Colorimetric Detection of Acenaphthene and Naphthalene Using Functionalized Gold Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
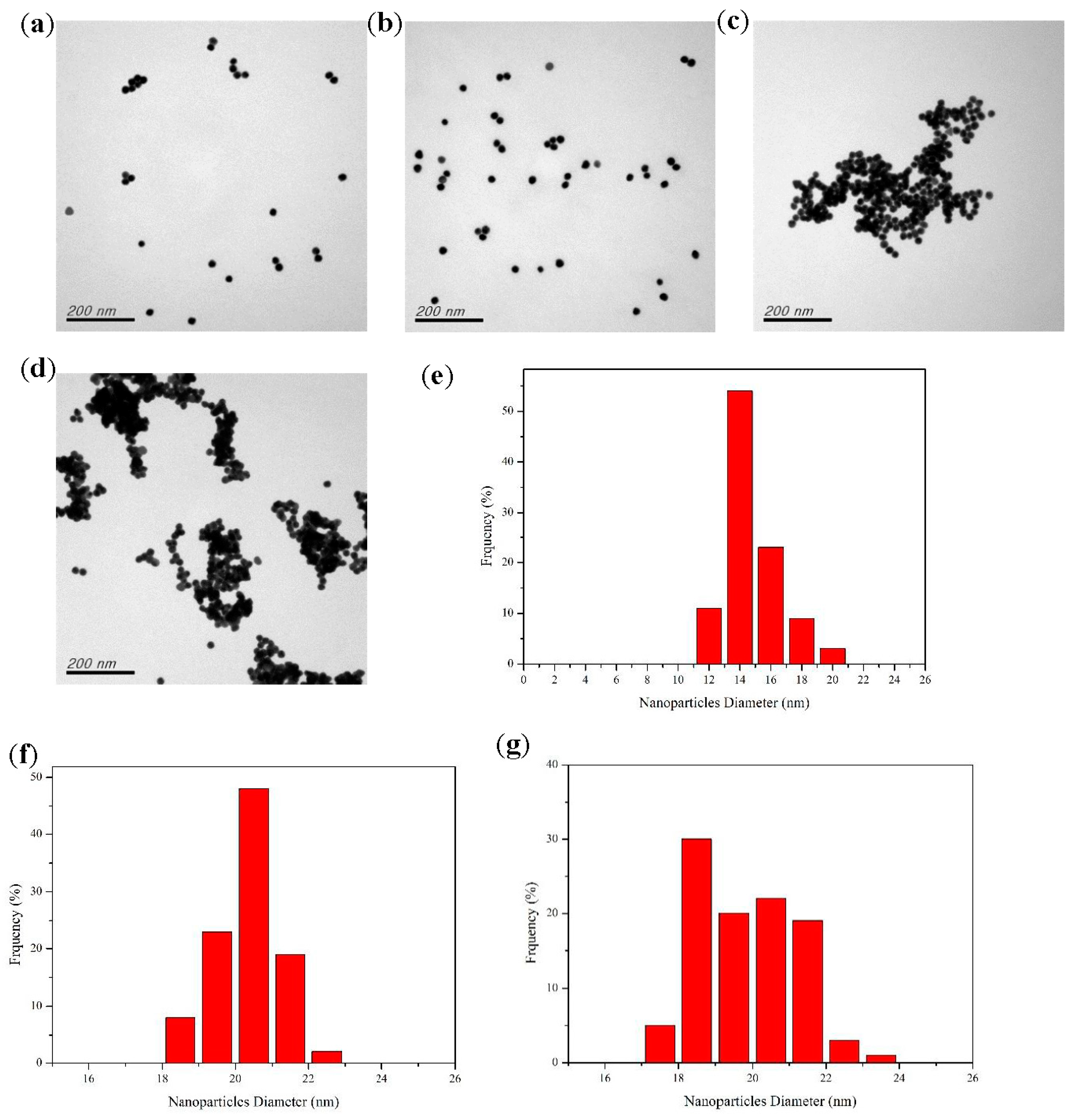
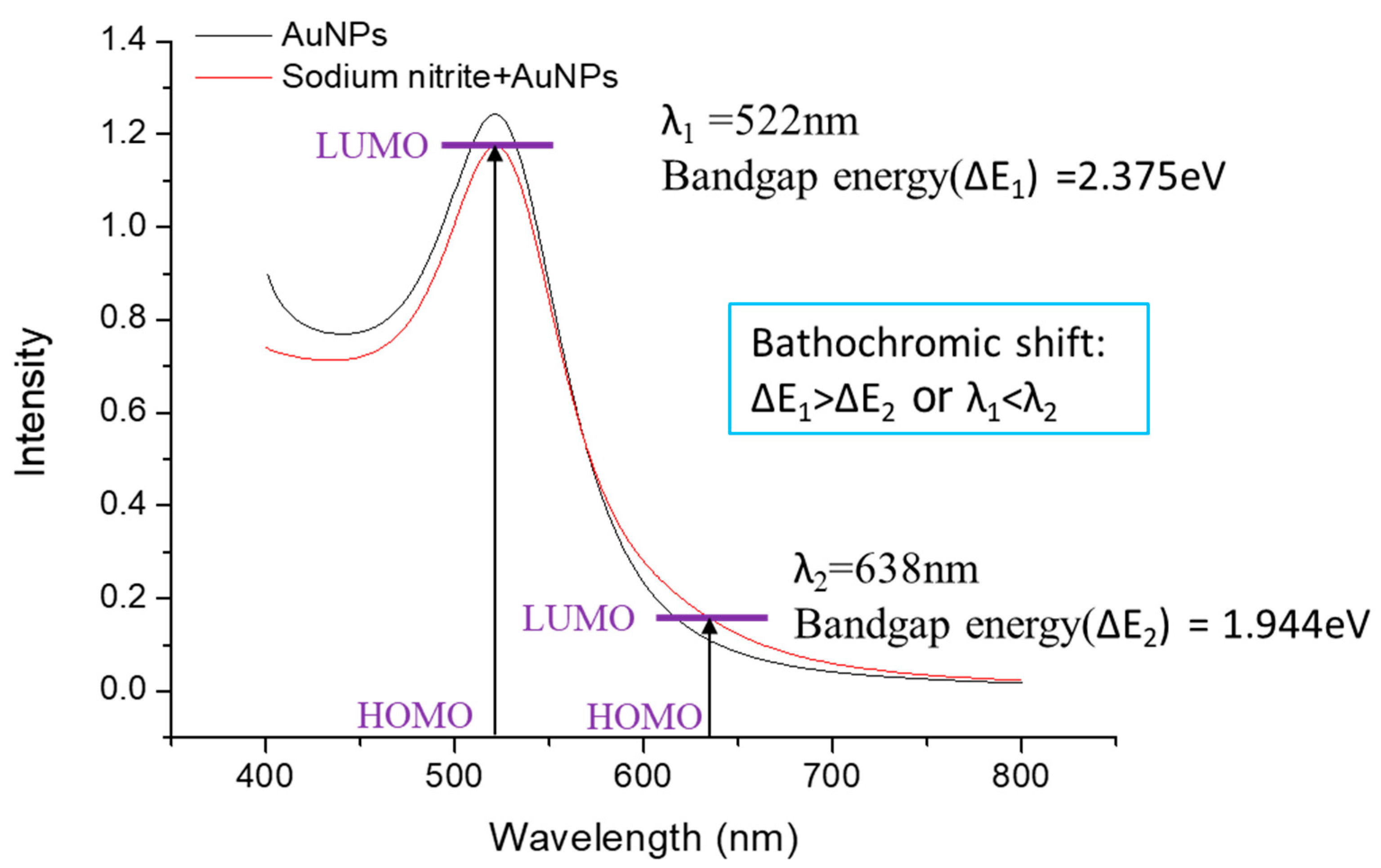
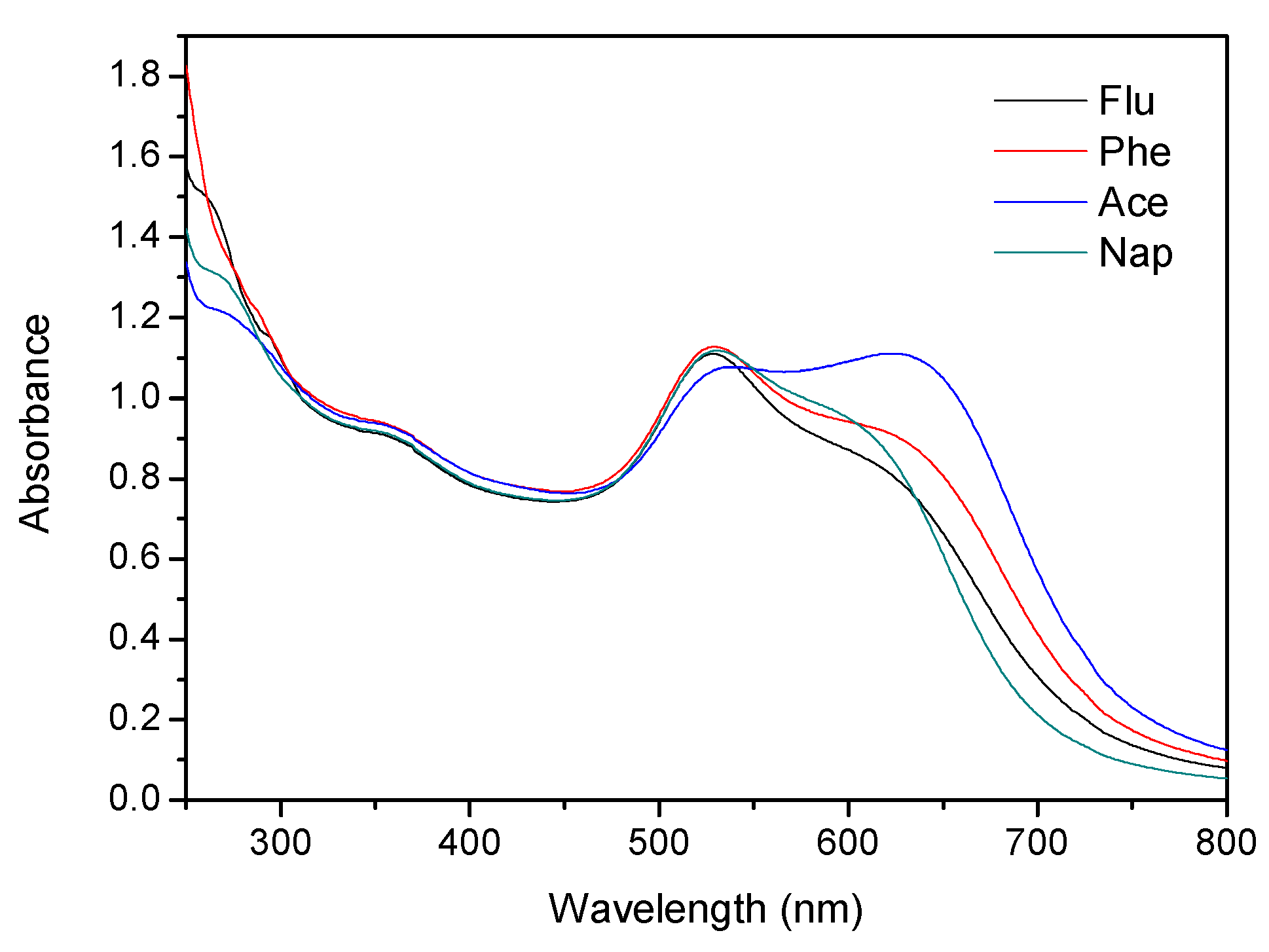
2.1. Characterization of Functionalized AuNPs
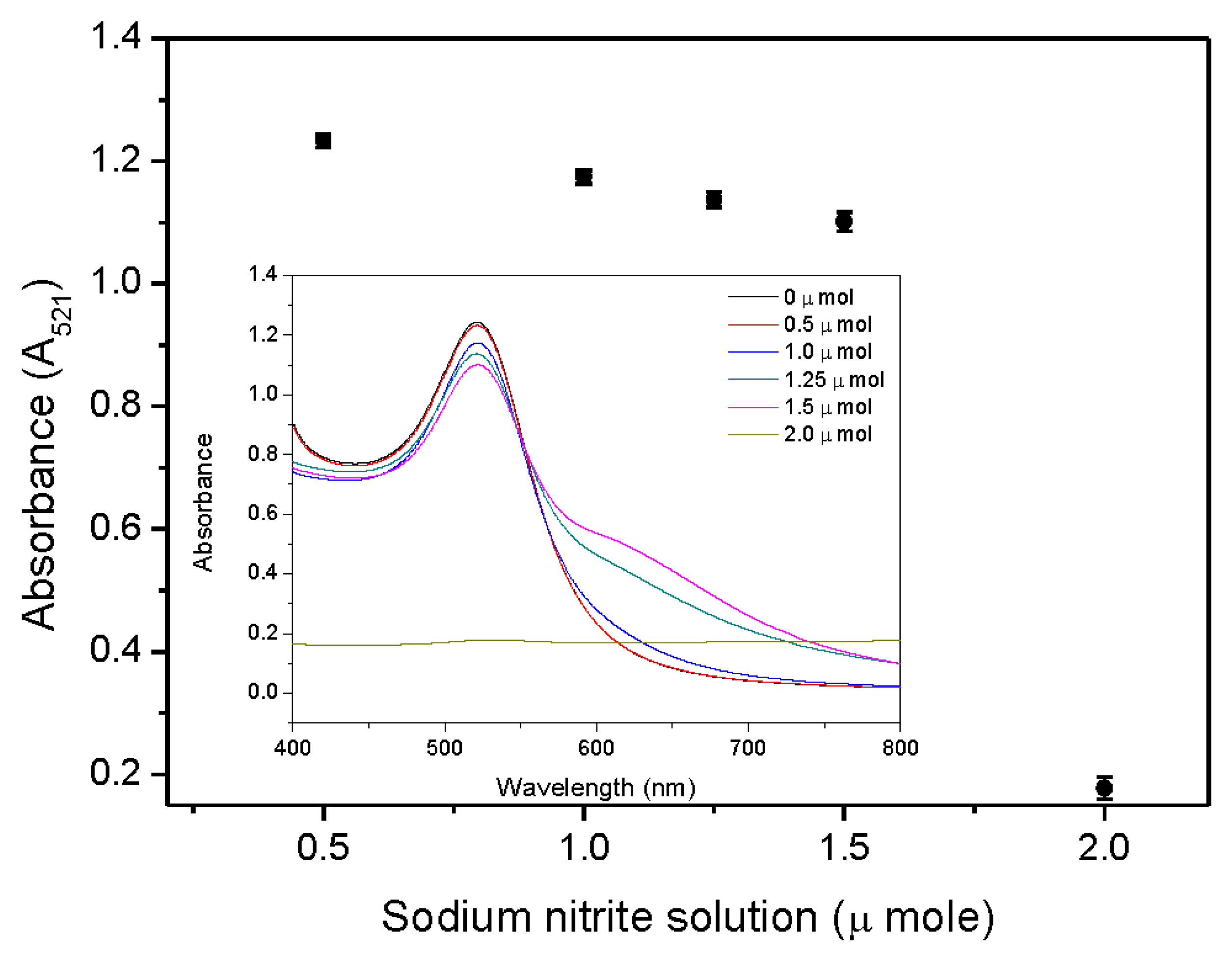
2.2. Optimization of the Amount of Sodium Nitrite
2.3. Optimization of the PBS Buffer Solution
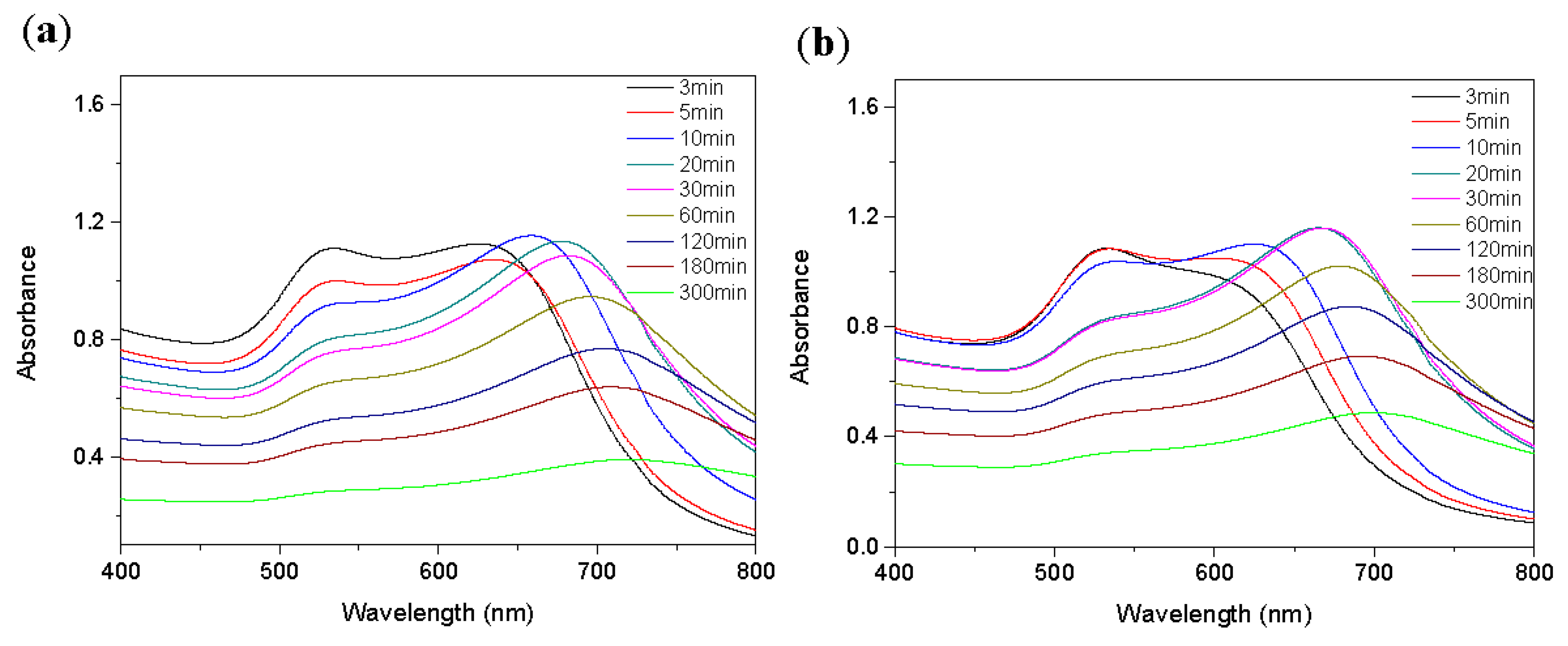
2.4. Optimization of the Detection Time
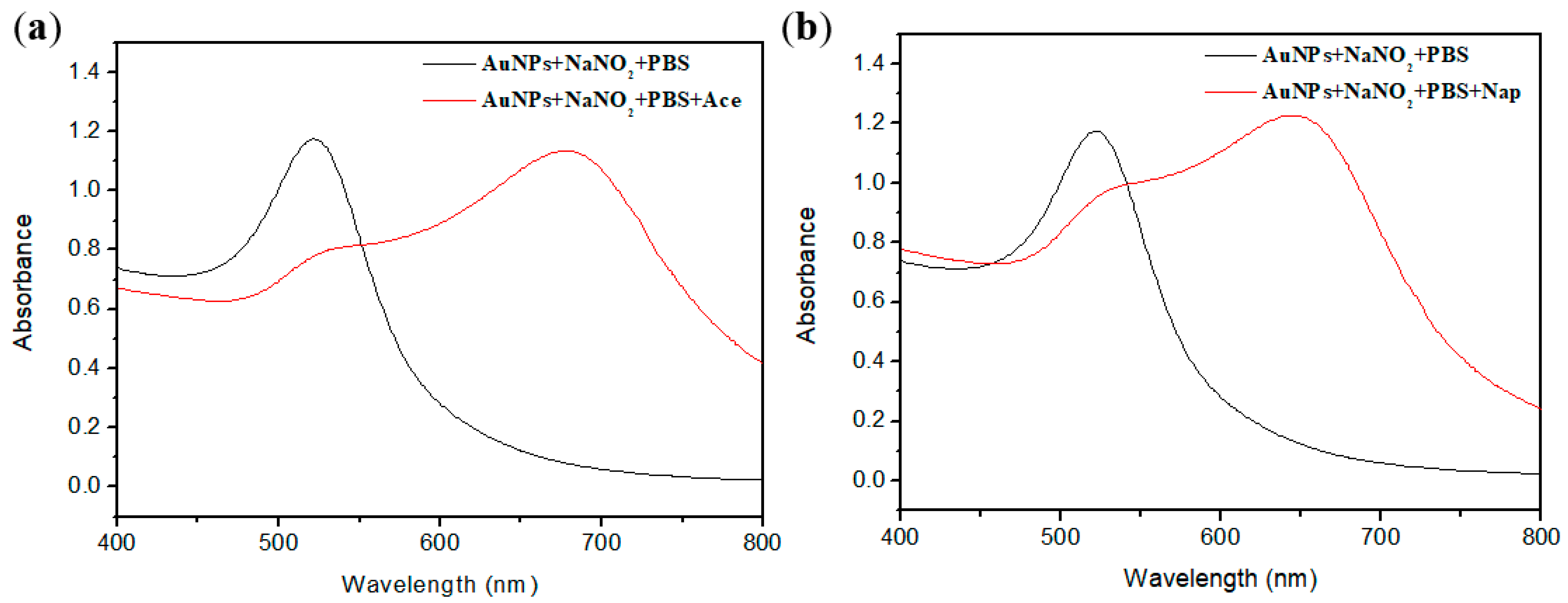
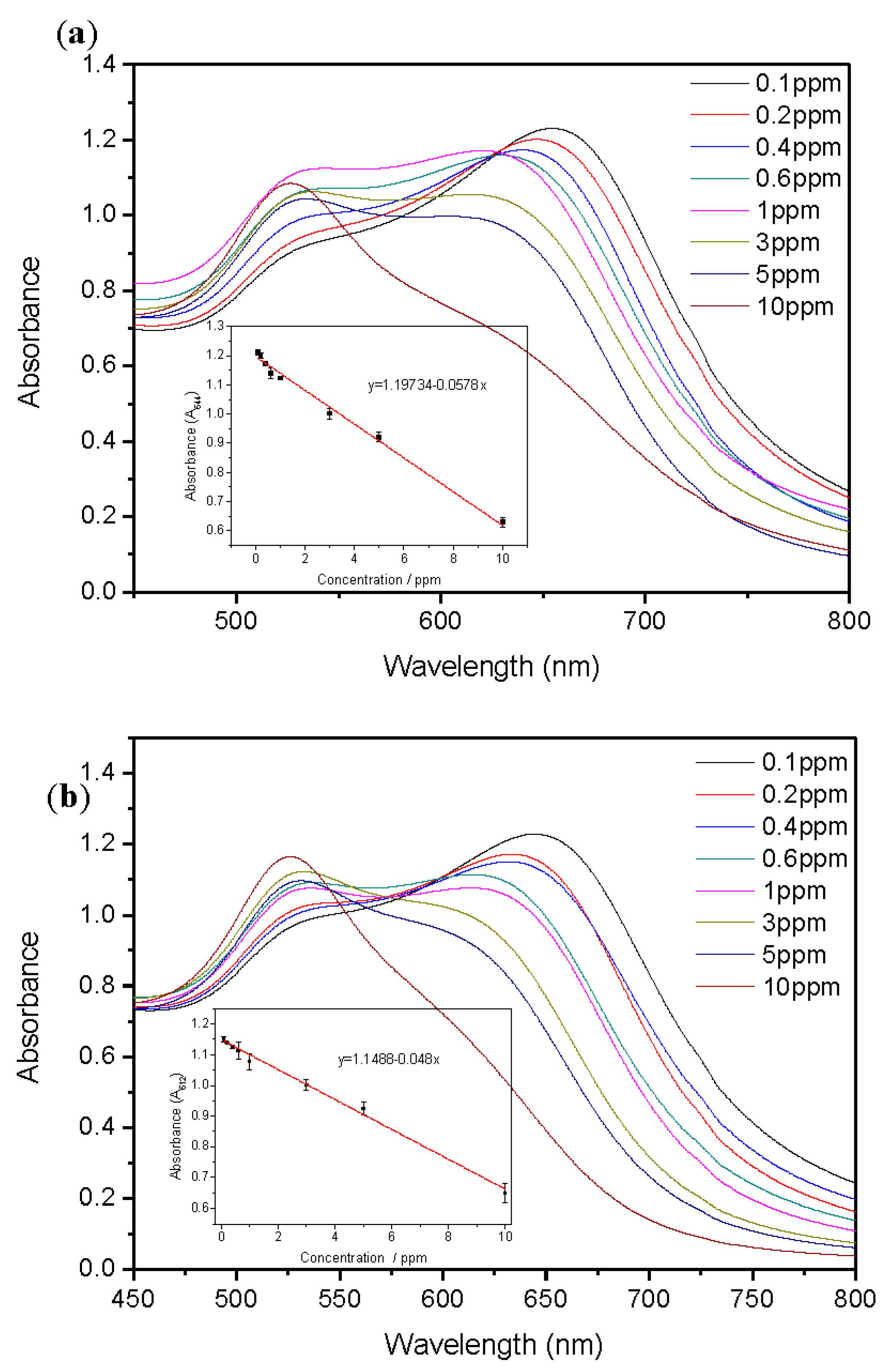
2.5. Sensitivity of Functionalized AuNPs
2.6. Sensitivity of Functionalized AuNPs
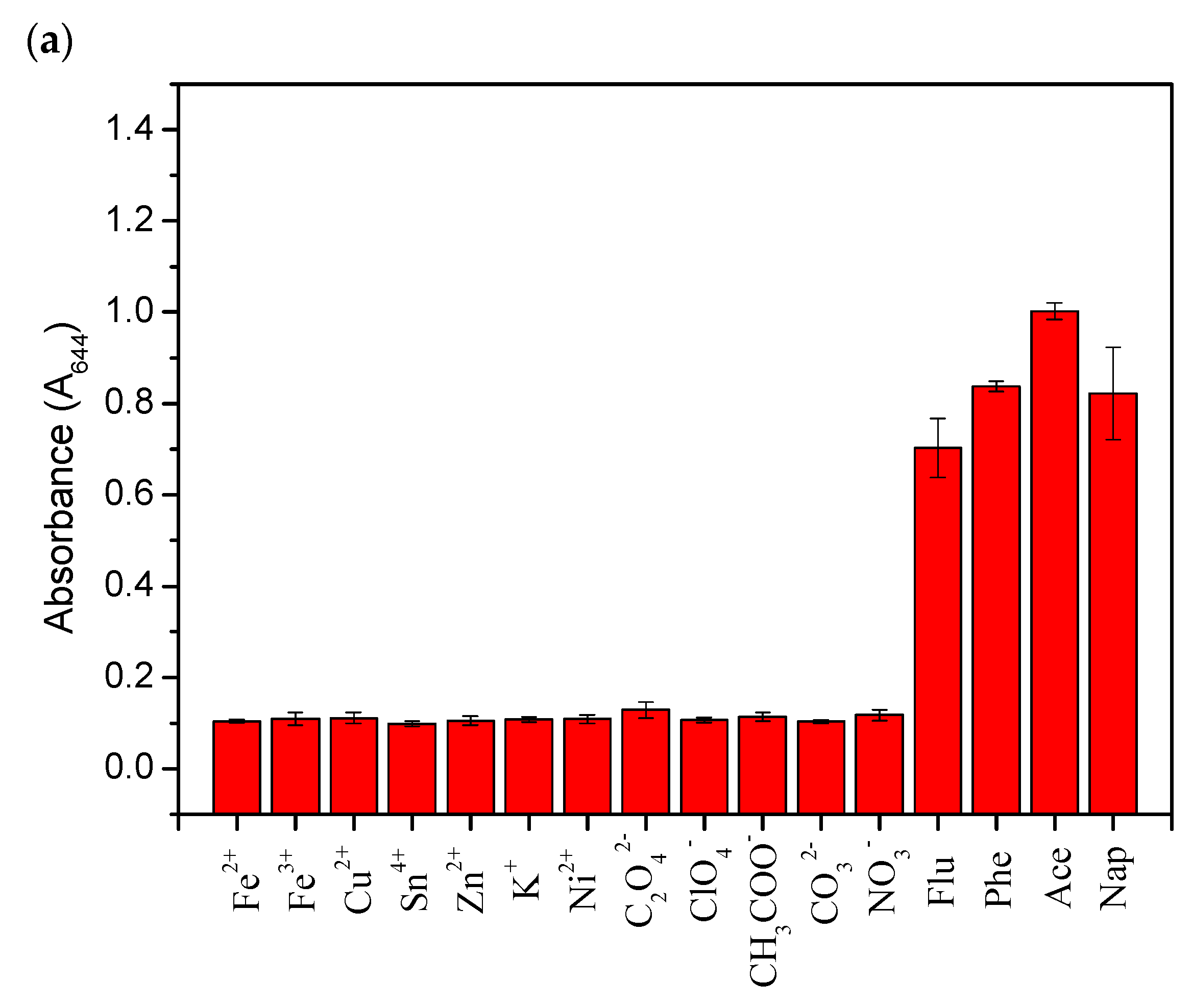
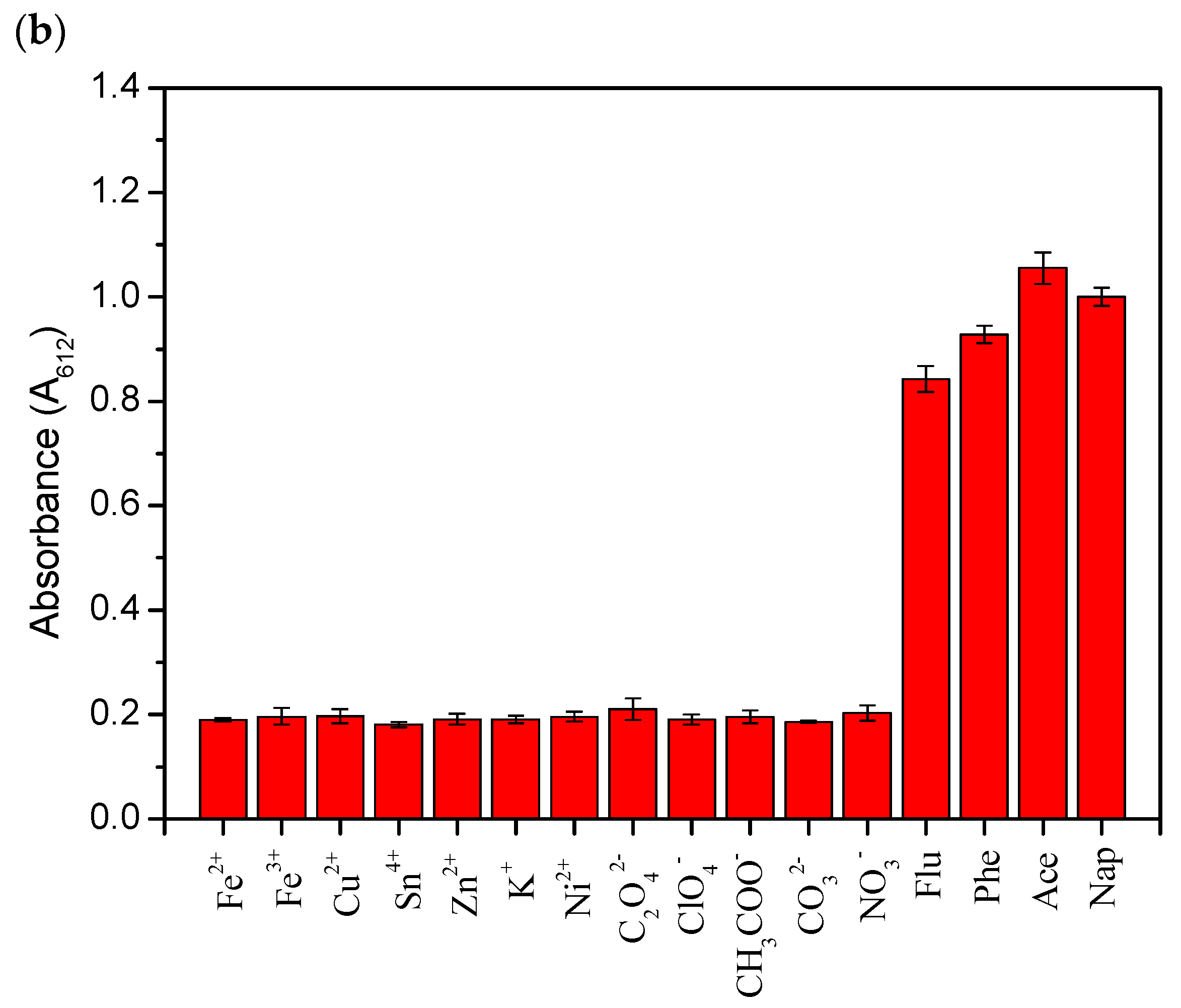
2.7. Detection of Ace and Nap in real water
3. Methods and Materials
3.1. Materials
3.2. Synthesis of functionalized AuNPs
- (1)
- Amount of sodium nitrite: different volumes of sodium nitrite solution (0.1 M) were added to the AuNP solution (100 µL of concentrated AuNP solution + 400 µL of DI water) and mixed well to test the absorbance change with or without the analyte.
- (2)
- Amount of buffer solution: after the addition of sodium nitrite solution was determined, the addition of PBS buffer solution could be optimized.
- (3)
- Detection time: the analyte (400 µL) was added to the mixed solution of concentrated AuNP (100 µL) and the optimal contents of sodium nitrite solution and PBS buffer solution. After the mixture solution was mixed well, the absorbance was recorded using a UV-vis spectrophotometer (Thermo Scientific, Braunschweig, Germany) at different detection times.
3.3. Analyte Detection Based on Functionalized AuNPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAHs | polycyclic aromatic hydrocarbons |
| AuNPs | gold nanoparticles |
| Nap | naphthalene |
| Ace | acenaphthene |
| LOD | limit of detection |
| EU | European Union |
| SCF | Scientific Committee on Food |
| GC/MS | gas chromatography/mass spectrometry |
| HPLC | high-performance liquid chromatography |
| LSPR | localized surface plasmon resonance |
| PBS | phosphate-buffered saline |
| IUPAC | International Union of Pure and Applied Chemistry |
| RSD | relative standard deviation |
References
- Souza, K.F.; Carvalho, L.R.; Allen, A.G.; Cardoso, A.A. Diurnal and nocturnal measurements of PAH, nitro-PAH, and oxy-PAH compounds in atmospheric particulate matter of a sugar cane burning region. Atmospheric Environ. 2014, 83, 193–201. [Google Scholar] [CrossRef]
- Idowu, O.; Carbery, M.; O’Connor, W.; Thavamani, P. Speciation and source apportionment of polycyclic aromatic compounds (PACs) in sediments of the largest salt water lake of Australia. Chemosphere 2019, 246, 125779. [Google Scholar] [CrossRef] [PubMed]
- Hasan, G.M.M.A.; Shaikh, A.A.; Satter, M.A.; Hossain, S. Detection of indicator polychlorinated biphenyls (I-PCBs) and polycyclic aromatic hydrocarbons (PAHs) in cow milk from selected areas of Dhaka, Bangladesh and potential human health risks assessment. Toxicol. Rep. 2022, 9, 1514–1522. [Google Scholar] [CrossRef]
- Imam, A.; Suman, S.K.; Kanaujia, P.K.; Ray, A. Biological machinery for polycyclic aromatic hydrocarbons degradation: A review. Bioresour. Technol. 2022, 343, 126121. [Google Scholar] [CrossRef]
- Gaur, N.; Narasimhulu, K.; Y, P. Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment. J. Clean. Prod. 2018, 198, 1602–1631. [Google Scholar] [CrossRef]
- Li, R.; Zhang, J.; Krebs, P. Global trade drives transboundary transfer of the health impacts of polycyclic aromatic hydrocarbon emissions. Commun. Earth Environ. 2022, 3, 170. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Zhang, Y. Analytical chemistry, formation, mitigation, and risk assessment of polycyclic aromatic hydrocarbons: From food processing to in vivo metabolic transformation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1422–1456. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU). Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards polycyclic aromatic hydrocarbons. Off. J. Eur. Union 2013, L328, 69–71. [Google Scholar]
- Product Safety Commission (AfPS). Testing and Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in the Awarding of GS Marks-Specification Pursuant to Article 21(1) no. 3 of the Product Safety Act (ProdSG), AfPS GS 2019:01 PAK; Federal Institute for Occupational Safety and Health (BAuA): Dortmund, Germany, 2020. [Google Scholar]
- Sommerfeld, T.; Jung, C.; Riedel, J.; Mauch, T.; Sauer, A.; Koch, M. Development of a certified reference material for the determination of polycyclic aromatic hydrocarbons (PAHs) in rubber toy. Anal. Bioanal. Chem. 2022, 414, 4369–4378. [Google Scholar] [CrossRef]
- Gu, Y.-G.; Li, H.-B.; Lu, H.-B. Polycyclic aromatic hydrocarbons (PAHs) in surface sediments from the largest deep plateau lake in China: Occurrence, sources and biological risk. Ecol. Eng. 2017, 101, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Ingenbleek, L.; Veyrand, B.; Adegboye, A.; Hossou, S.E.; Koné, A.Z.; Oyedele, A.D.; Kisito, C.S.K.J.; Dembélé, Y.K.; Eyangoh, S.; Verger, P.; et al. Polycyclic aromatic hydrocarbons in foods from the first regional total diet study in Sub-Saharan Africa: Contamination profile and occurrence data. Food Control 2019, 103, 133–144. [Google Scholar] [CrossRef]
- Drventić, I.; Šala, M.; Vidović, K.; Kroflič, A. Direct quantification of PAHs and nitro-PAHs in atmospheric PM by thermal desorption gas chromatography with electron ionization mass spectroscopic detection. Talanta 2023, 251, 123761. [Google Scholar] [CrossRef] [PubMed]
- Qazi, F.; Shahsavari, E.; Prawer, S.; Ball, A.S.; Tomljenovic-Hanic, S. Detection and identification of polyaromatic hydrocarbons (PAHs) contamination in soil using intrinsic fluorescence. Environ. Pollut. 2021, 272, 116010. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, T.; Zhao, W.; Cui, S.; Guo, R.; Li, D.; Kadasala, N.R.; Han, D.; Jiang, Y.; Liu, Y.; et al. Multifunctional magnetic Fe3O4/Cu2O-Ag nanocomposites with high sensitivity for SERS detection and efficient visible light-driven photocatalytic degradation of polycyclic aromatic hydrocarbons (PAHs). J. Colloid Interface Sci. 2022, 628, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhu, Q.; Wang, Y. Colorimetric detection of Gadidae species using probe-modified gold nanoparticles. Food Control 2020, 116, 107287. [Google Scholar] [CrossRef]
- Guo, R.-H.; Shu, C.-C.; Chuang, K.-J.; Hong, G.-B. Rapid colorimetric detection of phthalates using DNA-modified gold nanoparticles. Mater. Lett. 2021, 293, 129756. [Google Scholar] [CrossRef]
- Nguyen, D.K.; Jang, C.-H. Ultrasensitive colorimetric detection of amoxicillin based on Tris-HCl-induced aggregation of gold nanoparticles. Anal. Biochem. 2022, 645, 114634. [Google Scholar] [CrossRef]
- Hou, H.; Wang, P.; Zhang, J.; Li, C.; Jin, Y. Graphene Oxide-Supported Ag Nanoplates as LSPR Tunable and Reproducible Substrates for SERS Applications with Optimized Sensitivity. ACS Appl. Mater. Interfaces 2015, 7, 18038–18045. [Google Scholar] [CrossRef]
- Sang, Y.; Chen, X.; Zhang, L.; Li, D.; Xu, H. Electrospun polymeric nanofiber decorated with sea urchin-like gold nanoparticles as an efficient and stable SERS platform. J. Colloid Interface Sci. 2021, 590, 125–133. [Google Scholar] [CrossRef]
- Ma, C.-M.; Lin, L.-C.; Chuang, K.-J.; Hong, G.-B. Colorimetric detection of polycyclic aromatic hydrocarbons by using gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 268, 120701. [Google Scholar] [CrossRef]
- He, J.; Wang, W.; Zhang, H.; Ju, Y.; Yu, K.; Zhang, X.; Jiang, J. Nebulization dielectric barrier discharge ionization mass spectrometry: Rapid and sensitive analysis of acenaphthene. Talanta 2021, 222, 121681. [Google Scholar] [CrossRef] [PubMed]
- Zuazagoitia, D.; Millán, E.; García, R.; Zuazagoitia, D. A Screening Method for Polycyclic Aromatic Hydrocarbons Determination in Water by Headspace SPME with GC-FID. Chromatographia 2007, 66, 773–777. [Google Scholar] [CrossRef]
- Oliferova, L.; Statkus, M.; Tsysin, G.; Shpigun, O.; Zolotov, Y. On-line solid-phase extraction and HPLC determination of polycyclic aromatic hydrocarbons in water using fluorocarbon polymer sorbents. Anal. Chim. Acta 2005, 538, 35–40. [Google Scholar] [CrossRef]
- Rehwagen, M.; Müller, A.; Massolo, L.; Herbarth, O.; Ronco, A. Polycyclic aromatic hydrocarbons associated with particles in ambient air from urban and industrial areas. Sci. Total. Environ. 2005, 348, 199–210. [Google Scholar] [CrossRef]
- Valdman, E.; Battaglini, F.; Leite, S. Naphthalene detection by a bioluminescence sensor applied to wastewater samples. Sensors Actuators B Chem. 2004, 103, 7–12. [Google Scholar] [CrossRef]
- Shi, X.; Liu, S.; Han, X.; Ma, J.; Jiang, Y.; Yu, G. High-Sensitivity Surface-Enhanced Raman Scattering (SERS) Substrate Based on a Gold Colloid Solution with a pH Change for Detection of Trace-Level Polycyclic Aromatic Hydrocarbons in Aqueous Solution. Appl. Spectrosc. 2015, 69, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhuang, H.; Zhou, C. Detection of naphthalene by real-time immuno-PCR using molecular beacon. Mol. Cell. Probes 2009, 23, 29–34. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Hong, G.-B.; Hsu, J.-P.; Chuang, K.-J.; Ma, C.-M. Colorimetric Detection of 1-Naphthol and Glyphosate Using Modified Gold Nanoparticles. Sustainability 2022, 14, 10793. [Google Scholar] [CrossRef]


| Analytes | Methods | Linear Range (ppb) | LOD (ppb) | Detection Time | Detection Cost | Ref. |
|---|---|---|---|---|---|---|
| Ace | MS | 2.05–100 | 0.61 | >7 min | High | [22] |
| GC | 10–1000 | 0.2 | <10 min | High | [23] | |
| SPE-HPLC | 0.5–10 | 0.008 | <17 min | High | [24] | |
| HPLC | 1000–7000 | 880 | 15 min | High | [25] | |
| Colorimetric | 100–10,000 | 46 | Quick | Low | This study | |
| Nap | Bioluminescence | 50–500 | - | 70 min | Medium | [26] |
| SERS | 1.28–12.8 | 0.871 | <15 h | High | [27] | |
| SPE-HPLC | 0.5–10 | 0.04 | <17 min | High | [24] | |
| HPLC | 1000–7000 | 1320 | 15 min | High | [25] | |
| Immuno-PCR | 1–10,000 | - | Quick | Low | [28] | |
| Colorimetric | 100–10,000 | 1.5 | Quick | Low | This study |
| Samples | Analytes | Added (ppm) | Found (ppm) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Tap water | Ace | 3 | 2.97 ± 0.14 | 98.9 | ±0.81 |
| Mineral water | 3 | 2.95 ± 0.18 | 98.4 | ±0.98 | |
| Tap water | Nap | 3 | 3.09 ± 0.19 | 103.0 | ±0.82 |
| Mineral water | 3 | 3.01 ± 0.15 | 100.4 | ±0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, K.-J.; Dong, M.-R.; Laishram, P.; Hong, G.-B. Colorimetric Detection of Acenaphthene and Naphthalene Using Functionalized Gold Nanoparticles. Int. J. Mol. Sci. 2023, 24, 6635. https://doi.org/10.3390/ijms24076635
Chuang K-J, Dong M-R, Laishram P, Hong G-B. Colorimetric Detection of Acenaphthene and Naphthalene Using Functionalized Gold Nanoparticles. International Journal of Molecular Sciences. 2023; 24(7):6635. https://doi.org/10.3390/ijms24076635
Chicago/Turabian StyleChuang, Kai-Jen, Meng-Ru Dong, Purnima Laishram, and Gui-Bing Hong. 2023. "Colorimetric Detection of Acenaphthene and Naphthalene Using Functionalized Gold Nanoparticles" International Journal of Molecular Sciences 24, no. 7: 6635. https://doi.org/10.3390/ijms24076635






