Rats with Long-Term Cholestasis Have a Decreased Cytosolic but Maintained Mitochondrial Hepatic CoA Pool
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Fractionation of Liver Tissue
4.4. Determination of the CoA Content in Liver Homogenate and Liver Subcellular Fractions
4.5. Metabolism of Palmitate by Liver Homogenate and Liver Mitochondria
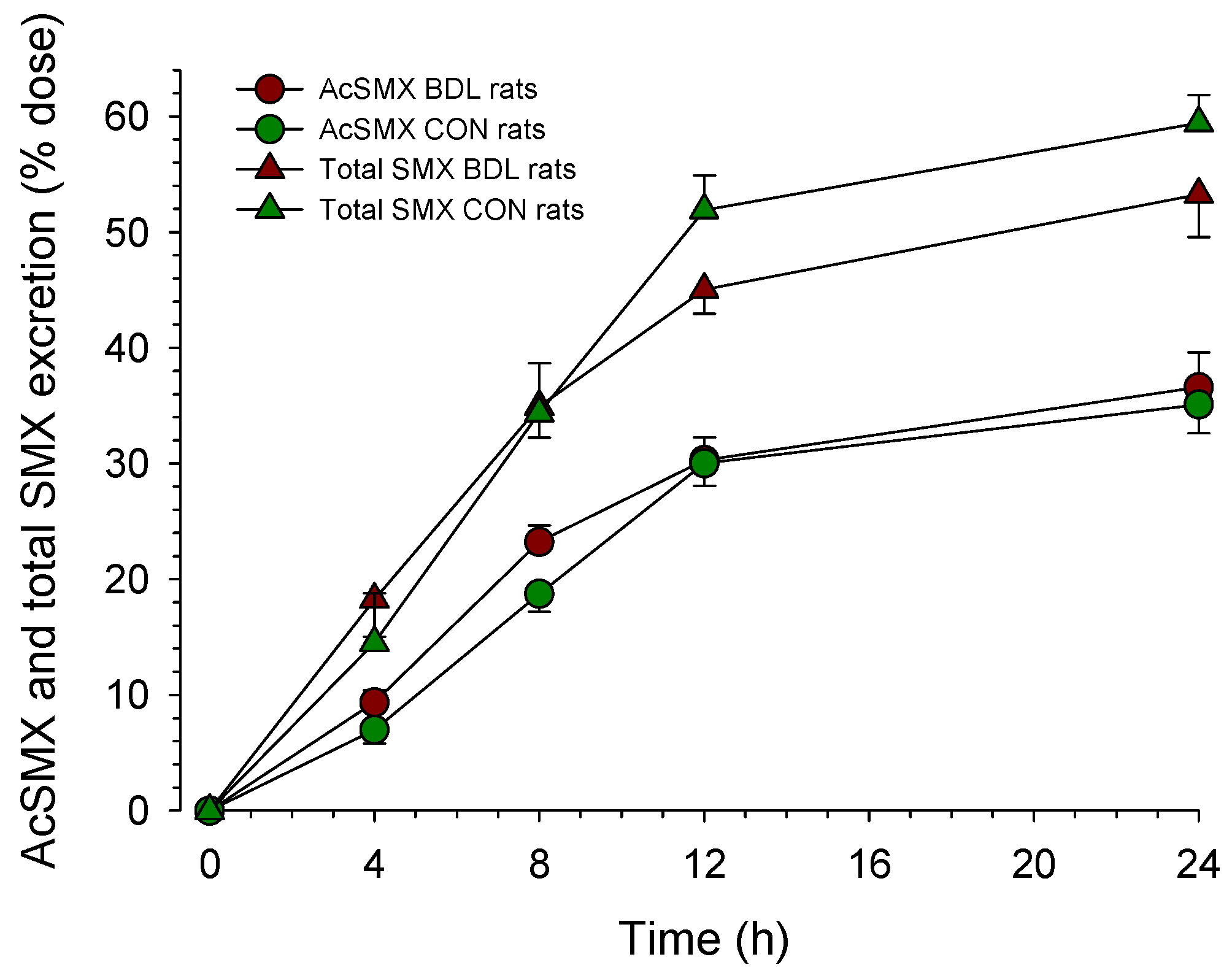
4.6. In Vivo Metabolism of Benzoate
4.7. In Vivo Metabolism of Sulfamethoxazole
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lipmann, F.; Kaplan, N.O.; Novelli, G.D.; Tuttle, L.C.; Guirard, B.M. Coenzyme for acetylation, a pantothenic acid derivative. J. Biol. Chem. 1947, 167, 869. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Zhang, Y.M.; Rock, C.O.; Jackowski, S. Coenzyme A: Back in action. Prog. Lipid Res. 2005, 44, 125–153. [Google Scholar] [CrossRef] [PubMed]
- Robishaw, J.D.; Neely, J.R. Coenzyme A metabolism. Am. J. Physiol. 1985, 248, E1–E9. [Google Scholar] [CrossRef]
- Naquet, P.; Kerr, E.W.; Vickers, S.D.; Leonardi, R. Regulation of coenzyme A levels by degradation: The ‘Ins and Outs’. Prog. Lipid Res. 2020, 78, 101028. [Google Scholar] [CrossRef]
- Domschke, W.; Liersch, M.; Decker, K. Lack of permeation of coenzyme A from blood into liver cells. Hoppe Seylers Z. Physiol. Chem. 1971, 352, 85–88. [Google Scholar] [CrossRef]
- Trefely, S.; Lovell, C.D.; Snyder, N.W.; Wellen, K.E. Compartmentalised acyl-CoA metabolism and roles in chromatin regulation. Mol. Metab. 2020, 38, 100941. [Google Scholar] [CrossRef]
- Wu, J.; Sandberg, M.; Weber, S.G. Integrated electroosmotic perfusion of tissue with online microfluidic analysis to track the metabolism of cystamine, pantethine, and coenzyme A. Anal. Chem. 2013, 85, 12020–12027. [Google Scholar] [CrossRef] [Green Version]
- Fiermonte, G.; Paradies, E.; Todisco, S.; Marobbio, C.M.; Palmieri, F. A novel member of solute carrier family 25 (SLC25A42) is a transporter of coenzyme A and adenosine 3’,5’-diphosphate in human mitochondria. J. Biol. Chem. 2009, 284, 18152–18159. [Google Scholar] [CrossRef] [Green Version]
- Agrimi, G.; Russo, A.; Scarcia, P.; Palmieri, F. The human gene SLC25A17 encodes a peroxisomal transporter of coenzyme A, FAD and NAD+. Biochem. J. 2012, 443, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Krahenbuhl, S.; Brass, E.P. Fuel homeostasis and carnitine metabolism in rats with secondary biliary cirrhosis. Hepatology 1991, 14, 927–934. [Google Scholar] [CrossRef]
- Krähenbühl, S.; Talos, C.; Reichen, J. Mechanisms of impaired hepatic fatty acid metabolism in rats with long-term bile duct ligation. Hepatology 1994, 19, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Krähenbühl, S.; Krähenbühl-Glauser, S.; Stucki, J.; Gehr, P.; Reichen, J. Stereological and functional analysis of liver mitochondria from rats with secondary biliary cirrhosis: Impaired mitochondrial metabolism and increased mitochondrial content per hepatocyte. Hepatology 1992, 15, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Gatley, S.J.; Sherratt, H.S. The synthesis of hippurate from benzoate and glycine by rat liver mitochondria. Submitochondrial localization and kinetics. Biochem. J. 1977, 166, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brass, E.P.; Ruff, L.J. Rat hepatic coenzyme A is redistributed in response to mitochondrial acyl-coenzyme A accumulation. J. Nutr. 1992, 122, 2094–2100. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Cano, M.L.; Potyraj, J. The relationship between metabolic state and total CoA content of rat liver and heart. J. Nutr. 1978, 108, 854–862. [Google Scholar] [CrossRef]
- Tokutake, Y.; Onizawa, N.; Katoh, H.; Toyoda, A.; Chohnan, S. Coenzyme A and its thioester pools in fasted and fed rat tissues. Biochem. Biophys. Res. Commun. 2010, 402, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Loud, A.V. A quantitative stereological description of the ultrastructure of normal rat liver parenchymal cells. J. Cell Biol. 1968, 37, 27–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robishaw, J.D.; Berkich, D.; Neely, J.R. Rate-limiting step and control of coenzyme A synthesis in cardiac muscle. J. Biol. Chem. 1982, 257, 10967–10972. [Google Scholar] [CrossRef]
- Gout, I. Coenzyme A, protein CoAlation and redox regulation in mammalian cells. Biochem. Soc. Trans. 2018, 46, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, Y.; Peak-Chew, S.Y.; Newell, C.; Miller-Aidoo, S.; Mangal, S.; Zhyvoloup, A.; Bakovic, J.; Malanchuk, O.; Pereira, G.C.; Kotiadis, V.; et al. Protein CoAlation: A redox-regulated protein modification by coenzyme A in mammalian cells. Biochem. J. 2017, 474, 2489–2508. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, Y.; Zhyvoloup, A.; Baković, J.; Thomas, N.; Yu, B.Y.K.; Das, S.; Orengo, C.; Newell, C.; Ward, J.; Saladino, G.; et al. Protein CoAlation and antioxidant function of coenzyme A in prokaryotic cells. Biochem. J. 2018, 475, 1909–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krähenbühl, S.; Talos, C.; Lauterburg, B.H.; Reichen, J. Reduced antioxidative capacity in liver mitochondria from bile duct ligated rats. Hepatology 1995, 22, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Hein, D.W.; Rustan, T.D.; Bucher, K.D.; Martin, W.J.; Furman, E.J. Acetylator phenotype-dependent and -independent expression of arylamine N-acetyltransferase isozymes in rapid and slow acetylator inbred rat liver. Drug Metab. Dispos. 1991, 19, 933–937. [Google Scholar] [PubMed]
- Sim, E.; Walters, K.; Boukouvala, S. Arylamine N-acetyltransferases: From structure to function. Drug Metab. Rev. 2008, 40, 479–510. [Google Scholar] [CrossRef] [PubMed]
- Svensson, C.K.; Tomilo, M. Effect of H2-receptor antagonists on rat liver cytosolic acetyl CoA:arylamine N-acetyltransferase activity. Drug Metab. Dispos. 1992, 20, 74–78. [Google Scholar] [PubMed]
- Yu, P.H.; Boulton, A.A. N-acylation of tyramines: Purification and characterization of an arylamine N-acetyltransferase from rat brain and liver. Can. J. Biochem. 1979, 57, 1204–1209. [Google Scholar] [CrossRef]
- Lewin, T.M.; Kim, J.H.; Granger, D.A.; Vance, J.E.; Coleman, R.A. Acyl-CoA synthetase isoforms 1, 4, and 5 are present in different subcellular membranes in rat liver and can be inhibited independently. J. Biol. Chem. 2001, 276, 24674–24679. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Nenkov, M.; Chen, Y.; Press, A.T.; Kaemmerer, E.; Gassler, N. Fatty acid metabolism and acyl-CoA synthetases in the liver-gut axis. World J. Hepatol. 2021, 13, 1512–1533. [Google Scholar] [CrossRef]
- Kim, J.H.; Lewin, T.M.; Coleman, R.A. Expression and characterization of recombinant rat Acyl-CoA synthetases 1, 4, and 5. Selective inhibition by triacsin C and thiazolidinediones. J. Biol. Chem. 2001, 276, 24667–24673. [Google Scholar] [CrossRef] [Green Version]
- Krähenbühl, L.; Reichen, J.; Talos, C.; Krähenbühl, S. Benzoic acid metabolism reflects hepatic mitochondrial function in rats with long-term extrahepatic cholestasis. Hepatology 1997, 25, 278–283. [Google Scholar] [CrossRef]
- Krähenbühl, L.; Schäfer, M.; Krähenbühl, S. Reversibility of hepatic mitochondrial damage in rats with long-term cholestasis. J. Hepatol. 1998, 28, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Krähenbühl, S.; Stucki, J.; Reichen, J. Reduced activity of the electron transport chain in liver mitochondria isolated from rats with secondary biliary cirrhosis. Hepatology 1992, 15, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, C.; DiMarco, J.P.; Tandler, B. Riboflavin and rat hepatic cell structure and function. Mitochondrial oxidative metabolism in deficiency states. J. Biol. Chem. 1979, 254, 4164–4170. [Google Scholar] [CrossRef] [PubMed]
- Srere, P. Citrate synthase. Methods Enzymol. 1969, 13, 3–11. [Google Scholar]
- Vassault, A. Lactate dhydrogenase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie GmbH: Weinheim, Germany, 1983; Volume 3, pp. 118–125. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Cederblad, G.; Carlin, J.I.; Constantin-Teodosiu, D.; Harper, P.; Hultman, E. Radioisotopic assays of CoASH and carnitine and their acetylated forms in human skeletal muscle. Anal. Biochem. 1990, 185, 274–278. [Google Scholar] [CrossRef]
- Friolet, R.; Hoppeler, H.; Krähenbühl, S. Relationship between the coenzyme A and the carnitine pools in human skeletal muscle at rest and after exhaustive exercise under normoxic and acutely hypoxic conditions. J. Clin. Investig. 1994, 94, 1490–1495. [Google Scholar] [CrossRef]
- Felser, A.; Stoller, A.; Morand, R.; Schnell, D.; Donzelli, M.; Terracciano, L.; Bouitbir, J.; Krähenbühl, S. Hepatic toxicity of dronedarone in mice: Role of mitochondrial β-oxidation. Toxicology 2014, 323, 1–9. [Google Scholar] [CrossRef]
- Reinartz, A.; Ehling, J.; Leue, A.; Liedtke, C.; Schneider, U.; Kopitz, J.; Weiss, T.; Hellerbrand, C.; Weiskirchen, R.; Knüchel, R.; et al. Lipid-induced up-regulation of human acyl-CoA synthetase 5 promotes hepatocellular apoptosis. Biochim. Biophys. Acta 2010, 1801, 1025–1035. [Google Scholar] [CrossRef]
- Dole, V.P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J. Clin. Investig. 1956, 35, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Ahern, D.A.; Mitchell, M.E. Liver function in protein-energy malnutrition measured by cinnamic acid tolerance and benzoic acid tolerance: Effect of carnitine supplementation. Br. J. Nutr. 1989, 61, 209–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arín, M.J.; Diez, M.T.; Resines, J.A. Rapid and simple method for the determination of urinary benzoic and phenylacetic acids and their glycine conjugates in ruminants by reversed-phase high-performance liquid chromatography. J. Chromatogr. 1992, 582, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Agarwal, K.C.; Beylot, M.; Soloviev, M.V.; David, F.; Reider, M.W.; Anderson, V.E.; Tserng, K.Y.; Brunengraber, H. Nonhomogeneous labeling of liver extra-mitochondrial acetyl-CoA. Implications for the probing of lipogenic acetyl-CoA via drug acetylation and for the production of acetate by the liver. J. Biol. Chem. 1994, 269, 11025–11029. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Opheim, K.E.; Siber, G.R.; Ericson, J.F.; Smith, A.L. High-performance liquid chromatographic quantitation of trimethoprim, sulfamethoxazole, and N4-acetylsulfamethoxazole in body fluids. J. Chromatogr. 1983, 278, 337–345. [Google Scholar] [CrossRef]

| Control (n = 5) | BDL Rats (n = 9) | |
|---|---|---|
| Body weight (g) | 339 ± 9 | 356 ± 8 |
| Liver weight (g/100 g body weight) | 3.69 ± 0.12 | 7.71 ± 0.15 * |
| Spleen weight (g/100 g body weight) | 0.20 ± 0.01 | 0.68 ± 0.02 * |
| Serum bilirubin (µmol/L) | 1 ± 1 | 114 ± 4 * |
| Serum bile acids (µmol/L) | 1 ± 1 | 31 ± 3 * |
| AST (U/L) | 72 ± 3 | 147 ± 6 * |
| Alkaline phosphatase (U/L) | 203 ± 20 | 460 ± 10 * |
| Control (n = 5) | BDL Rats (n = 9) | |
|---|---|---|
| Liver homogenate | ||
| CoASH | 43.9 ± 1.8 | 30.3 ± 1.3 * |
| SCA-CoA (% total CoA) | 143 ± 6 (68 ± 3) | 81.4 ± 2.1 * (64 ± 3) |
| TAS-CoA | 187 ± 8 | 112 ± 3 * |
| LCA-CoA | 23.2 ± 0.4 | 16.0 ± 0.3 * |
| Total CoA | 210 ± 9 | 128 ± 5 * |
| Liver mitochondria | ||
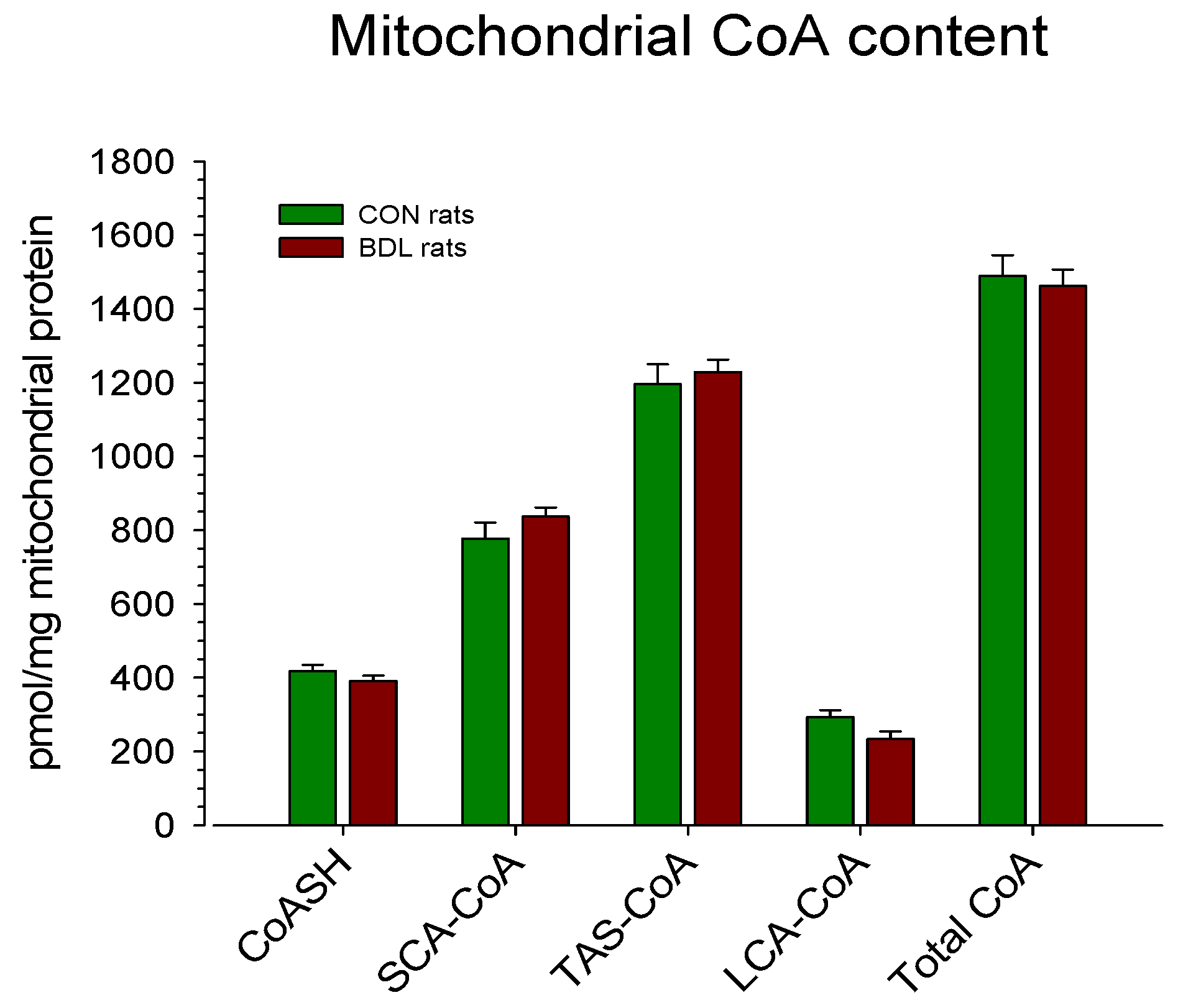
| CoASH | 31.4 ± 1.2 | 26.2 ± 1.0 * |
| SCA-CoA (% total CoA) | 58.2 ± 3.2 (52 ± 4) | 56.2 ± 1.6 (57 ± 3) |
| TAS-CoA | 89.6 ± 4.0 | 82.4 ± 2.3 |
| LCA-CoA | 21.9 ± 1.4 | 15.7 ± 1.4 * |
| Total CoA (% total liver) | 112 ± 5 (53 ± 4) | 98.1 ± 3.0 * (77 ± 4 *) |
| Liver cytoplasm | ||
| CoASH | 20.8 ± 3.0 | 4.18 ± 0.67 * |
| SCA-CoA (% total CoA) | 62.8 ± 3.2 (74 ± 4) | 18.6 ± 0.6 * (81 ± 3) |
| TAS-CoA | 83.6 ± 3.5 | 22.8 ± 0.8 * |
| LCA-CoA | 1.04 ± 0.13 | 0.08 ± 0.03 * |
| Total CoA (% total liver) | 84.6 ± 3.7 (41 ± 2) | 23.0 ± 0.9 * (18 ± 1 *) |
| Control (n = 5) | BDL Rats (n = 9) | |
|---|---|---|
| Formation of palmitoyl-CoA | ||
| Homogenate (no exogenous CoASH) | 48.2 ± 3.1 + | 8.1 ± 1.2 *+ (83%) |
| Homogenate (5 µM CoASH) | 152 ± 11 | 98.0 ± 5.1 * (36%) |
| Homogenate (150 µM CoASH) | 161 ± 10 | 102 ± 6 * (37%) |
| Mitochondria (150 µM CoASH) | 2.65 ± 0.15 | 2.58 ± 0.17 |
| Formation of acid-soluble products | ||
| Homogenate (no exogenous CoASH) | 25.4 ± 1.9 + | 4.7 ± 1.1 *+ (81%) |
| Homogenate (5 µM CoASH) | 75.2 ± 5.2 | 25.6 ± 2.1 * (66%) |
| Homogenate (150 µM CoASH) | 81.9 ± 4.8 | 30.1 ± 2.2 * (63%) |
| Mitochondria (150 µM CoASH) | 1.72 ± 0.09 | 1.21 ± 0.7 * (30%) |
| Control (n = 5) | BDL Rats (n = 9) | |
|---|---|---|
| L-glutamate 20 mM | ||
| State 3 | 95.6 ± 4.8 | 59.0 ± 3.1 * (38%) |
| RCR | 7.3 ± 0.4 | 6.7 ± 0.5 |
| ADP/O | 2.9 ± 0.2 | 2.6 ± 0.2 |
| Succinate 20 mM | ||
| State 3 | 199 ± 14 | 128 ± 9 * (36%) |
| RCR | 5.8 ± 0.4 | 4.9 ± 0.5 |
| ADP/O | 2.0 ± 0.2 | 1.9 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krähenbühl, L.; Krähenbühl, S. Rats with Long-Term Cholestasis Have a Decreased Cytosolic but Maintained Mitochondrial Hepatic CoA Pool. Int. J. Mol. Sci. 2023, 24, 4365. https://doi.org/10.3390/ijms24054365
Krähenbühl L, Krähenbühl S. Rats with Long-Term Cholestasis Have a Decreased Cytosolic but Maintained Mitochondrial Hepatic CoA Pool. International Journal of Molecular Sciences. 2023; 24(5):4365. https://doi.org/10.3390/ijms24054365
Chicago/Turabian StyleKrähenbühl, Lukas, and Stephan Krähenbühl. 2023. "Rats with Long-Term Cholestasis Have a Decreased Cytosolic but Maintained Mitochondrial Hepatic CoA Pool" International Journal of Molecular Sciences 24, no. 5: 4365. https://doi.org/10.3390/ijms24054365





