Molecular Advances in Sinusoidal Obstruction Syndrome/Veno-Occlusive Disease
Abstract
:1. Introduction
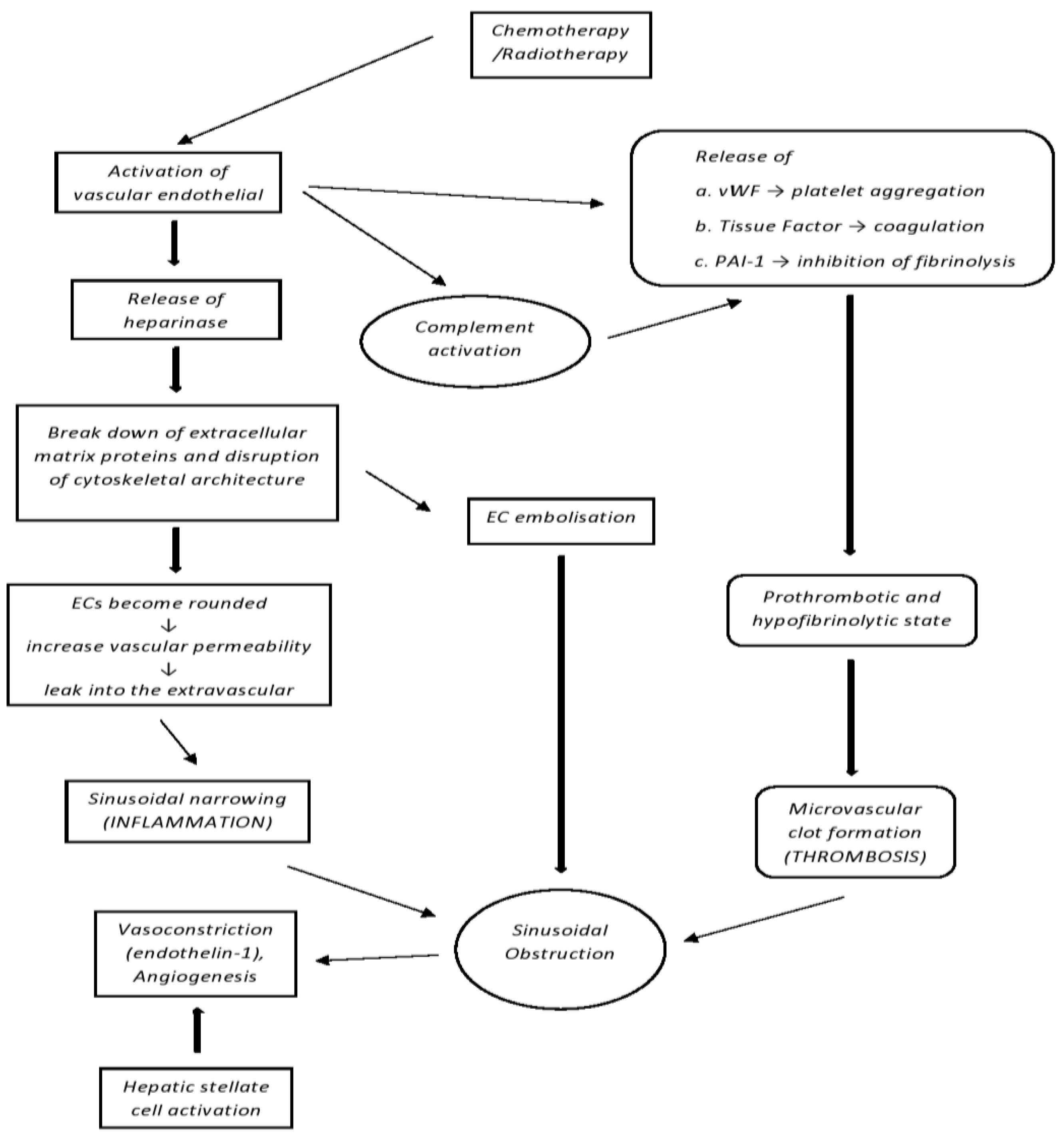
2. Pathophysiology
3. Clinical Presentation, Diagnostic Criteria and Risk Stratification
4. Risk Factors
5. Prophylaxis
5.1. Prostaglandin E1
5.2. Pentoxifylline, Antithrombin
5.3. Ursodeoxycholic Acid (UDCA)
5.4. Defibrotide
6. SOS/VOD Management
6.1. Defibrotide
6.2. Tissue Plasminogen Activator (TPA)
6.3. n-Acetyl-l-cysteine (NAC)
6.4. Recombinant Human Soluble Thrombomodulin Alpha (rhTM)
7. Complement Activation in SOS/VOD
Advances in Other Endothelial Injury Syndromes
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SOS/VOD | Sinusoidal Obstruction Syndrome/Veno-Occlusive Disease |
| HSCT | Hematopoietic Stem Cell Transplantation |
| MOD | Multi-Organ Dysfunction |
| HCT | Hematopoietic Cell Transplantation |
| EBMT | European Society for Blood and Marrow Transplantation |
| SEC | Sinusoidal Endothelial Cell |
| AML | Acute Myeloid Leukemia |
| B-ALL | B-cell Acute Lymphoblastic Leukemia |
| ECs | Endothelial Cells |
| VWF | Von Willebrand Factor |
| PAI-I | Plasminogen Activator Inhibitor-I |
| PH | Portal Hypertension |
| DILI | Drug-Induced Liver Injury |
| GVHD | Graft-Versus-Host Disease |
| RT | Refractory Thrombocytopenia |
| HVPG | Hepatic Vein Pressure Gradient |
| US | Ultrasound |
| CIBMTR | Center for International Blood and Marrow Transplant Research |
| EASIX | Endothelial Activation and Stress Index |
| LDH | Lactate Dehydrogenase |
| AlloSCT | Allogeneic Stem Cell Transplantation |
| GM | Gut Microbiome |
| BSI | Bloodstream Infection |
| LSM | Liver Stiffness Measurement |
| TE | Transient Elastography |
| MAC | Myeloablative Conditioning |
| GO | Gemtuzumab Ozogamicin |
| FDA | Food and Drug Administration |
| IO | Inotuzumab Ozogamicin |
| UFH | Unfractionated Heparin |
| LMWH | Low Molecular Weight Heparin |
| UDCA | Ursodeoxycholic Acid |
| BCSH | British Committee for Standards in Haematology |
| BSBMT | British Society for Blood and Marrow Transplantation |
| VOD | Veno-Occlusive Disease |
| CR | Complete Remission |
| TPA | Tissue Plasminogen Activator |
| NAC | n-Acetyl-l-Cysteine |
| RhTM | Recombinant human soluble Thrombomoduline alpha |
| HELLP | Hemolysis, Elevated Liver enzymes, and Low Platelet number syndrome |
| TA-TMA | Transplant-Associated Thrombotic Microangiopathy |
| ADAMTS13 | A Disintegrin and Metalloproteinase with Thrombospondin motifs |
| C1-INH-C | C1 Esterase Inhibitor |
| CFH | Complement Factor H |
| CFI | Complement Factor I |
| HLH | Hemophagocytic Lymphohistiocytosis |
| HUS | Hemolytic Uremic Syndrome |
| MASP-2 | Mannan-binding lectin-associated Serine Protease-2 |
References
- Mohty, M.; Malard, F.; Abecassis, M.M.; Aerts, E.; Alaskar, A.S.; Aljurf, M.; Arat, M.; Bader, P.; Baron, F.; Bazarbachi, A.; et al. Sinusoidal obstruction syndrome/veno-occlusive disease: Current situation and perspectives—A position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2015, 50, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.; Miller, J.L.; Uys, C.J.; Dietrich, B.E. Fatal veno-occlusive disease of the liver after chemotherapy, whole-body irradiation and bone marrow transplantation for refractory acute leukaemia. S. Afr. Med. J. 1979, 55, 5–10. [Google Scholar] [PubMed]
- Carreras, E.; Diaz-Ricart, M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant. 2011, 46, 1495–1502. [Google Scholar] [CrossRef] [Green Version]
- Mohty, M.; Malard, F.; Abecassis, M.; Aerts, E.; Alaskar, A.S.; Aljurf, M.; Arat, M.; Bader, P.; Baron, F.; Bazarbachi, A.; et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: A new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016, 51, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Kernan, N.A.; Grupp, S.; Smith, A.R.; Arai, S.; Triplett, B.; Antin, J.H.; Lehmann, L.; Shore, T.; Ho, V.T.; Bunin, N.; et al. Final results from a defibrotide treatment-IND study for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Br. J. Haematol. 2018, 181, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Coppell, J.A.; Richardson, P.G.; Soiffer, R.; Martin, P.L.; Kernan, N.A.; Chen, A.; Guinan, E.; Vogelsang, G.; Krishnan, A.; Giralt, S.; et al. Hepatic Veno-Occlusive Disease following Stem Cell Transplantation: Incidence, Clinical Course, and Outcome. Biol. Blood Marrow Transplant. 2010, 16, 157–168. Available online: https://www.sciencedirect.com/science/art-cle/pii/S1083879109004182 (accessed on 10 December 2022). [CrossRef] [Green Version]
- Haeger, M.; Unander, M.; Bengtsson, A. Enhanced anaphylatoxin and terminal C5b-9 complement complex formation in patients with the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet. Gynecol. 1990, 76, 698–702. [Google Scholar]
- Burwick, R.M.; Fichorova, R.N.; Dawood, H.Y.; Yamamoto, H.S.; Feinberg, B.B. Urinary excretion of C5b-9 in severe preeclampsia: Tipping the balance of complement activation in pregnancy. Hypertension 2013, 62, 1040–1045. [Google Scholar] [CrossRef] [Green Version]
- Vaught, A.J.; Gavriilaki, E.; Hueppchen, N.; Blakemore, K.; Yuan, X.; Seifert, S.M.; York, S.; Brodsky, R.A. Direct evidence of complement activation in HELLP syndrome: A link to atypical hemolytic uremic syndrome. Exp. Hematol. 2016, 44, 390–398. [Google Scholar] [CrossRef] [Green Version]
- Salmon, J.E.; Heuser, C.; Triebwasser, M.; Liszewski, M.K.; Kavanagh, D.; Roumenina, L.; Branch, D.W.; Goodship, T.; Fremeaux-Bacchi, V.; Atkinson, J.P. Mutations in Complement Regulatory Proteins Predispose to Preeclampsia: A Genetic Analysis of the PROMISSE Cohort. PLoS Med. 2011, 8, e1001013. [Google Scholar] [CrossRef] [Green Version]
- Vaught, A.J.; Braunstein, E.M.; Jasem, J.; Yuan, X.; Makhlin, I.; Eloundou, S.; Baines, A.C.; Merrill, S.A.; Chaturvedi, S.; Blakemore, K.; et al. Germline mutations in the alternative pathway of complement predispose to HELLP syndrome. JCI Insight 2018, 3, e99128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masetti, R.; Biagi, E.; Zama, D.; Muratore, E.; D’Amico, F.; Leardini, D.; Turroni, S.; Prete, A.; Brigidi, P.; Pession, A. Early modifications of the gut microbiome in children with hepatic sinusoidal obstruction syndrome after hematopoietic stem cell transplantation. Sci. Rep. 2021, 11, 14307. [Google Scholar] [CrossRef]
- Cooke, K.R.; Jannin, A.; Ho, V. The Contribution of Endothelial Activation and Injury to End-Organ Toxicity following Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2008, 14, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salat, C.; Holler, E.; Kolb, H.-J.; Reinhardt, B.; Pihusch, R.; Wilmanns, W.; Hiller, E. Plasminogen Activator Inhibitor-1 Confirms the Diagnosis of Hepatic Veno-Occlusive Disease in Patients With Hyperbilirubinemia After Bone Marrow Transplantation. Blood 1997, 89, 2184–2188. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Malard, F.; Abecasis, M.; Aerts, E.; Alaskar, A.S.; Aljurf, M.; Arat, M.; Bader, P.; Baron, F.; Basak, G.; et al. Prophylactic, preemptive, and curative treatment for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: A position statement from an international expert group. Bone Marrow Transplant. 2019, 55, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.J.; Lee, K.S.K.; Beschorner, W.E.; Vogel, V.G.; Grochow, L.B.; Braine, H.G.; Vogelsang, G.B.; Sensenbrenner, L.L.; Santos, G.W.; Saral, R. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 1987, 44, 778–783. [Google Scholar] [CrossRef]
- Mcdonald, G.B.; Sharma, P.; Matthews, D.E.; Shulman, H.M.; Thomas, E.D. Venocclusive Disease of the Liver after Bone Marrow Transplantation: Diagnosis, Incidence, and Predisposing Factors. Hepatology 1984, 4, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Corbacioglu, S.; Carreras, E.; Ansari, M.; Balduzzi, A.; Cesaro, S.; Dalle, J.-H.; Dignan, F.; Gibson, B.; Guengoer, T.; Gruhn, B.; et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: A new classification from the European society for blood and marrow transplantation. Bone Marrow Transplant. 2018, 53, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Embaby, M.; Rangarajan, H.G.; Abu-Arja, R.; Auletta, J.J.; Stanek, J.; Pai, V.; Nicol, K.K.; Bajwa, R.S. Refractory Thrombocytopenia Is a Valid Early Diagnostic Criteria for Hepatic Veno-Occlusive Disease in Children. Biol. Blood Marrow Transplant. 2020, 26, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Dignan, F.L.; Wynn, R.F.; Hadzic, N.; Karani, J.; Quaglia, A.; Pagliuca, A.; Veys, P.; Potter, M.N. BCSH/BSBMT guideline: Diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br. J. Haematol. 2013, 163, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, F.; Colecchia, A.; Alemanni, L.V.; Vestito, A.; Dajti, E.; Marasco, G.; Sessa, M.; Pession, A.; Bonifazi, F.; Festi, D. Role of imaging techniques in liver veno-occlusive disease diagnosis: Recent advances and literature review. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.; Colecchia, A.; Duarte, R.F.; Bonifazi, F.; Ravaioli, F.; Bourhis, J.H. Imaging in Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome. Biol. Blood Marrow Transplant. 2020, 26, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Strouse, C.; Zhang, Y.; Zhang, M.-J.; Digilio, A.; Pasquini, M.; Horowitz, M.M.; Lee, S.; Ho, V.; Ramanathan, M.; Chinratanalab, W.; et al. Risk Score for the Development of Veno-Occlusive Disease after Allogeneic Hematopoietic Cell Transplant. Biol. Blood Marrow Transplant. 2018, 24, 2072–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Penack, O.; Terzer, T.; Schult, D.; Majer-Lauterbach, J.; Radujkovic, A.; Blau, I.W.; Bullinger, L.; Müller-Tidow, C.; Dreger, P.; et al. Predicting sinusoidal obstruction syndrome after allogeneic stem cell transplantation with the EASIX biomarker panel. Haematologica 2021, 106, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Peled, J.U.; Gomes, A.L.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef]
- Kelly, M.S.; Ward, D.V.; Severyn, C.J.; Arshad, M.; Heston, S.M.; Jenkins, K.; Martin, P.L.; McGill, L.; Stokhuyzen, A.; Bhattarai, S.K.; et al. Gut Colonization Preceding Mucosal Barrier Injury Bloodstream Infection in Pediatric Hematopoietic Stem Cell Transplantation Recipients. Biol. Blood Marrow Transplant. 2019, 25, 2274–2280. [Google Scholar] [CrossRef]
- Biagi, E.; Zama, D.; Rampelli, S.; Turroni, S.; Brigidi, P.; Consolandi, C.; Severgnini, M.; Picotti, E.; Gasperini, P.; Merli, P.; et al. Early gut microbiota signature of aGvHD in children given allogeneic hematopoietic cell transplantation for hematological disorders. BMC Med Genom. 2019, 12, 49. [Google Scholar] [CrossRef]
- Zama, D.; Biagi, E.; Masetti, R.; Gasperini, P.; Prete, A.; Candela, M.; Brigidi, P.; Pession, A. Gut microbiota and hematopoietic stem cell transplantation: Where do we stand? Bone Marrow Transplant. 2017, 52, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Özkan, S.G.; Pata, C.; Şekuri, A.; Çınar, Y.; Özkan, H.A. Transient elastography of liver: Could it be a guide for diagnosis and management strategy in hepatic veno-occlusive disease (sinusoidal obstruction syndrome)? Transfus. Apher. Sci. 2022, 61, 103370. [Google Scholar] [CrossRef]
- Corbacioglu, S.; Jabbour, E.J.; Mohty, M. Risk Factors for Development of and Progression of Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome. Biol. Blood Marrow Transplant. 2019, 25, 1271–1280. [Google Scholar] [CrossRef] [Green Version]
- Roeker, L.; Kim, H.T.; Glotzbecker, B.; Nageshwar, P.; Nikiforow, S.; Koreth, J.; Armand, P.; Cutler, C.; Alyea, E.P.; Antin, J.H.; et al. Early Clinical Predictors of Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome after Myeloablative Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakushijin, K.; Atsuta, Y.; Doki, N.; Yokota, A.; Kanamori, H.; Miyamoto, T.; Ohwada, C.; Miyamura, K.; Nawa, Y.; Kurokawa, M.; et al. Sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation: Incidence, risk factors and outcomes. Bone Marrow Transplant. 2016, 51, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.Y.; Kim, S.-J.; Cheong, J.-W.; Kim, Y.; Jang, J.E.; Lee, J.Y.; Min, Y.H.; Yang, W.I.; Kim, J.S. High pre-transplant serum ferritin and busulfan-thiotepa conditioning regimen as risk factors for hepatic sinusoidal obstructive syndrome after autologous stem cell transplantation in patients with malignant lymphoma. Leuk. Lymphoma 2016, 57, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, I.D.; Singer, J.; Andrews, R.G.; Keating, A.; Powell, J.S.; Bjornson, B.H.; Cuttner, J.; Najfeld, V.; Reaman, G.; Raskind, W. Treatment of acute myeloid leukemia cells in vitro with a monoclonal antibody recognizing a myeloid differentiation antigen allows normal progenitor cells to be expressed. J. Clin. Investig. 1987, 79, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Norsworthy, K.J.; Ko, C.-W.; Lee, J.E.; Liu, J.; John, C.S.; Przepiorka, D.; Farrell, A.T.; Pazdur, R. FDA Approval Summary: Mylotarg for Treatment of Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia. Oncologist 2018, 23, 1103–1108. [Google Scholar] [CrossRef] [Green Version]
- US Food and Drug Administration (FDA). Mylotarg (Gemtuzumab Ozogamicin) [Prescribing Information]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761060s004lbl.pdf (accessed on 2 January 2023).
- Rajvanshi, P.; Shulman, H.M.; Sievers, E.; McDonald, G.B. Hepatic sinusoidal obstruction after gemtuzumab ozogamicin (Mylotarg) therapy. Blood 2002, 99, 2310–2314. [Google Scholar] [CrossRef]
- Castaigne, S.; Pautas, C.; Terré, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.-N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 2012, 379, 1508–1516, Erratum in Lancet 2018, 391, 838. [Google Scholar] [CrossRef] [PubMed]
- Battipaglia, G.; Labopin, M.; Candoni, A.; Fanin, R.; El Cheikh, J.; Blaise, D.; Michallet, M.; Ruggeri, A.; Contentin, N.; Ribera, J.-M.; et al. Risk of sinusoidal obstruction syndrome in allogeneic stem cell transplantation after prior gemtuzumab ozogamicin treatment: A retrospective study from the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2017, 52, 592–599. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; DeAngelo, D.J.; Advani, A.S.; Stelljes, M.; Kebriaei, P.; Cassaday, R.D.; Merchant, A.A.; Fujishima, N.; Uchida, T.; Calbacho, M.; et al. Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: Results from the open-label, randomised, phase 3 INO-VATE study. Lancet Haematol. 2017, 4, e387–e398. [Google Scholar] [CrossRef]
- Batsis, I.; Yannaki, E.; Kaloyannidis, P.; Sakellari, I.; Smias, C.; Georgoulis, I.; Fassas, A.; Anagnostopoulos, A. Veno-occlusive disease prophylaxis with fresh frozen plasma and heparin in bone marrow transplantation. Thromb. Res. 2006, 118, 611–618. [Google Scholar] [CrossRef]
- Imran, H.; Tleyjeh, I.; Zirakzadeh, A.; Rodriguez, V.; Khan, S.P. Use of prophylactic anticoagulation and the risk of hepatic veno-occlusive disease in patients undergoing hematopoietic stem cell transplantation: A systematic review and meta-analysis. Bone Marrow Transplant. 2006, 37, 677–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bearman, S.I.; Shen, D.D.; Hinds, M.S.; Hill, H.A.; McDonald, G.B. A phase I/II study of prostaglandin E1 for the prevention of hepatic venocclusive disease after bone marrow transplantation. Br. J. Haematol. 1993, 84, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Bordigoni, P.; Witz, F.; Von Bueltzingsloewen, A.; Schmitt, C.; Sommelet, D. Prostaglandin E1 (PGE1) induced arthritis following bone marrow transplantation. Br. J. Haematol. 1991, 78, 138–139. [Google Scholar] [CrossRef]
- Attal, M.; Huguet, F.; Rubie, H.; Charlet, J.P.; Schlaifer, D.; Huynh, A.; Laurent, G.; Pris, J. Prevention of regimen-related toxicities after bone marrow transplantation by pentoxifylline: A prospective, randomized trial. Blood 1993, 82, 732–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haussmann, U.; Fischer, J.; Eber, S.; Scherer, F.; Seger, R.; Güngör, T. Hepatic veno-occlusive disease in pediatric stem cell transplantation: Impact of pre-emptive antithrombin III replacement and combined antithrombin III/defibrotide therapy. Haematologica 2006, 91, 795–800. [Google Scholar]
- Tay, J.; Tinmouth, A.; Fergusson, D.; Huebsch, L.; Allan, D.S. Systematic Review of Controlled Clinical Trials on the Use of Ursodeoxycholic Acid for the Prevention of Hepatic Veno-occlusive Disease in Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2007, 13, 206–217. [Google Scholar] [CrossRef] [Green Version]
- Defitelio (Defibrotide Sodium) [Prescribing Information]; Jazz Pharmaceuticals: Palo Alto, CA, USA, 2016.
- Corbacioglu, S.; Cesaro, S.; Faraci, M.; Valteau-Couanet, D.; Gruhn, B.; Rovelli, A.; Boelens, J.J.; Hewitt, A.; Schrum, J.; Schulz, A.S.; et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: An open-label, phase 3, randomised controlled trial. Lancet 2012, 379, 1301–1309. [Google Scholar] [CrossRef]
- Richardson, P.G.; Triplett, B.; Ho, V.T.; Chao, N.; Dignan, F.L.; Maglio, M.; Mohty, M. Defibrotide sodium for the treatment of hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Expert Rev. Clin. Pharmacol. 2018, 11, 113–124. [Google Scholar] [CrossRef]
- Pescador, R.; Capuzzi, L.; Mantovani, M.; Fulgenzi, A.; Ferrero, M. Defibrotide: Properties and clinical use of an old/new drug. Vasc. Pharmacol. 2013, 59, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Eissner, G.; Multhoff, G.; Gerbitz, A.; Kirchner, S.; Bauer, S.; Haffner, S.; Sondermann, D.; Andreesen, R.; Holler, E. Fludarabine induces apoptosis, activation, and allogenicity in human endothelial and epithelial cells: Protective effect of defibrotide. Blood 2002, 100, 334–340. [Google Scholar] [CrossRef] [Green Version]
- Richardson, P.G.; Riches, M.L.; Kernan, N.A.; Brochstein, J.A.; Mineishi, S.; Termuhlen, A.M.; Arai, S.; Grupp, S.A.; Guinan, E.C.; Martin, P.L.; et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood 2016, 127, 1656–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbacioglu, S.; Carreras, E.; Mohty, M.; Pagliuca, A.; Boelens, J.J.; Damaj, G.; Iacobelli, M.; Niederwieser, D.; Olavarría, E.; Suarez, F.; et al. Defibrotide for the Treatment of Hepatic Veno-Occlusive Disease: Final Results From the International Compassionate-Use Program. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2016, 22, 1874–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, P.; Aggarwal, S.; Topaloglu, O.; Villa, K.F.; Corbacioglu, S. Systematic review of defibrotide studies in the treatment of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS). Bone Marrow Transplant. 2019, 54, 1951–1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strouse, C.; Richardson, P.; Prentice, G.; Korman, S.; Hume, R.; Nejadnik, B.; Horowitz, M.M.; Saber, W. Defibrotide for Treatment of Severe Veno-Occlusive Disease in Pediatrics and Adults: An Exploratory Analysis Using Data from the Center for International Blood and Marrow Transplant Research. Biol. Blood Marrow Transplant. 2016, 22, 1306–1312. [Google Scholar] [CrossRef] [Green Version]
- Schriber, J.; Milk, B.; Shaw, D.; Christiansen, N.; Baer, M.; Slack, J.; Tezcan, H.; Wetzler, M.; Herzig, G. Tissue plasminogen activator (tPA) as therapy for hepatotoxicity following bone marrow transplantation. Bone Marrow Transplant. 1999, 24, 1311–1314. [Google Scholar] [CrossRef] [Green Version]
- Bearman, S.I.; Lee, J.L.; Baroón, A.E.; McDonald, G.B. Treatment of hepatic venocclusive disease with recombinant human tissue plasminogen activator and heparin in 42 marrow transplant patients. Blood J. Am. Soc. Hematol. 1997, 89, 1501–1506. [Google Scholar]
- Yoon, J.-H.; Min, W.-S.; Kim, H.-J.; Kim, J.-H.; Shin, S.-H.; Yahng, S.-A.; Lee, S.-E.; Cho, B.-S.; Eom, K.-S.; Kim, Y.-J.; et al. Experiences of t-PA use in moderate-to-severe hepatic veno-occlusive disease after hematopoietic SCT: Is it still reasonable to use t-PA? Bone Marrow Transplant. 2013, 48, 1562–1568. [Google Scholar] [CrossRef]
- Barkholt, L.; Remberger, M.; Hassan, Z.; Fransson, K.; Omazic, B.; Svahn, B.-M.; Karlsson, H.; Brune, M.; Hassan, M.; Mattsson, J.; et al. A prospective randomized study using N-acetyl-L-cysteine for early liver toxicity after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008, 41, 785–790. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.C.-W.; Aung, L. Treatment of hepatic veno-occlusive disease in children with N -acetylcysteine. Pediatr. Blood Cancer 2019, 66, e27518. [Google Scholar] [CrossRef]
- Takagi, S.; Yuasa, M.; Uchida, N.; Yamaguchi, K.; Kageyama, K.; Kaji, D.; Taya, Y.; Nishida, A.; Ishiwata, K.; Yamamoto, H.; et al. Possible Therapeutic Potential of Recombinant Human Soluble Thrombomoduline Alpha for the Treatment of SOS/VOD: A Retrospective Study in Toranomon Hospital. Blood 2016, 128, 5747. [Google Scholar] [CrossRef]
- Qi, J.; Wang, J.; Chen, J.; Su, J.; Tang, Y.; Wu, X.; Ma, X.; Chen, F.; Ruan, C.; Zheng, X.L.; et al. Plasma levels of complement activation fragments C3b and sC5b-9 significantly increased in patients with thrombotic microangiopathy after allogeneic stem cell transplantation. Ann. Hematol. 2017, 96, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Heying, R.; Nürnberger, W.; Spiekerkötter, U.; Göbel, U. Hepatic veno-occlusive disease with severe capillary leakage after peripheral stem cell transplantation: Treatment with recombinant plasminogen activator and C1-esterase inhibitor concentrate. Bone Marrow Transplant. 1998, 21, 947–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucalossi, A.; Toraldo, F.; Tozzi, M.; Lenoci, M.; Castagnini, C.; Artuso, R.; Renieri, A.; Marotta, G. Is complement alternative pathway disregulation involved in veno-occlusive disease of the liver? Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2010, 16, 1749–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Changsirikulchai, S.; Myerson, D.; Guthrie, K.A.; McDonald, G.B.; Alpers, C.E.; Hingorani, S.R. Renal thrombotic microangiopathy after hematopoietic cell transplant: Role of GVHD in pathogenesis. Clin. J. Am. Soc. Nephrol. CJASN 2009, 4, 345–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamae, H.; Yamane, T.; Hasegawa, T.; Nakamae, M.; Terada, Y.; Hagihara, K.; Ohta, K.; Hino, M. Risk factor analysis for thrombotic microangiopathy after reduced-intensity or myeloablative allogeneic hematopoietic stem cell transplantation. Am. J. Hematol. 2006, 81, 525–531. [Google Scholar] [CrossRef]
- Willems, E.; Baron, F.; Seidel, L.; Frère, P.; Fillet, G.; Beguin, Y. Comparison of thrombotic microangiopathy after allogeneic hematopoietic cell transplantation with high-dose or nonmyeloablative conditioning. Bone Marrow Transplant. 2010, 45, 689–693. [Google Scholar] [CrossRef] [Green Version]
- Uderzo, C.; Bonanomi, S.; Busca, A.; Renoldi, M.; Ferrari, P.; Iacobelli, M.; Morreale, G.; Lanino, E.; Annaloro, C.; Della Volpe, A.; et al. Risk Factors and Severe Outcome in Thrombotic Microangiopathy After Allogeneic Hematopoietic Stem Cell Transplantation. Transplantation 2006, 82, 638–644. [Google Scholar] [CrossRef]
- Ho, V.T.; Cutler, C.; Carter, S.; Martin, P.; Adams, R.; Horowitz, M.; Ferrara, J.; Soiffer, R.; Giralt, S. Blood and Marrow Transplant Clinical Trials Network Toxicity Committee Consensus Summary: Thrombotic Microangiopathy after Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2005, 11, 571–575. [Google Scholar] [CrossRef] [Green Version]
- Jodele, S.; Davies, S.M.; Lane, A.; Khoury, J.; Dandoy, C.; Goebel, J.; Myers, K.; Grimley, M.; Bleesing, J.; El-Bietar, J.; et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: A study in children and young adults. Blood 2014, 124, 645–653. [Google Scholar] [CrossRef] [Green Version]
- Schoettler, M.; Lehmann, L.; Li, A.; Ma, C.; Duncan, C. Thrombotic Microangiopathy Following Pediatric Autologous Hematopoietic Cell Transplantation: A Report of Significant End-Organ Dysfunction in Eculizumab-Treated Survivors. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2019, 25, e163–e168. [Google Scholar] [CrossRef]
- Sakellari, I.; Barbouti, A.; I Bamichas, G.; Mallouri, D.; Kaloyannidis, P.; Fragidis, S.K.; Batsis, I.; Apostolou, C.; Karpouza, A.; Yannaki, E.; et al. GVHD-associated chronic kidney disease after allogeneic haematopoietic cell transplantation. Bone Marrow Transplant. 2013, 48, 1329–1334. [Google Scholar] [CrossRef]
- Gavriilaki, M.; Mainou, M.; Gavriilaki, E.; Haidich, A.; Papagiannopoulos, S.; Sakellari, I.; Anagnostopoulos, A.; Kimiskidis, V. Neurologic complications after allogeneic transplantation: A meta-analysis. Ann. Clin. Transl. Neurol. 2019, 6, 2037–2047. [Google Scholar] [CrossRef] [PubMed]
- Sakellari, I.; Gavriilaki, E.; Papagiannopoulos, S.; Gavriilaki, M.; Batsis, I.; Mallouri, D.; Vardi, A.; Constantinou, V.; Masmanidou, M.; Yannaki, E.; et al. Neurological adverse events post allogeneic hematopoietic cell transplantation: Major determinants of morbidity and mortality. J. Neurol. 2019, 266, 1960–1972. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, E.; Gkaliagkousi, E.; Grigoriadis, S.; Anyfanti, P.; Douma, S.; Anagnostopoulos, A. Hypertension in hematologic malignancies and hematopoietic cell transplantation: An emerging issue with the introduction of novel treatments. Blood Rev. 2019, 35, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Schoettler, M.; Carreras, E.; Cho, B.; Dandoy, C.; Ho, V.; Jodele, S.; Moissev, I.; Sanchez-Ortega, I.; Srivastava, A.; Atsuta, Y.; et al. Harmonizing Definitions for Diagnostic Criteria and Prognostic Assessment of Transplant Associated Thrombotic Microangiopathy: A Report on Behalf of the European Society for Blood and Marrow Transplantation (EBMT), American Society for Transplantation and Cellular Therapy (ASTCT), Asia-Pacific Blood and Marrow Transplantation Group (APBMT) and the Center for International Blood and Marrow Transplant Research (CIBMTR). Transplant. Cell Ther. 2023, 29, 151–163. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Anagnostopoulos, A.; Mastellos, D.C. Complement in Thrombotic Microangiopathies: Unraveling Ariadne’s Thread into the Labyrinth of Complement Therapeutics. Front. Immunol. 2019, 10, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavriilaki, E.; Brodsky, R.A. Complementopathies and precision medicine. J. Clin. Investig. 2020, 130, 2152–2163. [Google Scholar] [CrossRef] [PubMed]
- Jodele, S.; Zhang, K.; Zou, F.; Laskin, B.; Dandoy, C.; Myers, K.C.; Lane, A.; Meller, J.; Medvedovic, M.; Chen, J.; et al. The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood 2016, 127, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Rotz, S.J.; Luebbering, N.; Dixon, B.P.; Gavriilaki, E.; Brodsky, R.A.; Dandoy, C.E.; Jodele, S.; Davies, S.M. In vitro evidence of complement activation in transplantation-associated thrombotic microangiopathy. Blood Adv. 2017, 1, 1632–1634. [Google Scholar] [CrossRef] [Green Version]
- Gavriilaki, E.; Touloumenidou, T.; Sakellari, I.; Batsis, I.; Mallouri, D.; Psomopoulos, F.; Tsagiopoulou, M.; Koutra, M.; Yannaki, E.; Papalexandri, A.; et al. Pretransplant Genetic Susceptibility: Clinical Relevance in Transplant-Associated Thrombotic Microangiopathy. Thromb. Haemost. 2020, 120, 638–646. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Chrysanthopoulou, A.; Sakellari, I.; Batsis, I.; Mallouri, D.; Touloumenidou, T.; Papalexandri, A.; Mitsios, A.; Arampatzioglou, A.; Ritis, K.; et al. Linking Complement Activation, Coagulation, and Neutrophils in Transplant-Associated Thrombotic Microangiopathy. Thromb. Haemost. 2019, 119, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Jodele, S.; Medvedovic, M.; Luebbering, N.; Chen, J.; Dandoy, C.E.; Laskin, B.L.; Davies, S.M. Interferon-complement loop in transplant-associated thrombotic microangiopathy. Blood Adv. 2020, 4, 1166–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gloude, N.B.J.; Davies, S.M.; Marsh, R.A.; Jordan, M.B.; Chandra, S.; Jodele, S. Thrombotic Microangiopathy Can Occur Before Transplant in Children with HLH. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2017, 23, 233–234. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Yuan, X.; Ye, Z.; Ambinder, A.J.; Shanbhag, S.P.; Streiff, M.B.; Kickler, T.S.; Moliterno, A.R.; Sperati, C.J.; Brodsky, R.A. Modified Ham test for atypical hemolytic uremic syndrome. Blood 2015, 125, 3637–3646. [Google Scholar] [CrossRef] [Green Version]
- Osborne, A.J.; Breno, M.; Borsa, N.G.; Bu, F.; Frémeaux-Bacchi, V.; Gale, D.P.; Heuvel, L.P.V.D.; Kavanagh, D.; Noris, M.; Pinto, S.; et al. Statistical Validation of Rare Complement Variants Provides Insights into the Molecular Basis of Atypical Hemolytic Uremic Syndrome and C3 Glomerulopathy. J. Immunol. 2018, 200, 2464–2478. [Google Scholar] [CrossRef] [Green Version]
- Geerlings, M.; Volokhina, E.; De Jong, E.; Van De Kar, N.; Pauper, M.; Hoyng, C.; Heuvel, L.V.D.; Hollander, A.D. Genotype-phenotype correlations of low-frequency variants in the complement system in renal disease and age-related macular degeneration. Clin. Genet. 2018, 94, 330–338. [Google Scholar] [CrossRef]
- Legendre, C.M.; Licht, C.; Muus, P.; Greenbaum, L.A.; Babu, S.; Bedrosian, C.; Bingham, C.; Cohen, D.J.; Delmas, Y.; Douglas, K.; et al. Terminal Complement Inhibitor Eculizumab in Atypical Hemolytic–Uremic Syndrome. N. Engl. J. Med. 2013, 368, 2169–2181. [Google Scholar] [CrossRef] [Green Version]
- Rathbone, J.; Kaltenthaler, E.; Richards, A.; Tappenden, P.; Bessey, A.; Cantrell, A. A systematic review of eculizumab for atypical haemolytic uraemic syndrome (aHUS). BMJ Open 2013, 3, e003573. [Google Scholar] [CrossRef]
- Jodele, S.; Dandoy, C.E.; Danziger-Isakov, L.; Myers, K.C.; El-Bietar, J.; Nelson, A.; Wallace, G.; Teusink-Cross, A.; Davies, S.M. Terminal Complement Blockade after Hematopoietic Stem Cell Transplantation Is Safe without Meningococcal Vaccination. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2016, 22, 1337–1340. [Google Scholar] [CrossRef] [Green Version]
- Vasu, S.; Wu, H.; Satoskar, A.; Puto, M.; Roddy, J.; Blum, W.; Klisovic, R.; Andritsos, L.; Hofmeister, C.; Benson, D.M.; et al. Eculizumab therapy in adults with allogeneic hematopoietic cell transplant-associated thrombotic microangiopathy. Bone Marrow Transplant. 2016, 51, 1241–1244. [Google Scholar] [CrossRef] [Green Version]
- de Fontbrune, F.S.; Galambrun, C.; Sirvent, A.; Huynh, A.; Faguer, S.; Nguyen, S.; Bay, J.O.; Neven, B.; Moussi, J.; Simon, L.; et al. Use of Eculizumab in Patients with Allogeneic Stem Cell Transplant-Associated Thrombotic Microangiopathy: A Study From the SFGM-TC. Transplantation 2015, 99, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
- Bohl, S.R.; Kuchenbauer, F.; von Harsdorf, S.; Kloevekorn, N.; Schonsteiner, S.S.; Rouhi, A.; Schwarzwälder, P.; Döhner, H.; Bunjes, D.; Bommer, M. Thrombotic Microangiopathy after Allogeneic Stem Cell Transplantation: A Comparison of Eculizumab Therapy and Conventional Therapy. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2017, 23, 2172–2177. [Google Scholar] [CrossRef] [Green Version]
- Jodele, S.; Dandoy, C.E.; Lane, A.; Laskin, B.L.; Teusink-Cross, A.; Myers, K.C.; Wallace, G.H.; Nelson, A.; Bleesing, J.; Chima, R.S.; et al. Complement blockade for TA-TMA: Lessons learned from large pediatric cohort treated with eculizumab. Blood 2020, 135, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Rambaldi, A.K.S.; Smith, M.; Zecca, M.; Kwong, Y.L.; Claes, K.; Leung, N.; Whitaker, S. Improved survival following OMS721 treatment of hematopoieic stem cell transplant-associated thrombotic microangiopathy (HCT-TMA). Eur. Hematol. Assoc. Lib. 2018, 215162, PF724. [Google Scholar]
- Bonifazi, F.; Barbato, F.; Ravaioli, F.; Sessa, M.; DeFrancesco, I.; Arpinati, M.; Cavo, M.; Colecchia, A. Diagnosis and Treatment of VOD/SOS After Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2020, 11, 489. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Muñoz, M.E.; Forés, R.; Lario, A.; Bautista, G.; Bueno, J.L.; de Miguel, C.; Navarro, B.; De Laiglesia, A.; Sánchez-Guerrero, A.; Cabrera, J.R.; et al. Use of defibrotide to treat adult patients with transplant-associated thrombotic microangiopathy. Bone Marrow Transplant. 2019, 54, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Yeates, L.; Slatter, M.A.; Bonanomi, S.; Lim, F.L.W.I.; Ong, S.Y.; Dalissier, A.; Barberi, W.; Shulz, A.; Duval, M.; Heilmann, C.; et al. Use of defibrotide to treat transplant-associated thrombotic microangiopathy: A retrospective study of the Paediatric Diseases and Inborn Errors Working Parties of the European Society of Blood and Marrow Transplantation. Bone Marrow Transplant. 2017, 52, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Wong, K.; Lee, S.J.; Cushing-Haugen, K.L.; Flowers, M.E.; Friedman, D.L.; Leisenring, W.M.; Martin, P.J.; Mueller, B.A.; Baker, K.S. Late cardiovascular complications after hematopoietic cell transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2014, 20, 794–800. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.; Francisco, L.; Carter, A.; Sun, C.-L.; Baker, K.S.; Gurney, J.G.; McGlave, P.B.; Nademanee, A.; O’Donnell, M.; Ramsay, N.K.C.; et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: Report from the Bone Marrow Transplant Survivor Study. Blood 2007, 110, 3784–3792. [Google Scholar] [CrossRef] [Green Version]
- Sakellari, I.; Gavriilaki, E.; Batsis, I.; Mallouri, D.; Panteliadou, A.-K.; Lazaridou, A.; Vardi, A.; Constantinou, V.; Yannaki, E.; Papalexandri, A.; et al. Favorable impact of extracorporeal photopheresis in acute and chronic graft versus host disease: Prospective single-center study. J. Clin. Apher. 2018, 33, 654–660. [Google Scholar] [CrossRef]
- Sakellari, I.; Gavriilaki, E.; Kaliou, M.; Mallouri, D.; Batsis, I.; Yannaki, E.; Smias, C.; Sotiropoulos, D.; Tsorlini, E.; Anagnostopoulos, A. Candida is an emerging pathogen beyond the neutropenic period of allogeneic hematopoietic cell transplantation. Clin. Transplant. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Chatzidimitriou, D.; Gavriilaki, E.; Sakellari, I.; Diza, E. Hematopoietic cell transplantation and emerging viral infections. J. Med. Virol. 2010, 82, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Gkaliagkousi, E.; Gavriilaki, E.; Vasileiadis, I.; Nikolaidou, B.; Yiannaki, E.; Lazaridis, A.; Triantafyllou, A.; Anyfanti, P.; Markala, D.; Zarifis, I.; et al. Endothelial Microvesicles Circulating in Peripheral and Coronary Circulation Are Associated With Central Blood Pressure in Coronary Artery Disease. Am. J. Hypertens. 2019, 32, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Ishii, K.; Inami, N.; Kimura, Y.; Uoshima, N.; Ishida, H.; Yoshihara, T.; Urase, F.; Maeda, Y.; Hayashi, K. Evaluation of angiopoietins and cell-derived microparticles after stem cell transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2008, 14, 766–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pihusch, V.; Rank, A.; Steber, R.; Pihusch, M.; Pihusch, R.; Toth, B.; Hiller, E.; Kolb, H.-J. Endothelial Cell–Derived Microparticles in Allogeneic Hematopoietic Stem Cell Recipients. Transplantation 2006, 81, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, X.; Hong, M.; Luo, T.; Zhao, M.; Shen, H.; Fang, J.; Li, X.; Zang, S.; Chen, P.; et al. Endothelial microparticles delivering microRNA-155 into T lymphocytes are involved in the initiation of acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Oncotarget 2017, 8, 23360–23375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, V.T.; Revta, C.; Richardson, P.G. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: Update on defibrotide and other current investigational therapies. Bone Marrow Transplant. 2008, 41, 229–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Modified Seattle | Baltimore | EBMT–Classical | EBMT–Late Onset | EBMT–Pediatric |
|---|---|---|---|---|
| At least 2 of the following before day 20 post-HSCT | Bilirubin ≥ 2 mg/dL before day 21 post-HSCT AND at least 2 of the following: | Bilirubin ≥ 2 mg/dL before day 21 post-HSCT AND at least 2 of the following: | Classical SOS beyond day 21 OR Histologically proven SOS OR At least 2 of the following: | Presence of at least 2 of the following with no limitation for time of onset: |
| Bilirubin ≥ 2 mg/dL | Bilirubin ≥ 2 mg/dL | Rising bilirubin on at least 3 consecutive days OR Bilirubin ≥ 2 mg/dL within 72 h | ||
| Hepatomegaly, RUQ pain | Hepatomegaly | Painful hepatomegaly | Painful hepatomegaly | Hepatomegaly |
| Ascites with or w/o unexplained weight gain >2% from baseline | Ascites | Ascites | Ascites AND Hemodynamical and/or ultrasound evidence of SOS | Ascites Unexplained consumptive and transfusion-refractory thrombocytopenia |
| Weight gain > 5% from baseline | Weight gain > 5% from baseline | Weight gain > 5% from baseline | Unexplained weight gain on 3 consecutive days (despite diuretics) OR Weight gain > 5% from baseline |
| Mild | Moderate | Severe | Very Severe-MOD/MOF | |
|---|---|---|---|---|
| Time since first clinical symptoms of SOS/VOD | <7 Days | 5–7 Days | ≤4 Days | Any time |
| Bilirubin (mg/dL) | ≥2 and <3 | ≥3 and <5 | ≥5 and <8 | ≥8 |
| Bilirubin kinetics | Doubling within 48 h | |||
| Transaminases | ≤2 × normal | > 2 and ≤5 × normal | >5 and ≤8 × normal | >8 × normal |
| Weight increase | <5% | ≥5% and <10% | ≥5% and <10% | ≥10% |
| Renal function | <1.2 × baseline at transplant | ≥1.2 and <1.5 × baseline at transplant | ≥1.5 and <2 × baseline at transplant | ≥2 × baseline transplant or other signs of MOD/MOF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrikou, I.; Chatzidimitriou, D.; Skoura, L.; Nikolousis, E.; Sakellari, I.; Gavriilaki, E. Molecular Advances in Sinusoidal Obstruction Syndrome/Veno-Occlusive Disease. Int. J. Mol. Sci. 2023, 24, 5620. https://doi.org/10.3390/ijms24065620
Mavrikou I, Chatzidimitriou D, Skoura L, Nikolousis E, Sakellari I, Gavriilaki E. Molecular Advances in Sinusoidal Obstruction Syndrome/Veno-Occlusive Disease. International Journal of Molecular Sciences. 2023; 24(6):5620. https://doi.org/10.3390/ijms24065620
Chicago/Turabian StyleMavrikou, Ioulia, Dimitrios Chatzidimitriou, Lemonia Skoura, Emmanouil Nikolousis, Ioanna Sakellari, and Eleni Gavriilaki. 2023. "Molecular Advances in Sinusoidal Obstruction Syndrome/Veno-Occlusive Disease" International Journal of Molecular Sciences 24, no. 6: 5620. https://doi.org/10.3390/ijms24065620






