Activated Carbon-Enriched Electrospun-Produced Scaffolds for Drug Delivery/Release in Biological Systems
Abstract
:1. Introduction
2. Results and Discussion
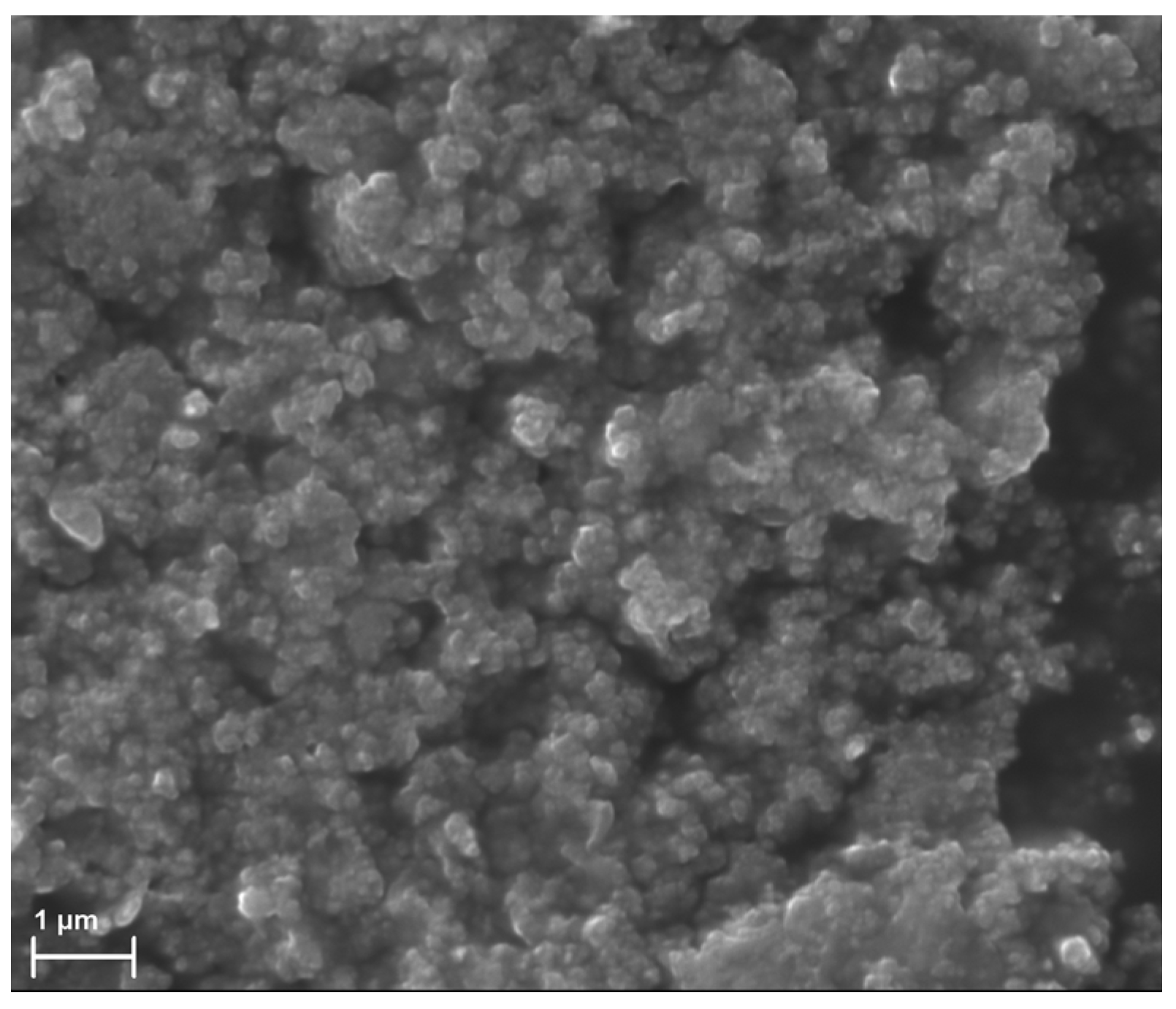
2.1. Preparation of ACN Suspensions
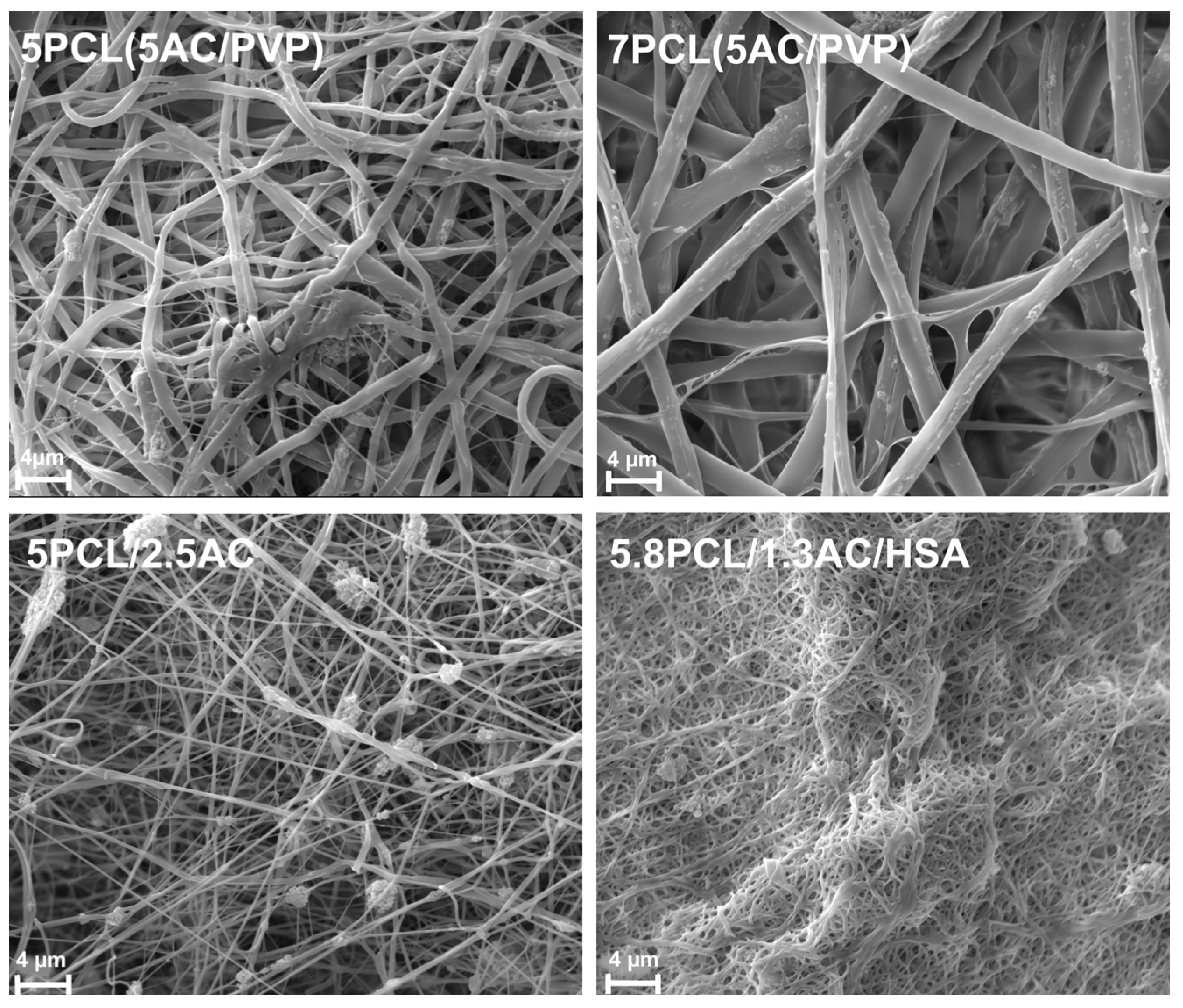
2.2. Preparation and Characterization of ACN-Enriched Scaffolds
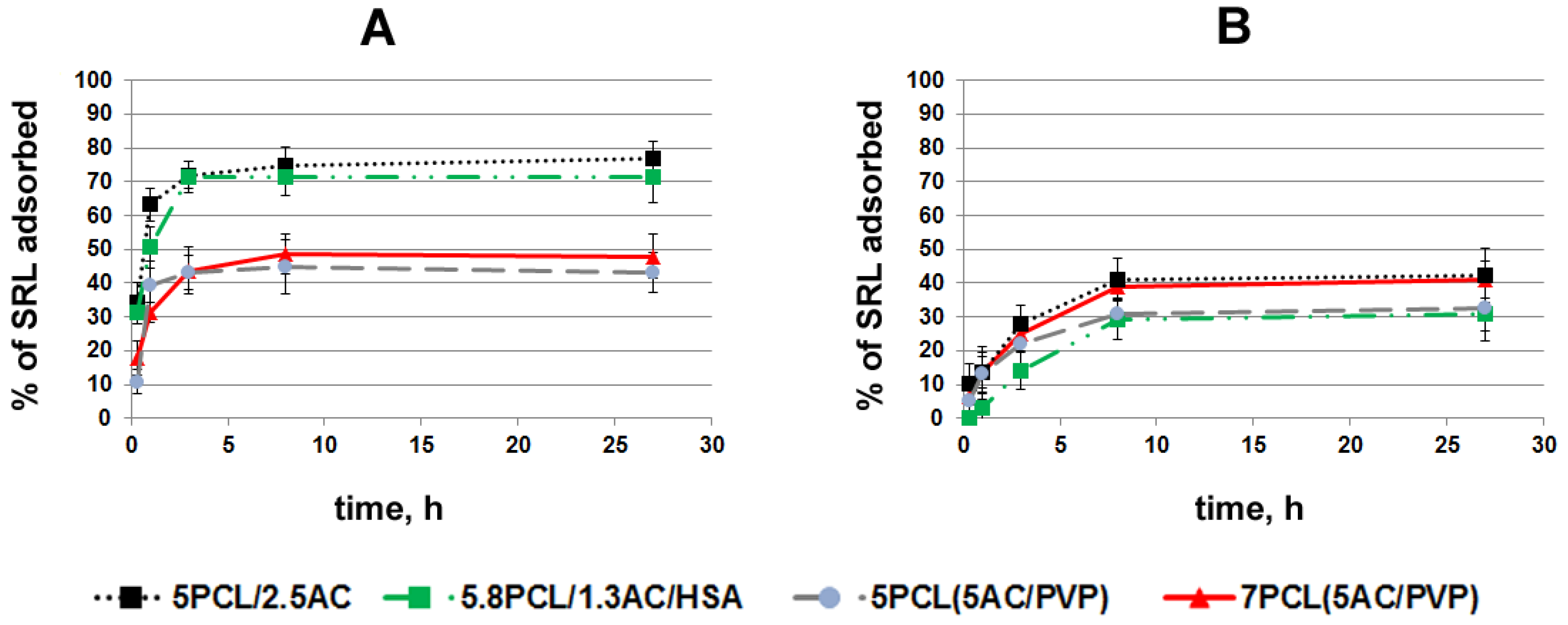
2.3. Study of Sirolimus Adsorption
2.4. Study of SRL Release
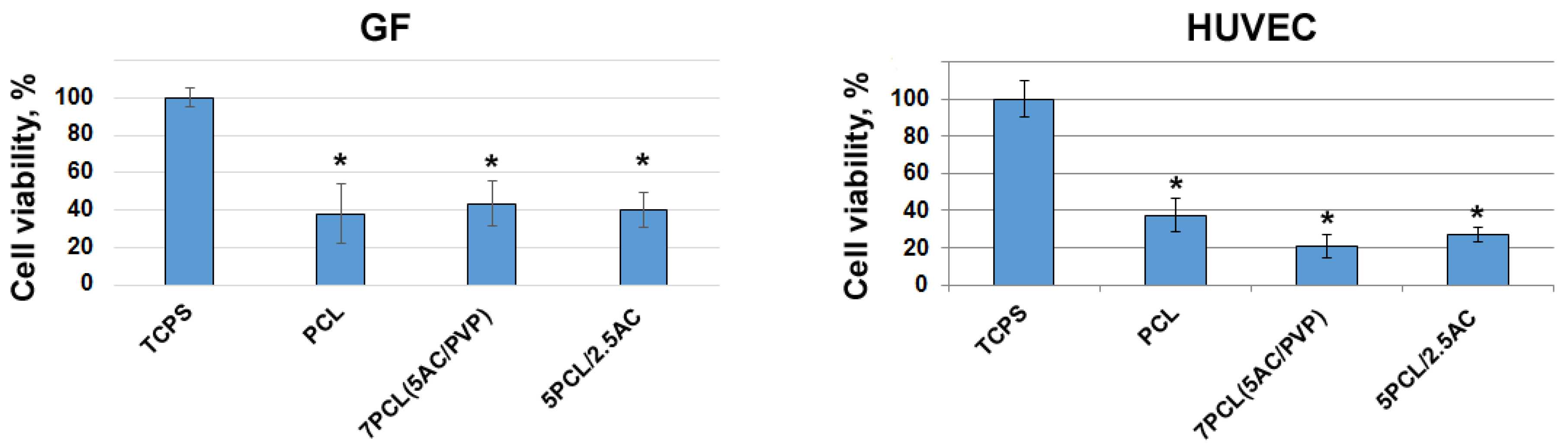
2.5. Cultivation of Cells on ACN-Enriched Scaffolds
3. Materials and Methods
3.1. Materials
3.2. Production of 3H-SRL
3.3. Preparation and Characterization of the ACN
3.4. Preparation of ACN Suspensions and Estimation of a Percolation Limit
3.5. Preparation of the Scaffolds by Electrospinning
3.6. Characterization of AC-Enriched Scaffolds
3.7. Study of SRL Binding and Release
3.8. Biocompatibility of the Scaffolds
3.9. Statistical Processing of Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Arun, A.; Malrautu, P.; Laha, A.; Luo, H.; Ramakrishna, S. Collagen nanoparticles in drug delivery systems and tissue engineering. Appl. Sci. 2021, 11, 11369. [Google Scholar] [CrossRef]
- Mohammadalizadeh, Z.; Bahremandi-Toloue, E.; Karbasi, S. Recent advances in modification strategies of pre- and post-electrospinning of nanofiber scaffolds in tissue engineering. React. Funct. Polym. 2022, 172, 105202. [Google Scholar] [CrossRef]
- Reis, K.; Sperling, L.; Teixeira, C.; Sommer, L.; Colombo, M.; Koester, L.; Pranke, P. VPA/PLGA microfibers produced by coaxial electrospinning for the treatment of central nervous system injury. Braz. J. Med. Biol. Res. 2020, 53, e8993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puhl, D.L.; Mohanraj, D.; Nelson, D.W.; Gilbert, R.J. Designing electrospun fiber platforms for efficient delivery of genetic material and genome editing tools. Adv. Drug Deliv. Rev. 2022, 183, 114161. [Google Scholar] [CrossRef] [PubMed]
- Kuno, T.; Ueyama, H.; Mikami, T.; Takagi, H.; Numasawa, Y.; Anzai, H.; Bangalore, S. Mortality in patients undergoing revascularization with paclitaxel eluting devices for infrainguinal peripheral artery disease: Insights from a network meta-analysis of randomized trials. Catheter. Cardiovasc. Interv. 2020, 96, E467–E478. [Google Scholar] [CrossRef]
- Macha, I.J.; Ben-Nissan, B.; Vilchevskaya, E.N.; Morozova, A.S.; Abali, B.E.; Wolfgang, H.; Müller, W.H.; Rickert, W. Drug delivery from polymer-based nanopharmaceuticals–an experimental study complemented by simulations of selected diffusion processes. Front. Bioeng. Biotechnol. 2019, 7, 37. [Google Scholar] [CrossRef]
- Li, D.; Wang, M.; Song, W.-L.; Yu, D.-G.; Bligh, S.W.A. Electrospun Janus Beads-On-A-String Structures for Different Types of Controlled Release Profiles of Double Drugs. Biomolecules 2021, 11, 635. [Google Scholar] [CrossRef]
- Yadavalli, T.; Ames, J.; Agelidis, A.; Suryawanshi, R.; Jaishankar, D.; Hopkins, J.; Thakkar, N.; Koujah, L.; Shukla, D. Drug-encapsulated carbon (DECON): A novel platform for enhanced drug delivery. Sci. Adv. 2019, 5, eaax0780. [Google Scholar] [CrossRef] [Green Version]
- Apul, O.G.; von Reitzenstein, N.H.; Schoepf, J.; Ladner, D.; Hristovski, K.D.; Westerhoff, P. Superfine powdered activated carbon incorporated into electrospun polystyrene fibers preserve adsorption capacity. Sci. Total Environ. 2017, 592, 458–464. [Google Scholar] [CrossRef]
- Li, F.; Chen, C.; Wang, Y.; Li, W.; Zhou, G.; Zhang, H.; Zhang, J.; Wang, J. Activated carbon-hybridized and amine-modified polyacrylonitrile nanofibers toward ultrahigh and recyclable metal ion and dye adsorption from wastewater. Front. Chem. Sci. Eng. 2021, 15, 984–997. [Google Scholar] [CrossRef]
- Chen, J.; Sun, J.; Luo, M.; Li, Y.; Wang, Z.; Wang, Y. As(III) oxidation and kinetic analysis by Herminiimonas arsenicoxydans-loaded electrospinning activated carbon fiber biofilms. Chemosphere 2022, 308, 136479. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, C.; Wang, B.; Quan, W.; Xu, C. AgNP-AC composite fibers and its adsorption and antibacterial properties. Front. Mater. 2022, 9, 894451. [Google Scholar] [CrossRef]
- Koriem, O.A.; Kamel, A.M.; Shaaban, W.; Elkady, M.F. Enhancement of dye separation performance of eco-friendly cellulose acetate-based embranes. Sustainability 2022, 14, 14665. [Google Scholar] [CrossRef]
- Malczewska, B. Preparation and performance of PAN–PAC nanofibers by electrospinning process to remove NOM from water. Materials 2021, 14, 4426. [Google Scholar] [CrossRef]
- Elmaghraby, N.A.; Omer, A.M.; Kenawy, E.-R.; Gaber, M.; Ragab, S.; Nemr, A.E. Composite nanofiber formation using a mixture of cellulose acetate and activated carbon for oil spill treatment. Environ. Sci. Pollut. Res. 2022, 7, 1–17. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.J. Colloid syringeless electrospinning toward nonwoven nanofiber web containing a massive amount of inorganic fillers. Macromol. Mater. Eng. 2022, 307, 2100818. [Google Scholar] [CrossRef]
- Lee, J.; Seo, J.; Cho, K.M.; Heo, J.; Jung, H.; Park, S.; Bae, J.; Lee, S.; Hong, J.; Kim, M.-K.; et al. Ultralight and Ultrathin Electrospun Membranes with Enhanced Air Permeability for Chemical and Biological Protection. ACS Appl. Mater. Interfaces 2022, 14, 32522–32532. [Google Scholar] [CrossRef]
- Hou, Z.; Itagaki, N.; Kobayashi, H.; Tanaka, K.; Takarada, W.; Kikutani, T.; Takasaki, M. Bamboo charcoal/poly(L-lactide) fiber webs prepared using laser-heated melt electrospinning. Polymers 2021, 13, 2776. [Google Scholar] [CrossRef]
- Nazarkina, Z.K.; Savostyanova, T.A.; Chelobanov, B.P.; Romanova, I.V.; Simonov, P.A.; Kvon, R.I.; Karpenko, A.A.; Laktionov, P.P. Activated Carbon for Drug Delivery from Composite Biomaterials: The Effect of Grinding on Sirolimus Binding and Release. Pharmaceutics 2022, 14, 1386. [Google Scholar] [CrossRef]
- Kuznetsov, K.A.; Murashov, I.S.; Chernonosova, V.S.; Chelobanov, B.P.; Stepanova, A.O.; Sergeevichev, D.S.; Karpenko, A.A.; Laktionov, P.P. Vascular Stents Coated with Electrospun Drug-Eluting Material: Functioning in Rabbit Iliac Artery. Polymers 2020, 12, 1741. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250. [Google Scholar] [CrossRef] [Green Version]
- Ko, Y.G.; Park, J.H.; Lee, J.B.; Oh, H.H.; Park, W.H.; Cho, D.; Kwon, O.H. Growth behavior of endothelial cells according to electrospun poly(D,L-Lactic-Co-Glycolic acid) fiber diameter as a tissue engineering scaffold. Tissue Eng. Regen. Med. 2016, 13, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Whited, B.M.; Rylander, M.N. The influence of electrospun scaffold topography on endothelial cell morphology, alignment, and adhesion in response to fluid flow. Biotechnol. Bioeng. 2014, 111, 184–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernonosova, V.S.; Laktionov, P.P. Structural Aspects of Electrospun Scaffolds Intended for Prosthetics of Blood Vessels. Polymers 2022, 14, 1698. [Google Scholar] [CrossRef]
- Luo, Y.; Hong, Y.; Shen, L.; Wu, F.; Lin, X. Multifunctional role of polyvinylpyrrolidone in pharmaceutical formulations. AAPS PharmSciTech 2021, 22, 34. [Google Scholar] [CrossRef]
- Nazarkina, Z.K.; Chelobanov, B.P.; Chernonosova, V.S.; Romanova, I.V.; Karpenko, A.A.; Laktionov, P.P. Siroli-mus-Eluting Electrospun-Produced Matrices as Coatings for Vascular Stents: Dependence of Drug Release on Matrix Structure and Composition of the External Environment. Materials 2020, 13, 2692. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef]
- Soliman, S.; Sant, S.; Nichol, J.W.; Khabiry, M.; Traversa, E.; Khademhosseini, A. Controlling the porosity of fibrous scaffolds by modulating the fiber diameter and packing density. J. Biomed. Mater. Res. A 2011, 96, 566–574. [Google Scholar] [CrossRef]
- Pryadko, A.S.; Botvin, V.V.; Mukhortova, Y.R.; Pariy, I.; Wagner, D.V.; Laktionov, P.P.; Chernonosova, V.S.; Chelobanov, B.P.; Chernozem, R.V.; Surmeneva, M.A.; et al. Core-Shell Magnetoactive PHB/Gelatin/Magnetite Composite Electrospun Scaffolds for Biomedical Applications. Polymers 2022, 14, 529. [Google Scholar] [CrossRef]
- Yazgan, G.; Dmitriev, R.I.; Tyagi, V.; Jenkins, J.; Rotaru, G.-M.; Rottmar, M.; Rossi, R.M.; Toncelli, C.; Papkovsky, D.B.; Maniura-Weber, K.; et al. Steering surface topographies of electrospun fibers: Understanding the mechanisms. Sci. Rep. 2017, 7, 158. [Google Scholar] [CrossRef] [Green Version]
- Peter, T., Jr. Ligand binding by albumin. In All about Albumin; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Xu, C.; Yang, F.; Wang, S.; Ramakrishna, S. In vitro study of human vascular endothelial cell function on materials with various surface roughness. J. Biomed. Mater. Res. A 2004, 71, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.O.; Laktionov, P.P.; Cherepanova, A.V.; Chernonosova, V.S.; Shevelev, G.Y.; Zaporozhchenko, I.A.; Karaskov, A.M.; Laktionov, P.P. General Study and Gene Expression Profiling of Endotheliocytes Cultivated on Electrospun Materials. Materials 2019, 12, 4082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidorov, V.N.; Polak, Y.V.; Laktionov, P.P.; Roshcke, V.V.; Kist, A.G. Method of Production of Tritium Labeled Organic Compounds and the Device for Its Implementation. SU Patent 1823961 A3, 18 January 1991. [Google Scholar]
- ISO Standard No. 7198; Cardiovascular implants and extracorporeal systems—Vascular prostheses—Tubular vascular grafts and vascular patches. International Organization for Standardization: Geneva, Switzerland. 2016. Available online: https://www.iso.org/standard/50661.html (accessed on 8 December 2022).
- Cherepanova, A.; Bushuev, A.; Kharkova, M.; Vlassov, V.V.; Laktionov, P.P. DNA inhibits dsRNA-induced secretion of proinflammatory cytokines by gingival fibroblasts. Immunobiology 2013, 218, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Minick, C.R. Culture of Human Endothelial Cells Derived from Umbilical Veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 1973, 52, 2745–2756. [Google Scholar]


| Composition of the Suspension | Solvent | |||||
|---|---|---|---|---|---|---|
| Ethanol | HFIP | TFE | H2O | Comments | ||
| PEG | ACN concentration, % | 0.01–2 | 0.01–2 | 0.01–2 | 0.01–2 | Suspensions are not stable in all ranges of concentrations |
| PEG concentration, % | 2–10 | 2–10 | 2–10 | 2–10 | ||
| Dispersibility * | + | + | + | + | ||
| suspension stability ** | − | ± | ± | − | ||
| PVP | ACN concentration, % | 0.01–2 | 0.01–10 | 0.01–2 | 0.01–2 | Optimal suspension contained 5% PVP, 5% ACNs (w/v) in HFIP |
| PVP concentration, % | 2–10 | 2/3.5/5/7/10 | 2–10 | 2–10 | ||
| dispersibility | + | + | + | + | ||
| suspension stability | − | −/±/+/+/+ | + | − | ||
| PVA | ACN concentration, % | − | 0.01–2 | − | − | Suspensions are not stable in all ranges of concentrations |
| PVA concentration, % | 2–10 | 2–10 | 2–10 | 2–10 | ||
| dispersibility | − | + | − | − | ||
| suspension stability | − | ± | − | − | ||
| H2O | ACN concentration, % | 2 | 2 | 2 | 2 | Suspensions are not stable in all ranges of concentrations |
| dispersibility | − | ± | ± | − | ||
| suspension stability | − | ± | ± | − | ||
| PCL | ACN concentration, % | − | 0.01–5 | 0.01–2 | − | Optimal suspension contained 5–7% PCL, 2.5% ACNs (w/v) in HFIP |
| PCL concentration, % | − | 5/7/10 | 5/7/10 | − | ||
| dispersibility | − | + | + | − | ||
| suspension stability | − | + | + | − | ||
| Treatment Suspension (% Weight/Volume) | 1000 g, 0.5 min, % ACN | 1000 g, 1 min, % ACN | 1000 g, 5 min, % ACN | 10,000 g, 5 min, % ACN | 12,000 g, 30 min, % ACN | Supernatant % ACN |
|---|---|---|---|---|---|---|
| 5% AC P *–5% PVP | 46.8 | 17.9 | 15.4 | 7.1 | 5.4 | 7.5 |
| 5% AC P–5% PVP | − | − | 85.8 | − | − | 14.2 |
| 5% AC W **–5% PVP | 30.7 | 27.2 | 30 | 6.2 | 2.9 | 3 |
| 5% AC W–5% PVP | − | − | 88.5 | − | − | 11.5 |
| 5% AC W–5% PCL | 23 | 22.8 | 43.4 | 8 | 0.3 | 2.5 |
| 5% AC W–5% PCL | − | − | 89.2 | − | − | 10.8 |
| Suspension, Composition | Suspension Resistance, kΩ/cm | Film Resistance, kΩ/cm |
|---|---|---|
| 2.5% AC–5% PCL | 860 | 10 |
| 2.5% AC–5% PVP | >2000 | 1600 |
| 5% AC–5% PVP, 1000 g, 1 min | >2000 | >2000 |
| 5% AC P–5% PVP, 1000 g, 1 min | >2000 | >2000 |
| Composition of Scaffold | Abbreviation | Content of Particles in Scaffold mg/cm2 | Feed Rate, mL/h Inner/Outer | Voltage, kV | Strength (MPa) | Maximum Elongation, % | Fiber Diameter (µm) | Pore Diameter (µm) | Porosity (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| coaxial fibers | 5% PCL-outer 5% AC/5% PVP-inner | 5PCL(5AC/PVP) | 0.40 ± 0.10 | 1/5 | 14 | 2.58 | 162% | 0.99 ± 0.65 | 1.5 ± 0.7 | 42% |
| 7% PCL-outer 5% AC/5% PVP-inner | 7PCL(5AC/PVP) | 0.35 ± 0.10 | 1/5 | 14 | 3.48 | 475% | 2.07 ± 0.3 | 7.2 ± 1.3 | 60% | |
| homogeneous fibers | 5% PCL/2.5% AC/0.1mMTEA * | 5PCL/2.5AC | 1.50 ± 0.20 | 0.7 | 24 | 4.65 | 175% | 0.21 ± 0.05 | 2 ± 0.4 | 37% |
| 5.8% PCL/1.3% AC/0.9% HSA *** | 5.8PCL/1.3AC/HSA | 0.80 ± 0.20 | 0.7 | 24 | ND ** | ND | 0.12 ± 0.02 | 0.3 ± 0.1 | 75% | |
| no AC | 5% PCL | 5 PCL | no AC | 1 | 26.5 | 15.04 | 200% | 0.36 ± 0.09 | 2.11 ± 0.34 | 61% |
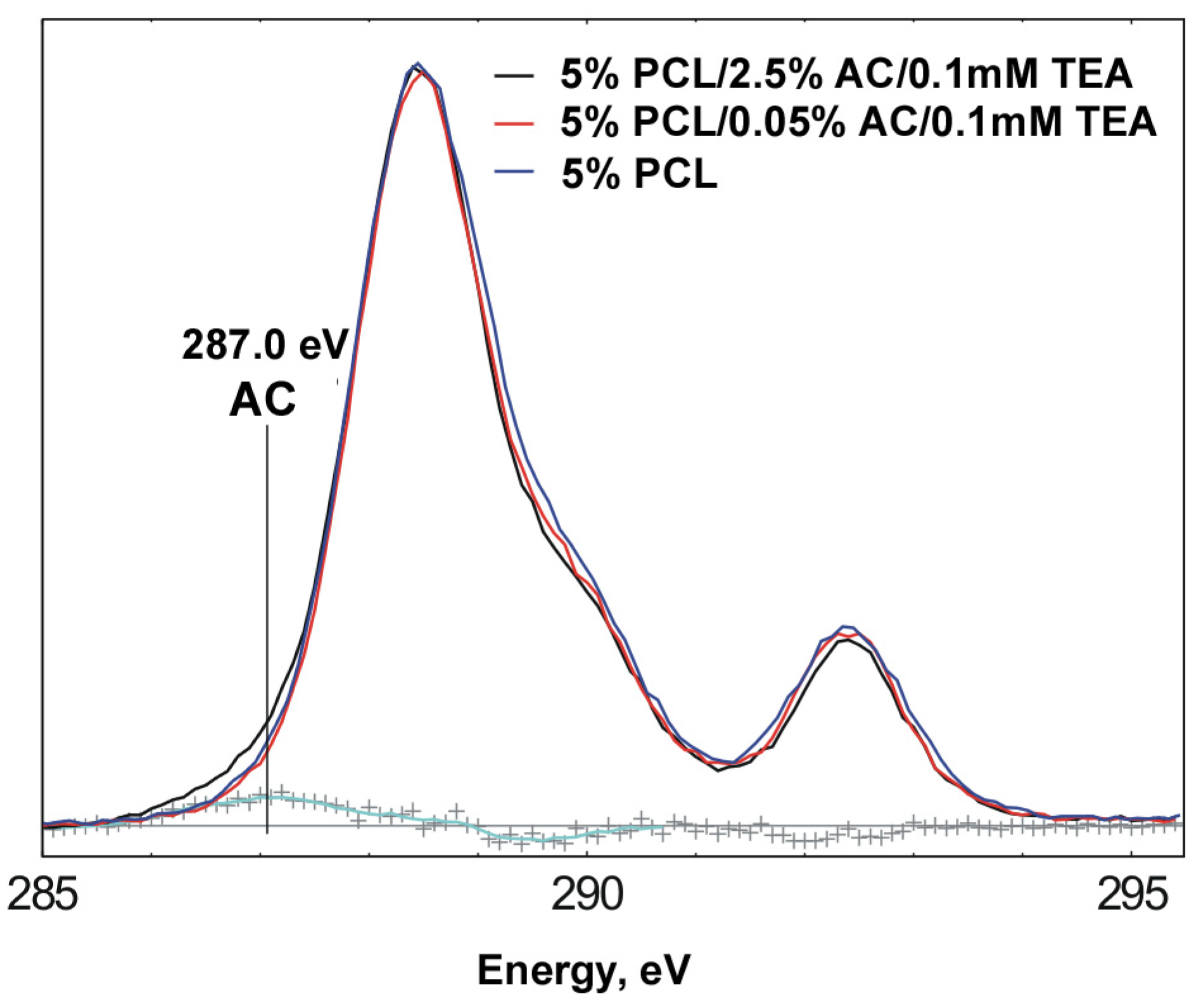
| O/C | P/C | N/C | |
|---|---|---|---|
| 5% PCL | 0.33 | - | - |
| 5% PCL/0.05% AC/0.1mMTEA | 0.35 | - | - |
| 5% PCL/2.5% AC/0.1mMTEA | 0.35 | - | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarkina, Z.K.; Stepanova, A.O.; Chelobanov, B.P.; Kvon, R.I.; Simonov, P.A.; Karpenko, A.A.; Laktionov, P.P. Activated Carbon-Enriched Electrospun-Produced Scaffolds for Drug Delivery/Release in Biological Systems. Int. J. Mol. Sci. 2023, 24, 6713. https://doi.org/10.3390/ijms24076713
Nazarkina ZK, Stepanova AO, Chelobanov BP, Kvon RI, Simonov PA, Karpenko AA, Laktionov PP. Activated Carbon-Enriched Electrospun-Produced Scaffolds for Drug Delivery/Release in Biological Systems. International Journal of Molecular Sciences. 2023; 24(7):6713. https://doi.org/10.3390/ijms24076713
Chicago/Turabian StyleNazarkina, Zhanna K., Alena O. Stepanova, Boris P. Chelobanov, Ren I. Kvon, Pavel A. Simonov, Andrey A. Karpenko, and Pavel P. Laktionov. 2023. "Activated Carbon-Enriched Electrospun-Produced Scaffolds for Drug Delivery/Release in Biological Systems" International Journal of Molecular Sciences 24, no. 7: 6713. https://doi.org/10.3390/ijms24076713





