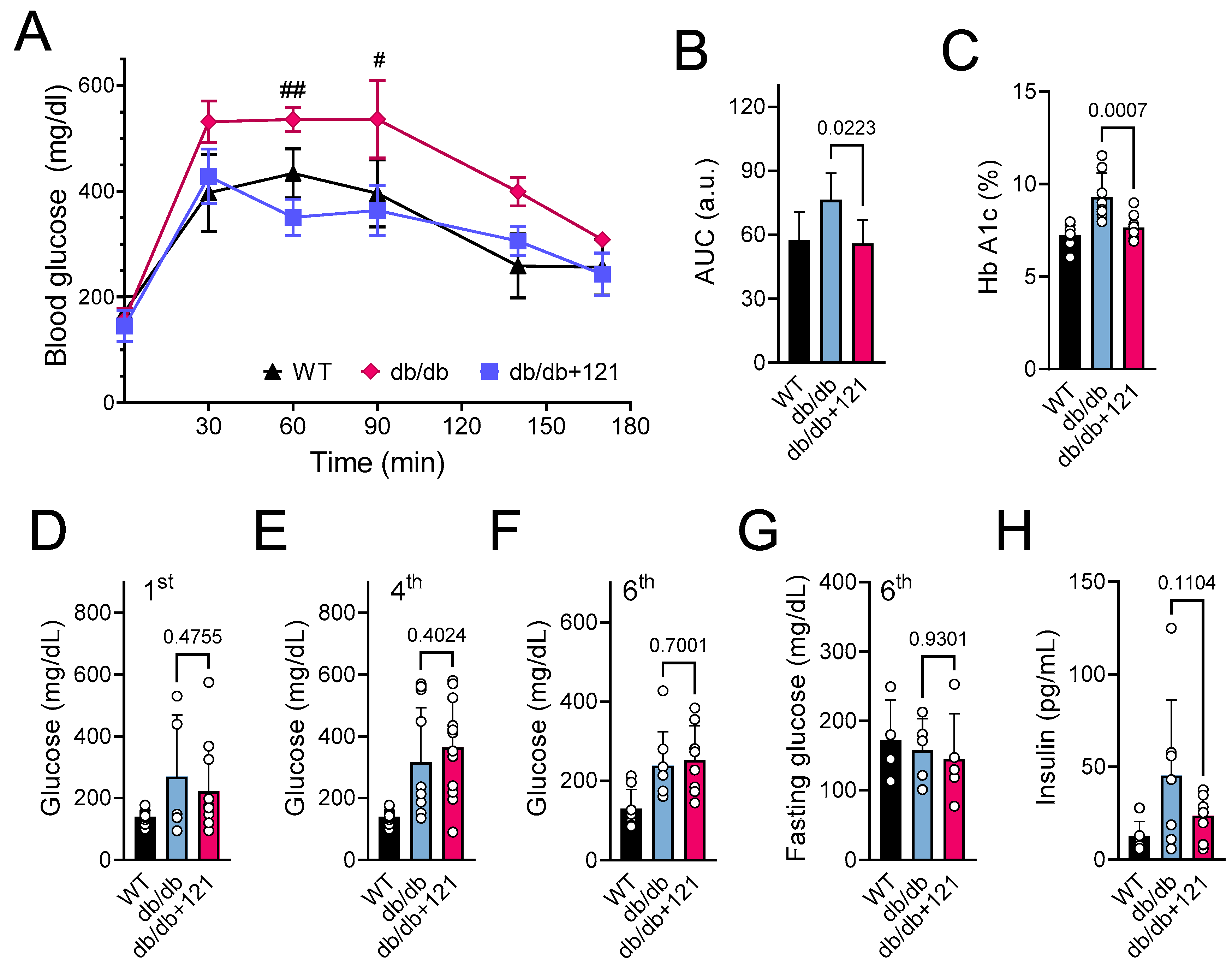
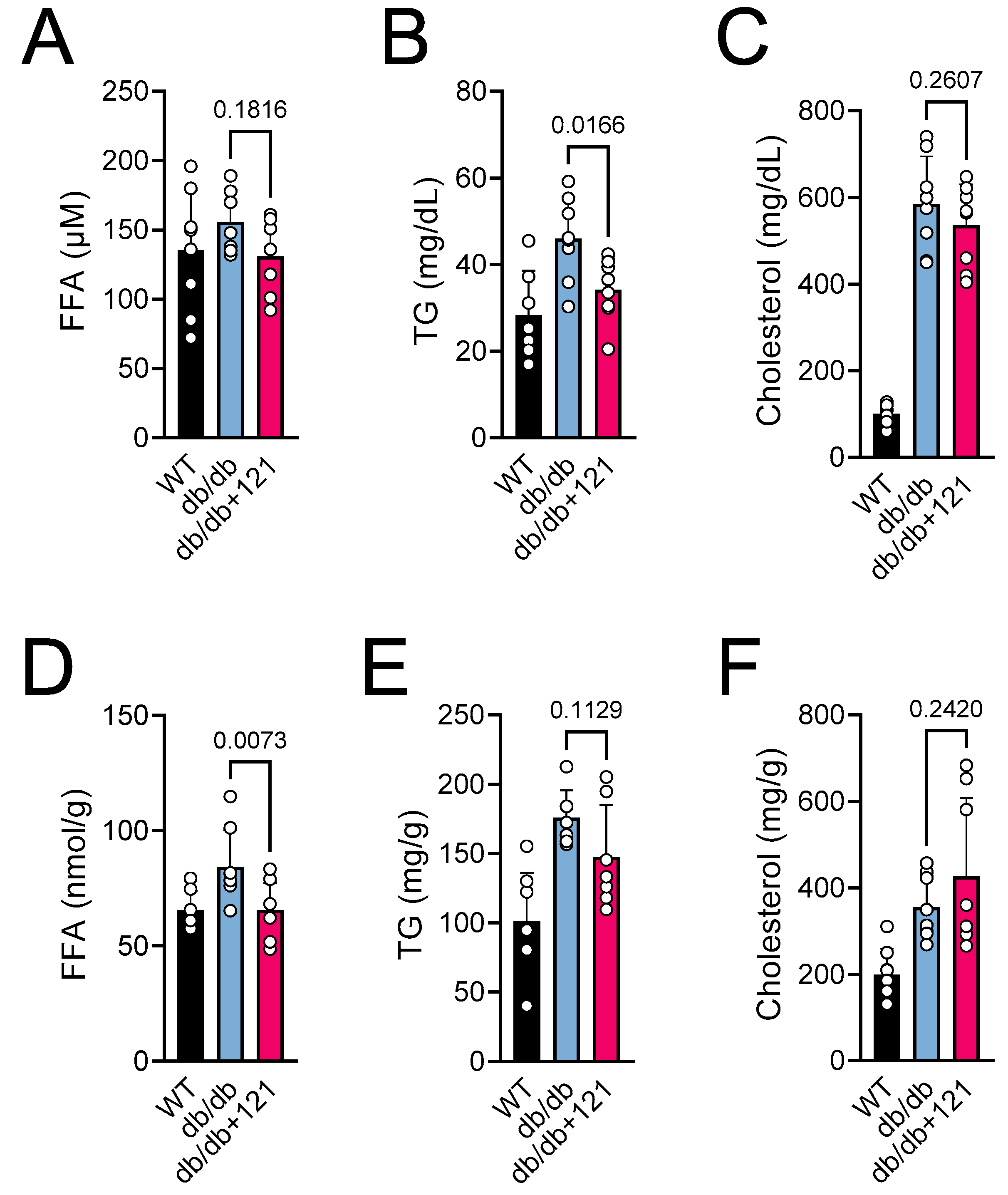
2.2. Glucose and Lipid Status
2.3. Liver Inflammation
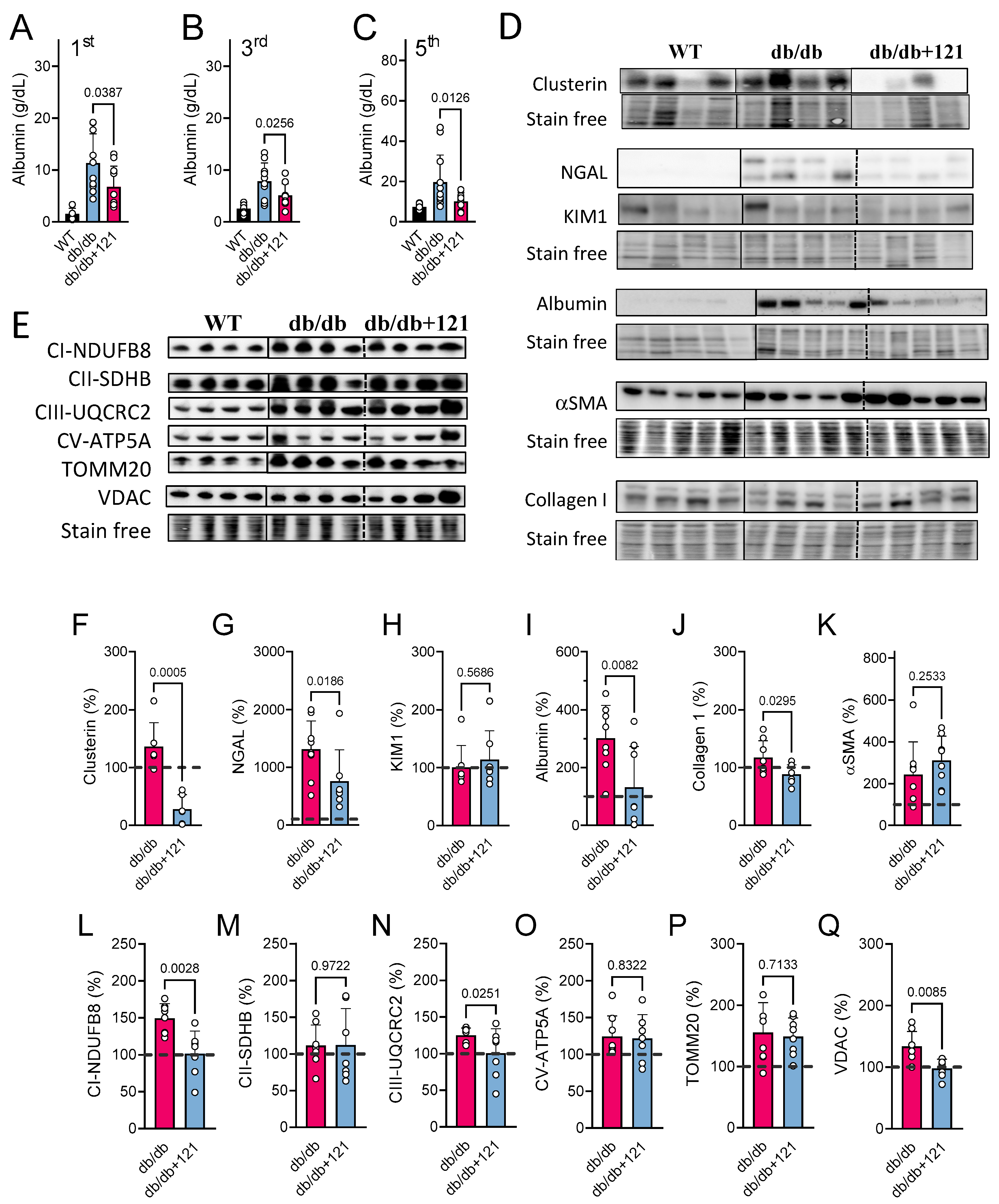
2.4. Kidney Markers
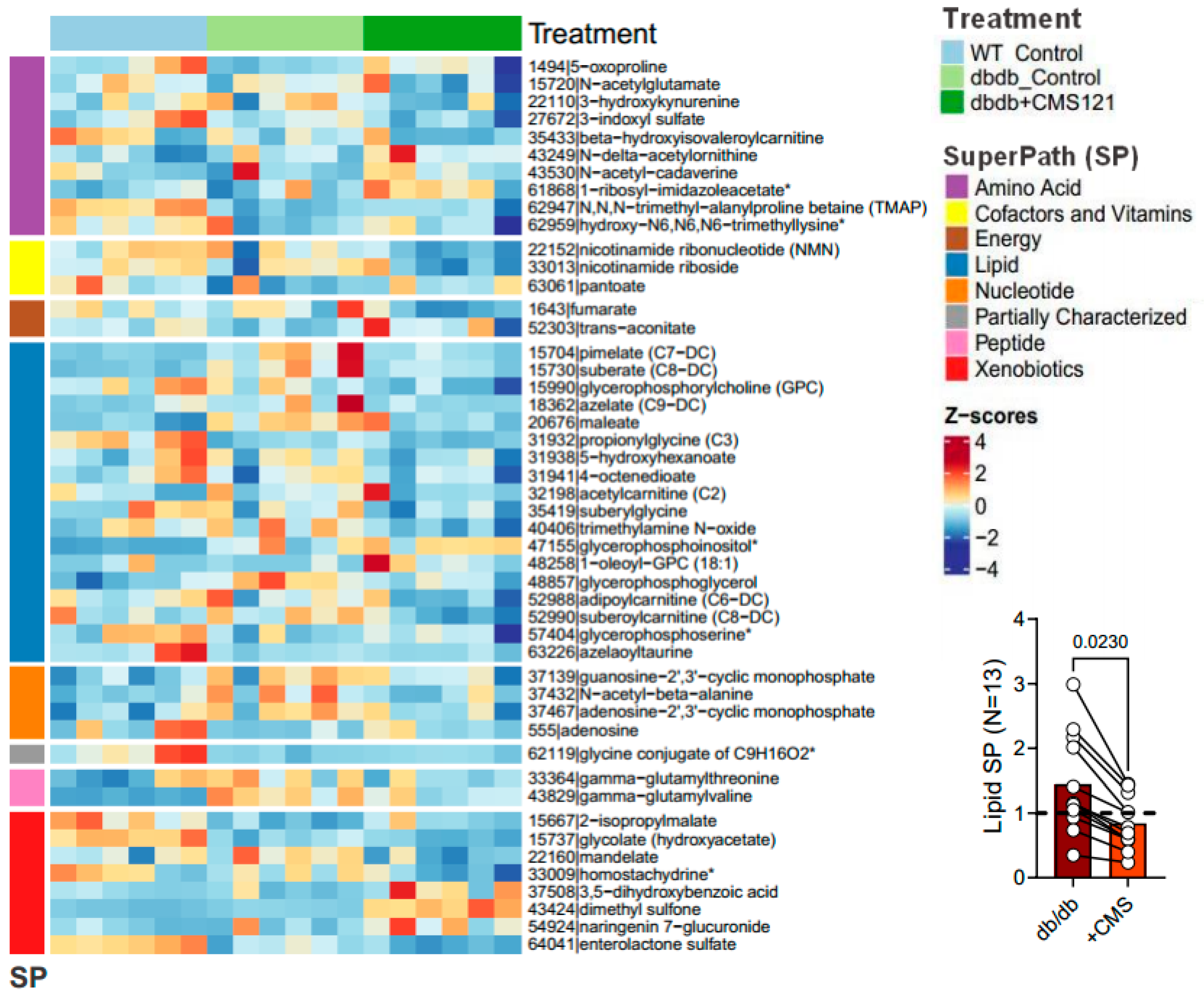
2.5. Urine Metabolome
2.5.1. Metabolomic Changes Associated with Fuel/Energy Metabolism
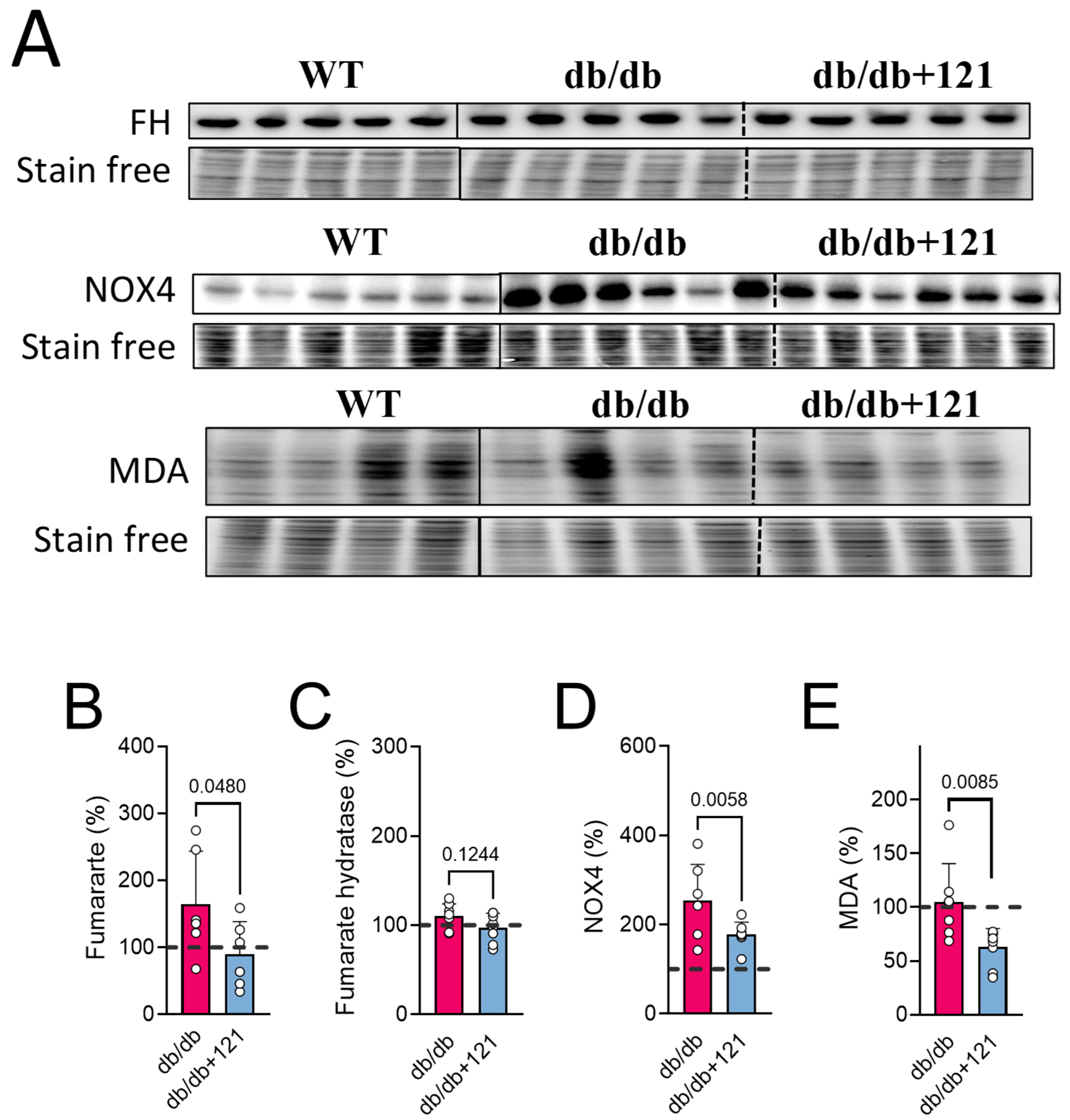
2.5.2. Metabolomic Changes Associated with Kidney Dysfunction
3. Discussion
3.1. Fuel and Energy Metabolism
3.2. Renal Protection
4. Material and Methods
4.1. Study Design
4.2. Body Mass and Metabolic Evaluation
4.3. Biochemical Analyses
4.4. Western Blotting
4.5. Metabolomic Analyses of Urine Samples
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- WHO World Health Organization. Screening for Type 2 Diabetes: Report of a World Health Organization and International Diabetes Federation Meeting. 2003. Available online: http://www.who.int/diabetes/publications/en/screening_mnc03.pdf (accessed on 5 January 2023).
- Jiang, Z.; Wang, Y.; Zhao, X.; Cui, H.; Han, M.; Ren, X.; Gang, X.; Wang, G. Obesity and Chronic Kidney Disease. Am. J. Physiol.-Endocrinol. Metab. 2023, 324, E24–E41. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.S.; Chang, A.R.; Shin, J.-I.; Reider, J.; Echouffo-Tcheugui, J.B.; Grams, M.E.; Selvin, E. Obesity and Chronic Kidney Disease in US Adults with Type 1 and Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2022, 107, 1247–1256. [Google Scholar] [CrossRef]
- Jangra, V.; Tople, J. Can Alzheimer’s Disease Be Secondary to Type-2 Diabetes Mellitus? Cureus 2022, 14, e31273. [Google Scholar] [CrossRef] [PubMed]
- Onaolapo, A.Y.; Ojo, F.O.; Adeleye, O.O.; Falade, J.; Onaolapo, O.J. Diabetes Mellitus and Energy Dysmetabolism in Alzheimer’s Disease: Understanding the Relationships and Potential Therapeutic Targets. Curr. Diabetes Rev. 2023, 19, e020123212333. [Google Scholar] [CrossRef]
- Čater, M.; Hölter, S.M. A Pathophysiological Intersection of Diabetes and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 11562. [Google Scholar] [CrossRef]
- Carvalho, C.; Moreira, P.I. Metabolic Defects Shared by Alzheimer’s Disease and Diabetes: A Focus on Mitochondria. Curr. Opin. Neurobiol. 2023, 79, 102694. [Google Scholar] [CrossRef]
- Marrano, N.; Biondi, G.; Borrelli, A.; Rella, M.; Zambetta, T.; Di Gioia, L.; Caporusso, M.; Logroscino, G.; Perrini, S.; Giorgino, F.; et al. Type 2 Diabetes and Alzheimer’s Disease: The Emerging Role of Cellular Lipotoxicity. Biomolecules 2023, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Barone, E.; Di Domenico, F.; Perluigi, M.; Butterfield, D.A. The Interplay among Oxidative Stress, Brain Insulin Resistance and AMPK Dysfunction Contribute to Neurodegeneration in Type 2 Diabetes and Alzheimer Disease. Free Radic. Biol. Med. 2021, 176, 16–33. [Google Scholar] [CrossRef]
- Maher, P.; Currais, A.; Schubert, D. Using the Oxytosis/Ferroptosis Pathway to Understand and Treat Age-Associated Neurodegenerative Diseases. Cell Chem. Biol. 2020, 27, 1456–1471. [Google Scholar] [CrossRef]
- Currais, A.; Huang, L.; Goldberg, J.; Petrascheck, M.; Ates, G.; Pinto-Duarte, A.; Shokhirev, M.N.; Schubert, D.; Maher, P. Elevating Acetyl-CoA Levels Reduces Aspects of Brain Aging. eLife 2019, 8, e47866. [Google Scholar] [CrossRef]
- Currais, A.; Goldberg, J.; Farrokhi, C.; Chang, M.; Prior, M.; Dargusch, R.; Daugherty, D.; Armando, A.; Quehenberger, O.; Maher, P.; et al. A Comprehensive Multiomics Approach toward Understanding the Relationship between Aging and Dementia. Aging 2015, 7, 937–952. [Google Scholar] [CrossRef] [Green Version]
- CDC Center for The Disease Control Nd Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. 2011. Available online: http://www.cdc.gov/DIABETES/pubs/factsheet11.htm (accessed on 5 January 2023).
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheer, R.; Nair, R.; Pasquale, M.K.; Evers, T.; Cockrell, M.; Gay, A.; Singh, R.; Schmedt, N. Predictive Risk Models to Identify Patients at High-Risk for Severe Clinical Outcomes with Chronic Kidney Disease and Type 2 Diabetes. J. Prim. Care Community Health 2022, 13, 21501319211063730. [Google Scholar] [CrossRef] [PubMed]
- Heng, X.-P.; Li, X.-J.; Li, L.; Yang, L.-Q.; Wang, Z.-T.; Huang, S.-P. Therapy to Obese Type 2 Diabetes Mellitus: How Far Will We Go Down the Wrong Road? Chin. J. Integr. Med. 2020, 26, 62–71. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive Blood-Glucose Control with Sulphonylureas or Insulin Compared with Conventional Treatment and Risk of Complications in Patients with Type 2 Diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Wen, J.; Yi, Z.; Chen, Y.; Huang, J.; Mao, X.; Zhang, L.; Zeng, Y.; Cheng, Q.; Ye, W.; Liu, Z.; et al. Efficacy of Metformin Therapy in Patients with Cancer: A Meta-Analysis of 22 Randomised Controlled Trials. BMC Med. 2022, 20, 402. [Google Scholar] [CrossRef]
- Bhori, M.; Rastogi, V.; Tungare, K.; Marar, T. A Review on Interplay between Obesity, Lipoprotein Profile and Nutrigenetics with Selected Candidate Marker Genes of Type 2 Diabetes Mellitus. Mol. Biol. Rep. 2022, 49, 687–703. [Google Scholar] [CrossRef]
- Bakhtiani, P.; Geffner, M.E. Early Anthropometric Indicators of Type 2 Diabetes Mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 52–56. [Google Scholar] [CrossRef]
- Thackrey, E.; Chen, J.; Martino, C.-R.; Preda, V. The Effects of Diet on Weight and Metabolic Outcomes in Patients with Double Diabetes: A Systematic Review. Nutrition 2022, 94, 111536. [Google Scholar] [CrossRef]
- Zeraattalab-Motlagh, S.; Jayedi, A.; Shab-Bidar, S. Mediterranean Dietary Pattern and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Eur. J. Nutr. 2022, 61, 1735–1748. [Google Scholar] [CrossRef]
- Laube, H.; Fussgänger, R.D.; Maier, V.; Pfeiffer, E.F. Hyperglucagonemia of the Isolated Perfused Pancreas of Diabetic Mice (Db-Db). Diabetologia 1973, 9, 400–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okajima, Y.; Matsuzaka, T.; Miyazaki, S.; Motomura, K.; Ohno, H.; Sharma, R.; Shimura, T.; Istiqamah, N.; Han, S.-I.; Mizunoe, Y.; et al. Morphological and Functional Adaptation of Pancreatic Islet Blood Vessels to Insulin Resistance Is Impaired in Diabetic Db/Db Mice. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166339. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chandrasekera, P.C.; Pippin, J.J. Leptin- and Leptin Receptor-Deficient Rodent Models: Relevance for Human Type 2 Diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Ding, X.; Wu, R.; Tong, B.; Zhao, L.; Lv, H.; Meng, X.; Liu, Y.; Ren, B.; Li, J.; et al. Novel Sesquiterpene Glycoside from Loquat Leaf Alleviates Type 2 Diabetes Mellitus Combined with Nonalcoholic Fatty Liver Disease by Improving Insulin Resistance, Oxidative Stress, Inflammation, and Gut Microbiota Composition. J. Agric. Food Chem. 2021, 69, 14176–14191. [Google Scholar] [CrossRef]
- Jiang, Y.; Sui, D.; Li, M.; Xu, H.; Yu, X.; Liu, J.; Yu, Q. Ginsenoside Re Improves Inflammation and Fibrosis in Hepatic Tissue by Upregulating PPARγ Expression and Inhibiting Oxidative Stress in Db/Db Mice. Evid. Based Complement Altern. Med. 2021, 2021, 9003603. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, L.; Wang, X.; Wang, W.; Wang, J. Inhibition of HMGB1 Involved in the Protective of Salidroside on Liver Injury in Diabetes Mice. Int. Immunopharmacol. 2020, 89, 106987. [Google Scholar] [CrossRef]
- Eddy, S.; Mariani, L.H.; Kretzler, M. Integrated Multi-Omics Approaches to Improve Classification of Chronic Kidney Disease. Nat. Rev. Nephrol. 2020, 16, 657–668. [Google Scholar] [CrossRef]
- Kalantari, S.; Nafar, M. An Update of Urine and Blood Metabolomics in Chronic Kidney Disease. Biomark. Med. 2019, 13, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Saucedo, A.L.; Perales-Quintana, M.M.; Paniagua-Vega, D.; Sanchez-Martinez, C.; Cordero-Perez, P.; Minsky, N.W. Chronic Kidney Disease and the Search for New Biomarkers for Early Diagnosis. Curr. Med. Chem. 2018, 25, 3719–3747. [Google Scholar] [CrossRef]
- Faaitiiti, K.L.; Jupiter, D.C. Diabetes-Specific Dementia: A Structured Literature Review of Cognitive Assessment Methods. J. Foot Ankle Surg. 2021, 61, 401–409. [Google Scholar] [CrossRef]
- Hadley, G.; Zhang, J.; Harris-Skillman, E.; Alexopoulou, Z.; DeLuca, G.C.; Pendlebury, S.T. Cognitive Decline and Diabetes: A Systematic Review of the Neuropathological Correlates Accounting for Cognition at Death. J. Neurol. Neurosurg. Psychiatry 2022, 93, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Tatavarthy, M.; Bennett, D.A. The Relation of Diabetes to Memory Function. Curr. Neurol. Neurosci. Rep. 2020, 20, 64. [Google Scholar] [CrossRef]
- Chiruta, C.; Schubert, D.; Dargusch, R.; Maher, P. Chemical Modification of the Multitarget Neuroprotective Compound Fisetin. J. Med. Chem. 2012, 55, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Prior, M.; Chiruta, C.; Currais, A.; Goldberg, J.; Ramsey, J.; Dargusch, R.; Maher, P.A.; Schubert, D. Back to the Future with Phenotypic Screening. ACS Chem. Neurosci. 2014, 5, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Castell, D.; Liang, Z.; Maher, P.; Currais, A. The Search for Anti-Oxytotic/Ferroptotic Compounds in the Plant World. Br. J. Pharmacol. 2021, 178, 3611–3626. [Google Scholar] [CrossRef]
- Ates, G.; Goldberg, J.; Currais, A.; Maher, P. CMS121, a Fatty Acid Synthase Inhibitor, Protects against Excess Lipid Peroxidation and Inflammation and Alleviates Cognitive Loss in a Transgenic Mouse Model of Alzheimer’s Disease. Redox Biol. 2020, 36, 101648. [Google Scholar] [CrossRef]
- Kepchia, D.; Currais, A.; Dargusch, R.; Finley, K.; Schubert, D.; Maher, P. Geroprotective Effects of Alzheimer’s Disease Drug Candidates. Aging 2021, 13, 3269–3289. [Google Scholar] [CrossRef]
- Maher, P.; Dargusch, R.; Ehren, J.L.; Okada, S.; Sharma, K.; Schubert, D. Fisetin Lowers Methylglyoxal Dependent Protein Glycation and Limits the Complications of Diabetes. PLoS ONE 2011, 6, e21226. [Google Scholar] [CrossRef]
- Tesch, G.H.; Lim, A.K.H. Recent Insights into Diabetic Renal Injury from the Db/Db Mouse Model of Type 2 Diabetic Nephropathy. Am. J. Physiol. Renal. Physiol. 2011, 300, F301–F310. [Google Scholar] [CrossRef]
- Korolenko, T.A.; Dubrovina, N.I.; Ovsyukova, M.V.; Bgatova, N.P.; Tenditnik, M.V.; Pupyshev, A.B.; Akopyan, A.A.; Goncharova, N.V.; Lin, C.-L.; Zavjalov, E.L.; et al. Treatment with Autophagy Inducer Trehalose Alleviates Memory and Behavioral Impairments and Neuroinflammatory Brain Processes in Db/Db Mice. Cells 2021, 10, 2557. [Google Scholar] [CrossRef]
- Wu, X.-L.; Deng, M.-Z.; Gao, Z.-J.; Dang, Y.-Y.; Li, Y.-C.; Li, C.-W. Neferine Alleviates Memory and Cognitive Dysfunction in Diabetic Mice through Modulation of the NLRP3 Inflammasome Pathway and Alleviation of Endoplasmic-Reticulum Stress. Int. Immunopharmacol. 2020, 84, 106559. [Google Scholar] [CrossRef]
- Suriano, F.; Vieira-Silva, S.; Falony, G.; Roumain, M.; Paquot, A.; Pelicaen, R.; Régnier, M.; Delzenne, N.M.; Raes, J.; Muccioli, G.G.; et al. Novel Insights into the Genetically Obese (Ob/Ob) and Diabetic (Db/Db) Mice: Two Sides of the Same Coin. Microbiome 2021, 9, 147. [Google Scholar] [CrossRef]
- Shahzad, K.; Fatima, S.; Al-Dabet, M.M.; Gadi, I.; Khawaja, H.; Ambreen, S.; Elwakiel, A.; Klöting, N.; Blüher, M.; Nawroth, P.P.; et al. CHOP-ASO Ameliorates Glomerular and Tubular Damage on Top of ACE Inhibition in Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2021, 32, 3066–3079. [Google Scholar] [CrossRef]
- Prasath, G.S.; Pillai, S.I.; Subramanian, S.P. Fisetin Improves Glucose Homeostasis through the Inhibition of Gluconeogenic Enzymes in Hepatic Tissues of Streptozotocin Induced Diabetic Rats. Eur. J. Pharmacol. 2014, 740, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Garris, D.R. Hypercytolipidemia-Induced Cellular Lipoapoptosis: Cytostructural and Endometabolic Basis of Progressive Organo-Involution Following Expression of Diabetes (Db/Db) and Obese (Ob/Ob) Mutation Syndromes. Prog. Histochem. Cytochem. 2006, 40, 181–231. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, Y.; Sun, Q.; Yang, Y.; Gao, Y.; Ge, W.; Liu, J.; Xu, X.; Zhang, J. An Intermediary Role of Adenine Nucleotides on Free Fatty Acids-Induced Hyperglycemia in Obese Mice. Front. Endocrinol. 2019, 10, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, Y.; Liu, X.; Yuan, X.; Xiang, M.; Liu, J.; Zhang, L.; Zhu, S.; Lu, J.; Tang, Q.; et al. Exercise and Metformin Intervention Prevents Lipotoxicity-Induced Hepatocyte Apoptosis by Alleviating Oxidative and ER Stress and Activating the AMPK/Nrf2/HO-1 Signaling Pathway in Db/Db Mice. Oxid. Med. Cell. Longev. 2022, 2022, 2297268. [Google Scholar] [CrossRef]
- Asadi, M.; Taghizadeh, S.; Kaviani, E.; Vakili, O.; Taheri-Anganeh, M.; Tahamtan, M.; Savardashtaki, A. Caspase-3: Structure, Function, and Biotechnological Aspects. Biotechnol. Appl. Biochem. 2022, 69, 1633–1645. [Google Scholar] [CrossRef]
- Chen, Q.; Ren, D.; Wu, J.; Yu, H.; Chen, X.; Wang, J.; Zhang, Y.; Liu, M.; Wang, T. Shenyan Kangfu Tablet Alleviates Diabetic Kidney Disease through Attenuating Inflammation and Modulating the Gut Microbiota. J. Nat. Med. 2021, 75, 84–98. [Google Scholar] [CrossRef]
- Huang, J.; Covic, M.; Huth, C.; Rommel, M.; Adam, J.; Zukunft, S.; Prehn, C.; Wang, L.; Nano, J.; Scheerer, M.F.; et al. Validation of Candidate Phospholipid Biomarkers of Chronic Kidney Disease in Hyperglycemic Individuals and Their Organ-Specific Exploration in Leptin Receptor-Deficient Db/Db Mouse. Metabolites 2021, 11, 89. [Google Scholar] [CrossRef]
- Cleveland, K.H.; Brosius, F.C.; Schnellmann, R.G. Regulation of Mitochondrial Dynamics and Energetics in the Diabetic Renal Proximal Tubule by the Β2-Adrenergic Receptor Agonist Formoterol. Am. J. Physiol. Renal. Physiol. 2020, 319, F773–F779. [Google Scholar] [CrossRef]
- Alfadhel, M.; Babiker, A. Inborn Errors of Metabolism Associated with Hyperglycaemic Ketoacidosis and Diabetes Mellitus: Narrative Review. Sudan. J. Paediatr. 2018, 18, 10–23. [Google Scholar] [CrossRef]
- Kido, J.; Matsumoto, S.; Nakamura, K. Carglumic Acid Contributes to a Favorable Clinical Course in a Case of Severe Propionic Acidemia. Case Rep. Pediatr. 2020, 2020, 4709548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korman, S.H.; Mandel, H.; Gutman, A. Characteristic Urine Organic Acid Profile in Peroxisomal Biogenesis Disorders. J. Inherit. Metab. Dis. 2000, 23, 425–428. [Google Scholar] [CrossRef]
- Liu, H.; Garrett, T.J.; Su, Z.; Khoo, C.; Zhao, S.; Gu, L. Modifications of the Urinary Metabolome in Young Women after Cranberry Juice Consumption Were Revealed Using the UHPLC-Q-Orbitrap-HRMS-Based Metabolomics Approach. Food Funct. 2020, 11, 2466–2476. [Google Scholar] [CrossRef]
- Pelantová, H.; Bugáňová, M.; Holubová, M.; Šedivá, B.; Zemenová, J.; Sýkora, D.; Kaválková, P.; Haluzík, M.; Železná, B.; Maletínská, L.; et al. Urinary Metabolomic Profiling in Mice with Diet-Induced Obesity and Type 2 Diabetes Mellitus after Treatment with Metformin, Vildagliptin and Their Combination. Mol. Cell. Endocrinol. 2016, 431, 88–100. [Google Scholar] [CrossRef]
- Yoshioka, K.; Shimojo, N.; Nakanishi, T.; Naka, K.; Okuda, K. Measurements of Urinary Adipic Acid and Suberic Acid Using High-Performance Liquid Chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1994, 655, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Qin, X.-J.; Jiang, H.; Chen, J.-F.; Wang, T.; Zhang, T.; Xu, S.-Z.; Song, J.-M. Detecting Serum and Urine Metabolic Profile Changes of CCl4-Liver Fibrosis in Rats at 12 Weeks Based on Gas Chromatography-Mass Spectrometry. Exp. Ther. Med. 2017, 14, 1496–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, M.; Wang, Q.; Kou, S.; Li, X.; Jiang, Z.; Sun, L.; Huang, X. A Sensitive UPLC-MS/MS Method for the Simultaneous Determination of the Metabolites in the Tryptophan Pathway in Rat Plasma. J. Pharm. Biomed. Anal. 2022, 219, 114979. [Google Scholar] [CrossRef] [PubMed]
- Barouei, J.; Bendiks, Z.; Martinic, A.; Mishchuk, D.; Heeney, D.; Hsieh, Y.-H.; Kieffer, D.; Zaragoza, J.; Martin, R.; Slupsky, C.; et al. Microbiota, Metabolome, and Immune Alterations in Obese Mice Fed a High-Fat Diet Containing Type 2 Resistant Starch. Mol. Nutr. Food Res. 2017, 61, 1700184. [Google Scholar] [CrossRef]
- Bernard, L.; Zhou, L.; Surapaneni, A.; Chen, J.; Rebholz, C.M.; Coresh, J.; Yu, B.; Boerwinkle, E.; Schlosser, P.; Grams, M.E. Serum Metabolites and Kidney Outcomes: The Atherosclerosis Risk in Communities Study. Kidney Med. 2022, 4, 100522. [Google Scholar] [CrossRef] [PubMed]
- Posada-Ayala, M.; Zubiri, I.; Martin-Lorenzo, M.; Sanz-Maroto, A.; Molero, D.; Gonzalez-Calero, L.; Fernandez-Fernandez, B.; de la Cuesta, F.; Laborde, C.M.; Barderas, M.G.; et al. Identification of a Urine Metabolomic Signature in Patients with Advanced-Stage Chronic Kidney Disease. Kidney Int. 2014, 85, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Heiss, G.; Alexander, D.; Grams, M.E.; Boerwinkle, E. Associations Between the Serum Metabolome and All-Cause Mortality Among African Americans in the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Epidemiol. 2016, 183, 650–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Wang, C.; Yang, H.; Liu, S.; Lu, Y.; Fu, P.; Liu, J. Metabolomics Reveal Mitochondrial and Fatty Acid Metabolism Disorders That Contribute to the Development of DKD in T2DM Patients. Mol. BioSyst. 2017, 13, 2392–2400. [Google Scholar] [CrossRef]
- Saulnier, P.; Darshi, M.; Wheelock, K.M.; Looker, H.C.; Fufaa, G.D.; Knowler, W.C.; Weil, E.J.; Tanamas, S.K.; Lemley, K.V.; Saito, R.; et al. Urine Metabolites Are Associated with Glomerular Lesions in Type 2 Diabetes. Metabolomics 2018, 14, 84. [Google Scholar] [CrossRef]
- Sekula, P.; Goek, O.-N.; Quaye, L.; Barrios, C.; Levey, A.S.; Römisch-Margl, W.; Menni, C.; Yet, I.; Gieger, C.; Inker, L.A.; et al. A Metabolome-Wide Association Study of Kidney Function and Disease in the General Population. J. Am. Soc. Nephrol. 2016, 27, 1175–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyarzún, C.; Garrido, W.; Alarcón, S.; Yáñez, A.; Sobrevia, L.; Quezada, C.; San Martín, R. Adenosine Contribution to Normal Renal Physiology and Chronic Kidney Disease. Mol. Asp. Med. 2017, 55, 75–89. [Google Scholar] [CrossRef] [Green Version]
- Yap, S.C.; Lee, H.T. Adenosine and Protection from Acute Kidney Injury. Curr. Opin. Nephrol. Hypertens. 2012, 21, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, E.K.; Mi, Z.; Janesko-Feldman, K.; Jackson, T.C.; Kochanek, P.M. 2′,3′-CGMP Exists in Vivo and Comprises a 2′,3′-CGMP-Guanosine Pathway. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R783–R790. [Google Scholar] [CrossRef]
- Mishima, E.; Fukuda, S.; Shima, H.; Hirayama, A.; Akiyama, Y.; Takeuchi, Y.; Fukuda, N.N.; Suzuki, T.; Suzuki, C.; Yuri, A.; et al. Alteration of the Intestinal Environment by Lubiprostone Is Associated with Amelioration of Adenine-Induced CKD. J. Am. Soc. Nephrol. 2015, 26, 1787–1794. [Google Scholar] [CrossRef] [Green Version]
- Rajaram, R.D.; Dissard, R.; Jaquet, V.; de Seigneux, S. Potential Benefits and Harms of NADPH Oxidase Type 4 in the Kidneys and Cardiovascular System. Nephrol. Dial. Transplant. 2019, 34, 567–576. [Google Scholar] [CrossRef] [PubMed]
- You, Y.-H.; Quach, T.; Saito, R.; Pham, J.; Sharma, K. Metabolomics Reveals a Key Role for Fumarate in Mediating the Effects of NADPH Oxidase 4 in Diabetic Kidney Disease. JASN 2016, 27, 466–481. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Liu, A.B.; Sun, S.; Ajami, N.J.; Ross, M.C.; Wang, H.; Zhang, L.; Reuhl, K.; Kobayashi, K.; Onishi, J.C.; et al. Green Tea Polyphenols Modify the Gut Microbiome in Db/Db Mice as Co-Abundance Groups Correlating with the Blood Glucose Lowering Effect. Mol. Nutr. Food Res. 2019, 63, 1801064. [Google Scholar] [CrossRef]
- Zeng, H.; Li, X.; Zhou, D.; Wang, N.; Yu, X.; Long, L.; Cheng, H.; Zhou, S.; Shen, Z.; Zhou, W. Qihu Preparation Ameliorates Diabetes by Activating the AMPK Signaling Pathway in Db/Db Mice. DMSO 2021, 14, 3229–3241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wu, X.; Wang, W.; Yang, C.S.; Zhang, J. Tea Drinking Alleviates Diabetic Symptoms via Upregulating Renal Water Reabsorption Proteins and Downregulating Renal Gluconeogenic Enzymes in Db/Db Mice. Mol. Nutr. Food Res. 2020, 64, e2000505. [Google Scholar] [CrossRef]
- Maher, P. Preventing and Treating Neurological Disorders with the Flavonol Fisetin. Brain Plast. 2021, 6, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Prasath, G.S.; Subramanian, S.P. Modulatory Effects of Fisetin, a Bioflavonoid, on Hyperglycemia by Attenuating the Key Enzymes of Carbohydrate Metabolism in Hepatic and Renal Tissues in Streptozotocin-Induced Diabetic Rats. Eur. J. Pharmacol. 2011, 668, 492–496. [Google Scholar] [CrossRef]
- Kan, E.; Kiliçkan, E.; Ayar, A.; Çolak, R. Effects of Two Antioxidants; α-Lipoic Acid and Fisetin against Diabetic Cataract in Mice. Int. Ophthalmol. 2015, 35, 115–120. [Google Scholar] [CrossRef]
- Wei, X.; Song, H.; Yin, L.; Rizzo, M.G.; Sidhu, R.; Covey, D.F.; Ory, D.S.; Semenkovich, C.F. Fatty Acid Synthesis Configures the Plasma Membrane for Inflammation in Diabetes. Nature 2016, 539, 294–298. [Google Scholar] [CrossRef] [Green Version]
- O’Farrell, M.; Duke, G.; Crowley, R.; Buckley, D.; Martins, E.B.; Bhattacharya, D.; Friedman, S.L.; Kemble, G. FASN Inhibition Targets Multiple Drivers of NASH by Reducing Steatosis, Inflammation and Fibrosis in Preclinical Models. Sci. Rep. 2022, 12, 15661. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Deng, Y.; Yang, L.; Ou, W.; Xie, M.; Ding, L.; Jiang, C.; Yu, H.; Li, Q.; et al. Mitochondrial Protein Hyperacetylation Underpins Heart Failure with Preserved Ejection Fraction in Mice. J. Mol. Cell. Cardiol. 2022, 165, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-M.; Kang, H.-A.; Cominguez, D.C.; Kim, S.-H.; An, H.-J. Papain Ameliorates Lipid Accumulation and Inflammation in High-Fat Diet-Induced Obesity Mice and 3T3-L1 Adipocytes via AMPK Activation. Int. J. Mol. Sci. 2021, 22, 9885. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Yang, S.; Wu, X.; He, D.; Cao, K.; Zhang, W. Emerging Role of Ferroptosis in Acute Kidney Injury. Oxid. Med. Cell. Longev. 2019, 2019, 8010614. [Google Scholar] [CrossRef] [Green Version]
- Bugnicourt, J.-M.; Godefroy, O.; Chillon, J.-M.; Choukroun, G.; Massy, Z.A. Cognitive Disorders and Dementia in CKD: The Neglected Kidney-Brain Axis. J. Am. Soc. Nephrol. 2013, 24, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Miranda, A.S.; Cordeiro, T.M.; Dos Santos Lacerda Soares, T.M.; Ferreira, R.N.; Simões, E.; Silva, A.C. Kidney-Brain Axis Inflammatory Cross-Talk: From Bench to Bedside. Clin. Sci. 2017, 131, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Li, S.; Zhang, C.; Chen, H.; Wang, N.; Feng, Y. Clinical Efficacies, Underlying Mechanisms and Molecular Targets of Chinese Medicines for Diabetic Nephropathy Treatment and Management. Acta Pharm. Sin. B 2021, 11, 2749–2767. [Google Scholar] [CrossRef] [PubMed]
- Mizdrak, M.; Kumrić, M.; Kurir, T.T.; Božić, J. Emerging Biomarkers for Early Detection of Chronic Kidney Disease. J. Pers. Med. 2022, 12, 548. [Google Scholar] [CrossRef]
- Ntrinias, T.; Papasotiriou, M.; Balta, L.; Kalavrizioti, D.; Vamvakas, S.; Papachristou, E.; Goumenos, D.S. Biomarkers in Progressive Chronic Kidney Disease. Still a Long Way to Go. PRILOZI 2019, 40, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, H.Y.; Ford Versypt, A.N. Pathophysiology of Mesangial Expansion in Diabetic Nephropathy: Mesangial Structure, Glomerular Biomechanics, and Biochemical Signaling and Regulation. J. Biol. Eng. 2022, 16, 19. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, G. Update on Pathogenesis of Glomerular Hyperfiltration in Early Diabetic Kidney Disease. Front. Endocrinol. 2022, 13, 872918. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liang, X.; Ladeiras, D.; Fellay, B.; Ming, X.-F.; Yang, Z. Role of Tubular Epithelial Arginase-II in Renal Inflammaging. NPJ Aging Mech. Dis. 2021, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Pernow, J.; Jung, C. The Emerging Role of Arginase in Endothelial Dysfunction in Diabetes. Curr. Vasc. Pharmacol. 2016, 14, 155–162. [Google Scholar] [CrossRef]
- Polis, B.; Srikanth, K.D.; Elliott, E.; Gil-Henn, H.; Samson, A.O. L-Norvaline Reverses Cognitive Decline and Synaptic Loss in a Murine Model of Alzheimer’s Disease. Neurotherapeutics 2018, 15, 1036–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, N.S.; Stamper, B.D.; Elbarbry, F.; Nguyen, V.; Lopez, S.; Kawasaki, Y.; Poormohamadian, R.; Roberts, S.C. Natural Products That Target the Arginase in Leishmania Parasites Hold Therapeutic Promise. Microorganisms 2021, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Jia, Q.; Cao, H.; Chen, C.; Xing, S.; Huang, Y.; Shen, D. Fisetin Ameliorates Atherosclerosis by Regulating PCSK9 and LOX-1 in ApoE-/- Mice. Exp. Ther. Med. 2021, 21, 25. [Google Scholar] [CrossRef]
- Galvan, D.L.; Mise, K.; Danesh, F.R. Mitochondrial Regulation of Diabetic Kidney Disease. Front. Med. 2021, 8, 745279. [Google Scholar] [CrossRef]
- Friederich-Persson, M.; Persson, P. Mitochondrial Angiotensin II Receptors Regulate Oxygen Consumption in Kidney Mitochondria from Healthy and Type 1 Diabetic Rats. Am. J. Physiol. Renal. Physiol. 2020, 318, F683–F688. [Google Scholar] [CrossRef]
- Wu, M.; Li, S.; Yu, X.; Chen, W.; Ma, H.; Shao, C.; Zhang, Y.; Zhang, A.; Huang, S.; Jia, Z. Mitochondrial Activity Contributes to Impaired Renal Metabolic Homeostasis and Renal Pathology in STZ-Induced Diabetic Mice. Am. J. Physiol. Renal. Physiol. 2019, 317, F593–F605. [Google Scholar] [CrossRef]
- Lindblom, R.; Higgins, G.; Coughlan, M.; de Haan, J.B. Targeting Mitochondria and Reactive Oxygen Species-Driven Pathogenesis in Diabetic Nephropathy. Rev. Diabet. Stud. 2015, 12, 134–156. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Wu, F.; Wang, J.; Gao, L.; Jiang, L.; Li, H.-D.; Ma, Q.; Liu, X.; Wei, B.; Zhou, L.; et al. Nox4 in Renal Diseases: An Update. Free. Radic. Biol. Med. 2018, 124, 466–472. [Google Scholar] [CrossRef]
- Jo, H.A.; Hyeon, J.S.; Yang, S.H.; Jung, Y.; Ha, H.; Jeong, C.W.; Kwak, C.; Kim, Y.; Lee, H.; Lee, J.P.; et al. Fumarate Modulates Phospholipase A2 Receptor Autoimmunity-Induced Podocyte Injury in Membranous Nephropathy. Kidney Int. 2021, 99, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Liu, S.; Gurung, R.L.; Ching, J.; Kovalik, J.P.; Tan, T.Y.; Lim, S.C. Urine Tricarboxylic Acid Cycle Metabolites Predict Progressive Chronic Kidney Disease in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 4357–4364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mina, A.I.; LeClair, R.A.; LeClair, K.B.; Cohen, D.E.; Lantier, L.; Banks, A.S. CalR: A Web-Based Analysis Tool for Indirect Calorimetry Experiments. Cell Metab. 2018, 28, 656–666.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Court, M.; Selevsek, N.; Matondo, M.; Allory, Y.; Garin, J.; Masselon, C.D.; Domon, B. Toward a Standardized Urine Proteome Analysis Methodology. Proteomics 2011, 11, 1160–1171. [Google Scholar] [CrossRef]
- Benedé-Ubieto, R.; Estévez-Vázquez, O.; Ramadori, P.; Cubero, F.J.; Nevzorova, Y.A. Guidelines and Considerations for Metabolic Tolerance Tests in Mice. Diabetes Metab. Syndr. Obes. 2020, 13, 439–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, P.; Currais, A. Large-Scale Metabolome Analysis. Available online: Bio-protocol.org/prep102 (accessed on 5 January 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahid, S.; Dafre, A.L.; Currais, A.; Yu, J.; Schubert, D.; Maher, P. The Geroprotective Drug Candidate CMS121 Alleviates Diabetes, Liver Inflammation, and Renal Damage in db/db Leptin Receptor Deficient Mice. Int. J. Mol. Sci. 2023, 24, 6828. https://doi.org/10.3390/ijms24076828
Zahid S, Dafre AL, Currais A, Yu J, Schubert D, Maher P. The Geroprotective Drug Candidate CMS121 Alleviates Diabetes, Liver Inflammation, and Renal Damage in db/db Leptin Receptor Deficient Mice. International Journal of Molecular Sciences. 2023; 24(7):6828. https://doi.org/10.3390/ijms24076828
Chicago/Turabian StyleZahid, Saadia, Alcir L. Dafre, Antonio Currais, Jingting Yu, David Schubert, and Pamela Maher. 2023. "The Geroprotective Drug Candidate CMS121 Alleviates Diabetes, Liver Inflammation, and Renal Damage in db/db Leptin Receptor Deficient Mice" International Journal of Molecular Sciences 24, no. 7: 6828. https://doi.org/10.3390/ijms24076828






