Identification and Characterization of 5-HT Receptor 1 from Scylla paramamosain: The Essential Roles of 5-HT and Its Receptor Gene during Aggressive Behavior in Crab Species
Abstract
:1. Introduction
2. Results
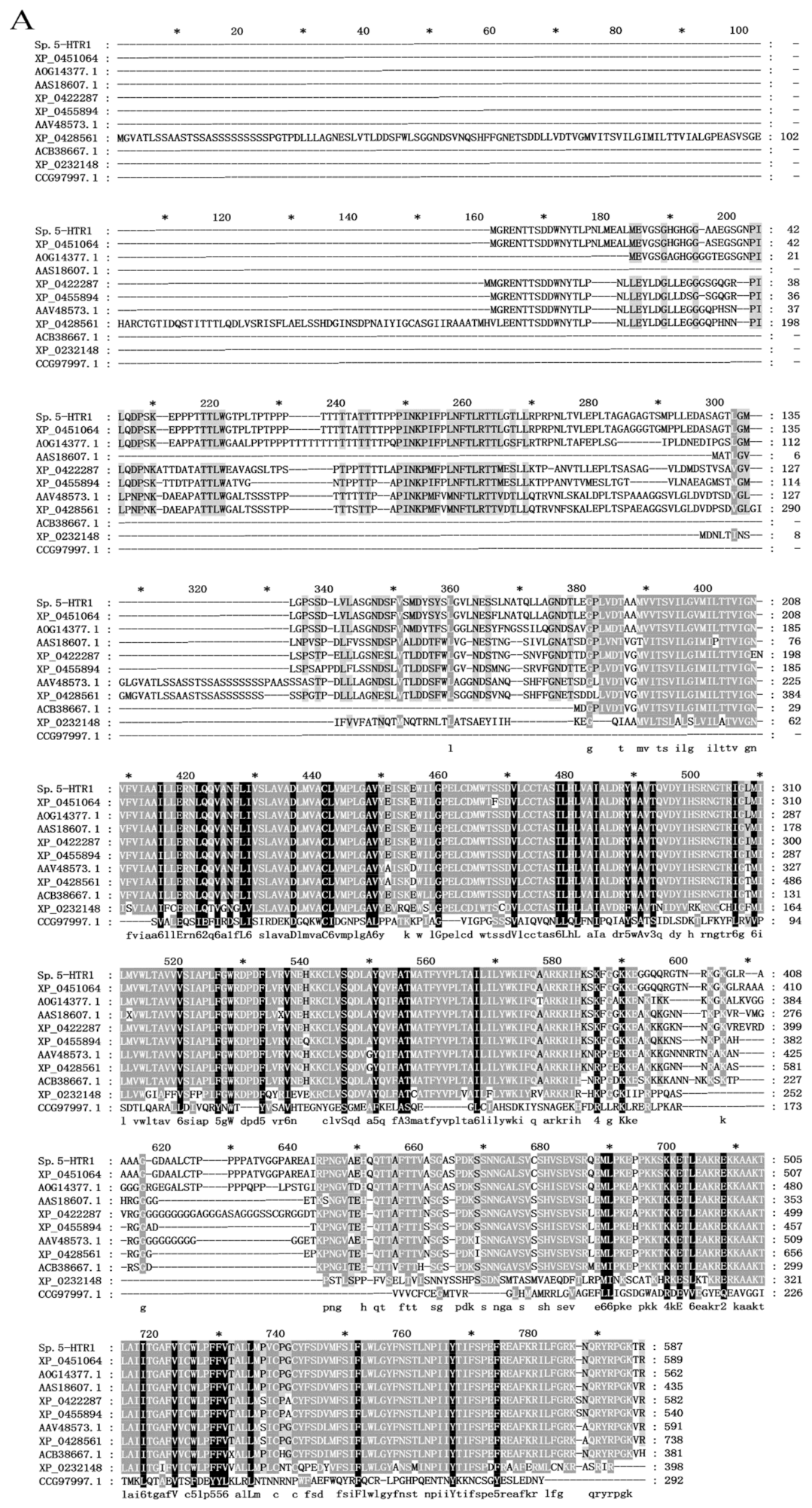
2.1. Cloning of the Full-Length cDNA of Sp5-HTR1 from S. paramamosain
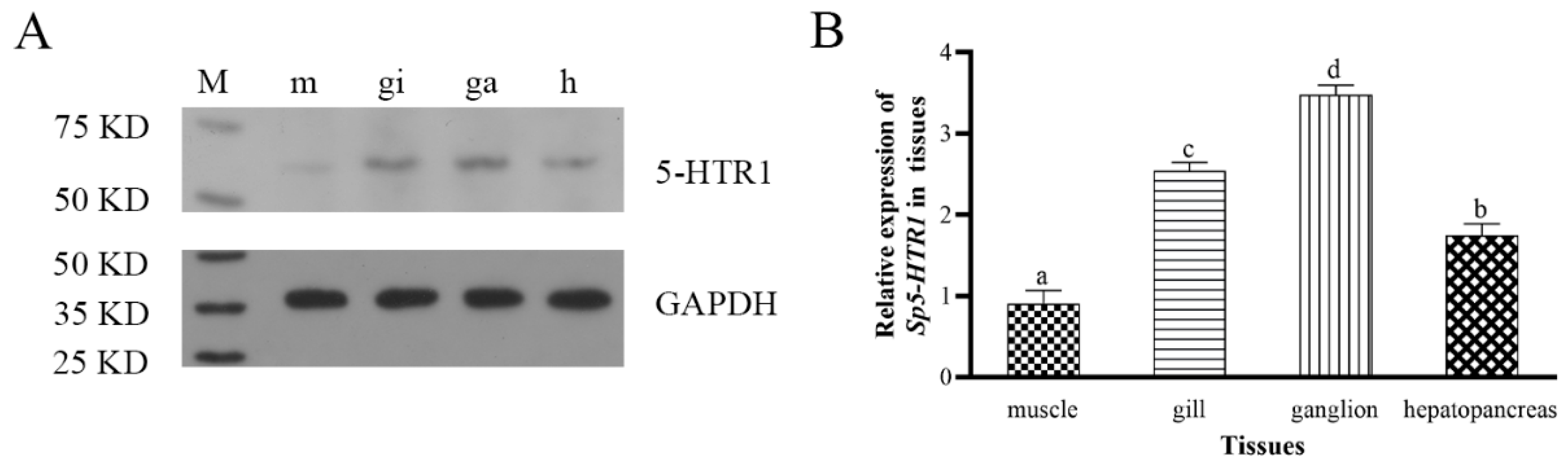
2.2. Peptide-Coupled Antigen and Rabbit Polyclonal Antibody Analysis
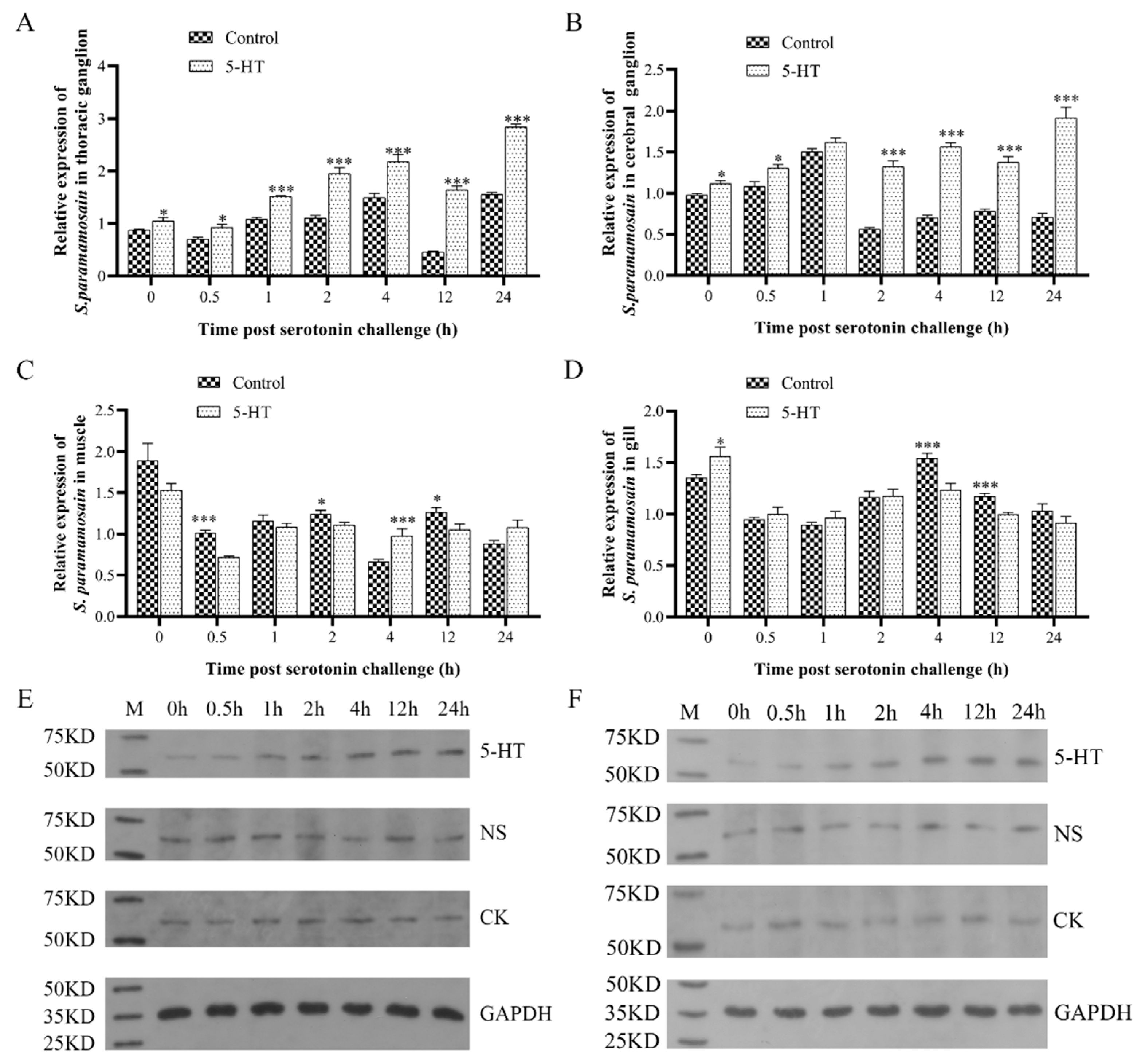
2.3. Sp5-HTR1 Response to 5-HT Injection
2.4. Behavioral Response to Different Concentrations of 5-HT Injection in S. paramamosain
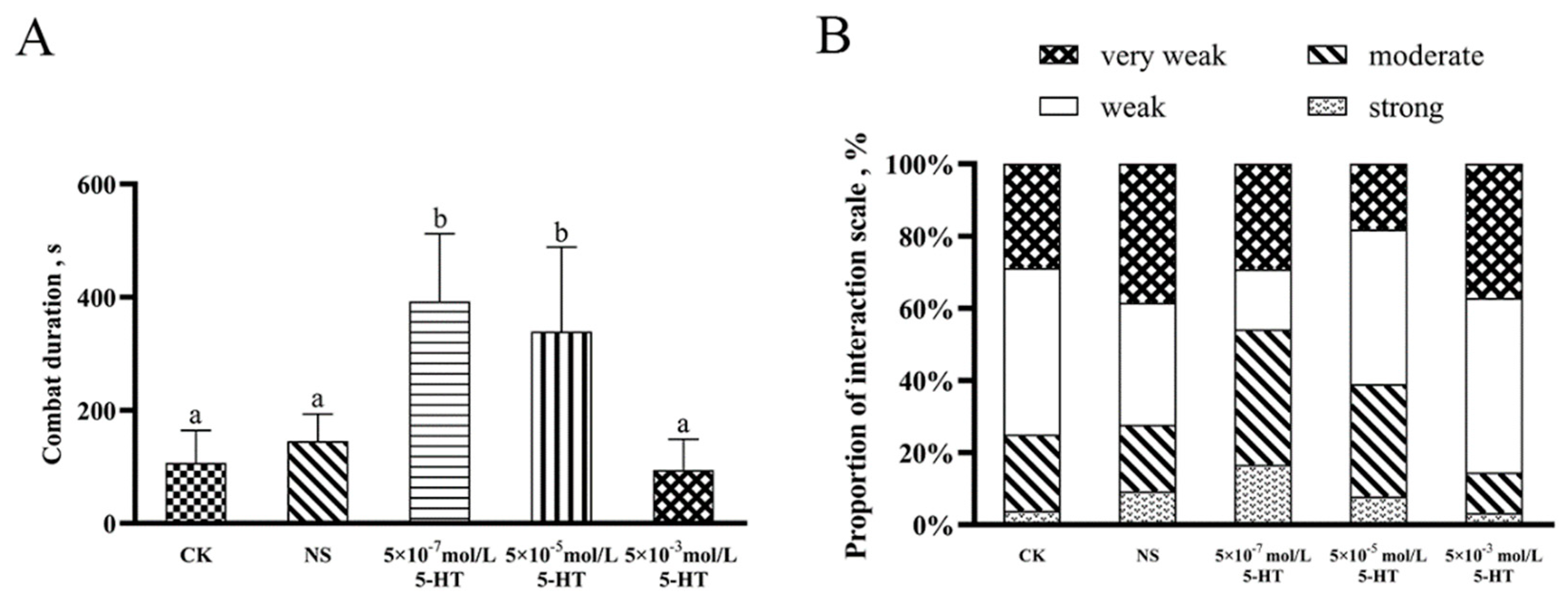
2.5. Effects of Different Concentrations of 5-HT on the Aggressive Behavior of S. paramamosain
3. Discussion
3.1. Cloning and Evolutionary Analysis of Sp5-HTR1 Sequence
3.2. Sp5-HTR1 Is Involved in the Regulation of Competitive Behavior of S. paramamosain
3.3. Effect of Exogenous Injection of 5-HT on S. paramamosain
4. Materials and Methods
4.1. Animal Collection and Maintenance
4.2. Rapid Amplification of cDNA Ends
4.3. Sequence Analysis
4.4. Peptide-Coupled Antigen and Preparation of the Antibody
4.5. Sp5-HTR1 Expression in the Tissues of S. paramamosain
4.6. Western Blotting
4.7. Effect of 5-HT on the Behavior of S. paramamosain after Injection
4.8. Observation of Aggressive Behaviors
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Sun, X. Agonistic behaviors of aquatic animals. Zool. Res. 2013, 34, 214–220. [Google Scholar] [CrossRef]
- Glass, C.; Huntingford, F. Initiation and resolution of fights between swimming crabs (Liocarcinus depurator). Ethology 1988, 77, 237–249. [Google Scholar] [CrossRef]
- Correa, C.; Baeza, J.A.; Hinojosa, I.A.; Thiel, M. Male dominance hierarchy and mating tactics in the rock shrimp Rhynchocinetes typus (Decapoda: Caridea). J. Crustac. Biol. 2003, 23, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Matheson, K.; Gagnon, P. Effects of temperature, body size, and chela loss on competition for a limited food resource between indigenous rock crab (Cancer irroratus Say) and recently introduced green crab (Carcinus maenas L.). J. Exp. Mar. Biol. Ecol. 2012, 428, 49–56. [Google Scholar] [CrossRef]
- Briffa, M.; de la Haye, K.; Munday, P.L. High CO2 and marine animal behaviour: Potential mechanisms and ecological consequences. Mar. Pollut. Bull. 2012, 64, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Farca Luna, A.J.; Hurtado-Zavala, J.I.; Reischig, T.; Heinrich, R. Circadian regulation of agonistic behavior in groups of parthenogenetic marbled crayfish, Procambarus sp. J. Biol. Rhythms 2009, 24, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, K.; Sugiyama, A.; Jinbo, T.; Murakami, K. The influence of male size on competitive mating success in the Japanese spiny lobster Panulirus japonicus (von Siebold, 1824)(Decapoda: Palinuridae): Implications for broodstock management techniques. J. Crustac. Biol. 2018, 38, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Tina, F.W.; Jaroensutasinee, M.; Keeratipattarakarn, K.; Jaroensutasinee, K. Sex and burrow/chimney ownership affecting time allocation for surface activities in Uca rosea (Tweedie, 1937)(Brachyura, Ocypodidae). Crustaceana 2018, 91, 51–62. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Cannibalism of decapod crustaceans and implications for their aquaculture: A review of its prevalence, influencing factors, and mitigating methods. Rev. Fish. Sci. Aquac. 2017, 25, 42–69. [Google Scholar] [CrossRef]
- Courtene-Jones, W.; Briffa, M. Boldness and asymmetric contests: Role-and outcome-dependent effects of fighting in hermit crabs. Behav. Ecol. 2014, 25, 1073–1082. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Pang, Y.; Huang, G.; Xu, M.; Zhang, C.; He, L.; Lv, J.; Song, Y.; Song, X.; Cheng, Y. The serotonin or dopamine by cyclic adenosine monophosphate-protein kinase A pathway involved in the agonistic behaviour of Chinese mitten crab, Eriocheir sinensis. Physiol. Behav. 2019, 209, 112621. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, J.; Liu, D. Research progresses in crustacean agonistic behavior. Period. Ocean. Univ. China 2020, 50, 31–36. [Google Scholar] [CrossRef]
- Sloley, B.; Juorio, A. Monoamine neurotransmitters in invertebrates and vertebrates: An examination of the diverse enzymatic pathways utilized to synthesize and inactivate blogenic amines. Int. Rev. Neurobiol. 1995, 38, 253–303. [Google Scholar] [CrossRef] [PubMed]
- Fingerman, M. Crustacean endocrinology: A retrospective, prospective, and introspective analysis. Physiol. Zool. 1997, 70, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, M.; Pivac, N.; Seler, D.M.; Perkovic, M.N.; Pessia, M.; Di Giovanni, G. The role of the serotonergic system at the interface of aggression and suicide. Neuroscience 2013, 236, 160–185. [Google Scholar] [CrossRef] [Green Version]
- Kong, Q.; Tai, F. The neural mechanism of aggressive behavior. Prog. Mod. Biomed. 2006, 6, 55–58. [Google Scholar] [CrossRef]
- Kravitz, E.A. Hormonal control of behavior: Amines and the biasing of behavioral output in lobsters. Science 1988, 241, 1775–1781. [Google Scholar] [CrossRef]
- Huber, R.; Panksepp, J.; Yue, Z.; Delago, A.; Moore, P. Dynamic interactions of behavior and amine neurochemistry in acquisition and maintenance of social rank in crayfish. Brain. Behav. Evol. 2001, 57, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Lesch, K.P.; Gutknecht, L. Pharmacogenetics of the serotonin transporter. Prog. Neuro-Psychoph. 2005, 29, 1062–1073. [Google Scholar] [CrossRef]
- Li, Z.; Chalazonitis, A.; Huang, Y.; Mann, J.J.; Margolis, K.G.; Yang, Q.M.; Kim, D.O.; Côté, F.; Mallet, J.; Gershon, M.D. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J. Neurosci. 2011, 31, 8998–9009. [Google Scholar] [CrossRef]
- Ongvarrasopone, C.; Roshorm, Y.; Somyong, S.; Pothiratana, C.; Petchdee, S.; Tangkhabuanbutra, J.; Sophasan, S.; Panyim, S. Molecular cloning and functional expression of the Penaeus monodon 5-HT receptor. BBA Gene Struct. Expr. 2006, 1759, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.C.; Taylor, K.; Krasne, F.B. Reciprocal stimulation of decay between serotonergic facilitation and depression of synaptic transmission. J. Neurophysiol. 2008, 100, 1113–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saudou, F.; Amara, D.A.; Dierich, A.; LeMeur, M.; Ramboz, S.; Segu, L.; Buhot, M. -C.; Hen, R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science 1994, 265, 1875–1878. [Google Scholar] [CrossRef]
- Filby, A.L.; Paull, G.C.; Hickmore, T.F.; Tyler, C.R. Unravelling the neurophysiological basis of aggression in a fish model. BMC Genomics 2010, 11, 498. [Google Scholar] [CrossRef] [Green Version]
- Loveland, J.L.; Uy, N.; Maruska, K.P.; Carpenter, R.E.; Fernald, R.D. Social status differences regulate the serotonergic system of a cichlid fish, Astatotilapia burtoni. J. Exp. Biol. 2014, 217, 2680–2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Benton, J.L.; Beltz, B.S. 5-HT receptors mediate lineage-dependent effects of serotonin on adult neurogenesis in Procambarus clarkii. Neural Dev. 2011, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Northcutt, A.J.; Lett, K.M.; Garcia, V.B.; Diester, C.M.; Lane, B.J.; Marder, E.; Schulz, D.J. Deep sequencing of transcriptomes from the nervous systems of two decapod crustaceans to characterize genes important for neural circuit function and modulation. BMC Genom. 2016, 17, 2. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Acevedo, N.; Reyes-Colón, D.; Ruíz-Rodríguez, E.A.; Rivera, N.M.; Rosenthal, J.; Kohn, A.B.; Moroz, L.L.; Sosa, M.A. Cloning and immunoreactivity of the 5-HT1Mac and 5-HT2Mac receptors in the central nervous system of the freshwater prawn Macrobrachium rosenbergii. J. Comp. Neurol. 2009, 513, 399–416. [Google Scholar] [CrossRef] [Green Version]
- Zheng, P.; Han, T.; Li, X.; Wang, J.; Su, H.; Xu, H.; Wang, Y.; Wang, C. Dietary protein requirement of juvenile mud crab Scylla paramamosain. Aquaculture 2020, 518, 734852. [Google Scholar] [CrossRef]
- Aramant, R.; Elofsson, R. Monoaminergic neurons in the nervous system of crustaceans. Cell Tissue Res. 1976, 170, 231–251. [Google Scholar] [CrossRef]
- Beltz, B.; Eisen, J.S.; Flamm, R.; Harris-Warrick, R.; Hooper, S.L.; Marder, E. Serotonergic innervation and modulation of the stomatogastric ganglion of three decapod crustaceans (Panulirus interruptus, Homarus americanus and Cancer irroratus). J. Exp. Biol. 1984, 109, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Fingerman, M. Roles of neurotransmitters in regulating reproductive hormone release and gonadal maturation in decapod crustaceans. Invertebr. Reprod. Dev. 1997, 31, 47–54. [Google Scholar] [CrossRef]
- Luttrell, L.M.; Lefkowitz, R.J. The role of β-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002, 115, 455–465. [Google Scholar] [CrossRef]
- Masson, J.; Emerit, M.B.; Hamon, M.; Darmon, M. Serotonergic signaling: Multiple effectors and pleiotropic effects. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 685–713. [Google Scholar] [CrossRef]
- Montalbano, A.; Corradetti, R.; Mlinar, B. Pharmacological characterization of 5-HT1A autoreceptor-coupled GIRK channels in rat dorsal raphe 5-HT neurons. PLoS ONE 2015, 10, e0140369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Y.; He, L.; Song, Y.; Song, X.; Lv, J.; Cheng, Y.; Yang, X. Identification and integrated analysis of microRNA and mRNA expression profiles during agonistic behavior in Chinese mitten crab (Eriocheir sinensis) using a deep sequencing approach. Front. Genet. 2020, 11, 321. [Google Scholar] [CrossRef] [Green Version]
- Ślifirski, G.; Król, M.; Turło, J. 5-HT receptors and the development of new antidepressants. Int. J. Mol. Sci. 2021, 22, 9015. [Google Scholar] [CrossRef]
- Shah, P.A.; Park, C.J.; Shaughnessy, M.P.; Cowles, R.A. Serotonin as a mitogen in the gastrointestinal tract: Revisiting a familiar molecule in a new role. Cell. Mol. Gastroente. 2021, 12, 1093–1104. [Google Scholar] [CrossRef]
- Ago, Y.; Araki, R.; Tanaka, T.; Sasaga, A.; Nishiyama, S.; Takuma, K.; Matsuda, T. Role of social encounter-induced activation of prefrontal serotonergic systems in the abnormal behaviors of isolation-reared mice. Neuropsychopharmacol 2013, 38, 1535–1547. [Google Scholar] [CrossRef] [Green Version]
- de Haas, E.N.; van der Eijk, J.A. Where in the serotonergic system does it go wrong? Unravelling the route by which the serotonergic system affects feather pecking in chickens. Neurosci. Biobehav. Rev. 2018, 95, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.N.; Kabelik, D. The effects of dopamine receptor 1 and 2 agonists and antagonists on sexual and aggressive behaviors in male green anoles. PLoS ONE 2017, 12, e0172041. [Google Scholar] [CrossRef]
- McAllister-Williams, R.; Kelly, J. The modulation of calcium channel currents recorded from adult rat dorsal raphe neurones by 5-HT1A receptor or direct G-protein activation. Neuropharmacology 1995, 34, 1491–1506. [Google Scholar] [CrossRef]
- Zhuang, X.; Gross, C.; Santarelli, L.; Compan, V.; Trillat, A.-C.; Hen, R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacol 1999, 21, 52S–60S. [Google Scholar] [CrossRef] [Green Version]
- Korte, S.M.; Meijer, O.C.; De Kloet, E.R.; Buwalda, B.; Keijser, J.; Sluyter, F.; van Oortmerssen, G.; Bohus, B. Enhanced 5-HT1A receptor expression in forebrain regions of aggressive house mice. Brain Res. 1996, 736, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Boschert, U.; Amara, D.A.; Segu, L.; Hen, R. The mouse 5-hydroxytryptamine 1B receptor is localized predominantly on axon terminals. Neuroscience 1994, 58, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Ramboz, S.; Saudou, F.; Amara, D.A.; Belzung, C.; Segu, L.; Misslin, R.; Buhot, M.-C.; Hen, R. 5-HT1B receptor knock out—Behavioral consequences. Behav. Brain Res. 1995, 73, 305–312. [Google Scholar] [CrossRef]
- Theodoridi, A.; Tsalafouta, A.; Pavlidis, M. Acute exposure to fluoxetine alters aggressive behavior of zebrafish and expression of genes involved in serotonergic system regulation. Front. Neurosci. 2017, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Briffa, M.; Elwood, R.W. Monoamines and decision making during contests in the hermit crab Pagurus bernhardus. Anim. Behav. 2007, 73, 605–612. [Google Scholar] [CrossRef]
- Sun, Y.; Guan, L.; Xiong, J.; Xi, Q.; Zhang, Y. Effects of L-tryptophan-supplemented dietary on growth performance and 5-HT and GABA levels in juvenile Litopenaeus vannamei. Aquac. Int. 2015, 23, 235–251. [Google Scholar] [CrossRef]
- Christie, A.E. Crustacean neuroendocrine systems and their signaling agents. Cell Tissue Res. 2011, 345, 41–67. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, L.U.; Taylor, A.C.; Huntingford, F.A.; Watson, D.G. Agonistic behaviour and biogenic amines in shore crabs Carcinus maenas. J. Exp. Biol. 2000, 203, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, E.; Gherardi, F. Biogenic amines influence aggressiveness in crayfish but not their force or hierarchical rank. Anim. Behav. 2007, 74, 1715–1724. [Google Scholar] [CrossRef] [Green Version]
- Huber, R.; Smith, K.; Delago, A.; Isaksson, K.; Kravitz, E.A. Serotonin and aggressive motivation in crustaceans: Altering the decision to retreat. Proc. Natl. Acad. Sci. USA 1997, 94, 5939–5942. [Google Scholar] [CrossRef] [Green Version]
- Antonsen, B.L.; Paul, D.H. Serotonin and octopamine elicit stereotypical agonistic behaviors in the squat lobster Munida quadrispina (Anomura, Galatheidae). J. Comp. Physiol. A 1997, 181, 501–510. [Google Scholar] [CrossRef]
- Bacqué-Cazenave, J.; Cattaert, D.; Delbecque, J.P.; Fossat, P. Alteration of size perception: Serotonin has opposite effects on the aggressiveness of crayfish confronting either a smaller or a larger rival. J. Exp. Biol. 2018, 221, jeb177840. [Google Scholar] [CrossRef] [Green Version]
- Laranja, J.L.Q., Jr.; Quinitio, E.T.; Catacutan, M.R.; Coloso, R.M. Effects of dietary L-tryptophan on the agonistic behavior, growth and survival of juvenile mud crab Scylla serrata. Aquaculture 2010, 310, 84–90. [Google Scholar] [CrossRef]
- Harlıoğlu, M.M.; Harlıoğlu, A.G.; Mişe Yonar, S.; Çakmak Duran, T. Effects of dietary l-tryptophan on the agonistic behavior, growth, and survival of freshwater crayfish Astacus leptodactylus Eschscholtz. Aquac. Int. 2014, 22, 733–748. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Huang, G.; Xu, M.; Cheng, Y.; Yang, X. Effects of dietary L-tryptophan supplementation on growth performance, food intake, digestive enzyme activity and serotonin (5-HT) levels in juvenile Chinese mitten crab (Eriocheir sinensis). Aquac. Nutr. 2021, 27, 1602–1611. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, X.; Meng, X.; Luo, K.; Luan, S.; Cao, B.; Kong, J. cDNA cloning and expression analysis of a phosphopyruvate hydratase gene from the chinese shrimp Fenneropenaeus chinensis. Fish Shellfish Immunol. 2017, 63, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.; Mangiamele, L. Effects of serotonin and serotonin analogs on posture and agonistic behavior in crayfish. J. Comp. Physiol. A 2001, 187, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.-I.; Tomita, T.; Miyamoto, K. A mighty claw: Pinching force of the coconut crab, the largest terrestrial crustacean. PLoS ONE 2016, 11, e0166108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Zhao, C.; Xiong, Z.; Mu, C.; Xu, S.; Wang, D. Analysis of the agonistic behaviour and behaviour pattern of Portunus trituberculatus. Aquac. Res. 2021, 52, 2233–2242. [Google Scholar] [CrossRef]
- Muramatsu, D.; Koga, T. Fighting with an unreliable weapon: Opponent choice and risk avoidance in fiddler crab contests. Behav. Ecol. Sociobiol. 2016, 70, 713–724. [Google Scholar] [CrossRef] [Green Version]



| Primer | Sequence (5′–3′) | Sequence Information |
|---|---|---|
| m5-HT-1-F | CTACACGCTGCCCAACCTG | RT-PCR Primer |
| m5-HT-1-R | GATGGAGAACATGACATCTGAGAAG | RT-PCR Prime |
| r5-HT-1-F1 | CAAGGGAAGCCATCAGACCCAACGGTGTA | 5-RACE Primer |
| r5-HT-1-F2 | CGTCCCCAGACAAGTCCTCTAACAATGG | 5-RACE Primer |
| r5-HT-1-R1 | GGGGTGGGGGTGAGTGGAGTACC | 3-RACE Primer |
| r5-HT-1-R2 | GCTGGGGTCCTGCAGGATGGGGTTG | 3-RACE Primer |
| 5HT1-F | GAGGTGATGAGTGTTGGTAATACC | Specific primer (for real-time PCR) |
| 5HT1-R | CCTTTCCTTGAGTTTCCAGAACAG | Specific primer (for real-time PCR) |
| GAPDH-F | CTAAGGCTGTAGGCAAGGTCATT | Primers for GAPDH |
| GAPDH-R | CCAGAATGCAAGTCAGGTCAAC | Primers for GAPDH |
| Behavior | Description |
|---|---|
| Move to | One crab approaches the other crab. |
| Move away | One crab moves away from the other crab after aggressive behaviors. |
| Chelipeds display | Chelipeds are held out in front. |
| Push | One crab uses its chelipeds to push the opponent away. |
| Strike | One crab strikes the opponent with its chelipeds. |
| Grasp | One crab uses its chelipeds to pinch the carapace, chelipeds, or legs of the opponent. |
| Climb on | One crab climbs on top of the other crab. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Fu, Y.; Zhai, W.; Wang, X.; Zhou, Y.; Liu, L.; Wang, C. Identification and Characterization of 5-HT Receptor 1 from Scylla paramamosain: The Essential Roles of 5-HT and Its Receptor Gene during Aggressive Behavior in Crab Species. Int. J. Mol. Sci. 2023, 24, 4211. https://doi.org/10.3390/ijms24044211
Huang X, Fu Y, Zhai W, Wang X, Zhou Y, Liu L, Wang C. Identification and Characterization of 5-HT Receptor 1 from Scylla paramamosain: The Essential Roles of 5-HT and Its Receptor Gene during Aggressive Behavior in Crab Species. International Journal of Molecular Sciences. 2023; 24(4):4211. https://doi.org/10.3390/ijms24044211
Chicago/Turabian StyleHuang, Xinlian, Yuanyuan Fu, Wei Zhai, Xiaopeng Wang, Yueyue Zhou, Lei Liu, and Chunlin Wang. 2023. "Identification and Characterization of 5-HT Receptor 1 from Scylla paramamosain: The Essential Roles of 5-HT and Its Receptor Gene during Aggressive Behavior in Crab Species" International Journal of Molecular Sciences 24, no. 4: 4211. https://doi.org/10.3390/ijms24044211






