Functional Roles of Connexins and Gap Junctions in Osteo-Chondral Cellular Components
Abstract
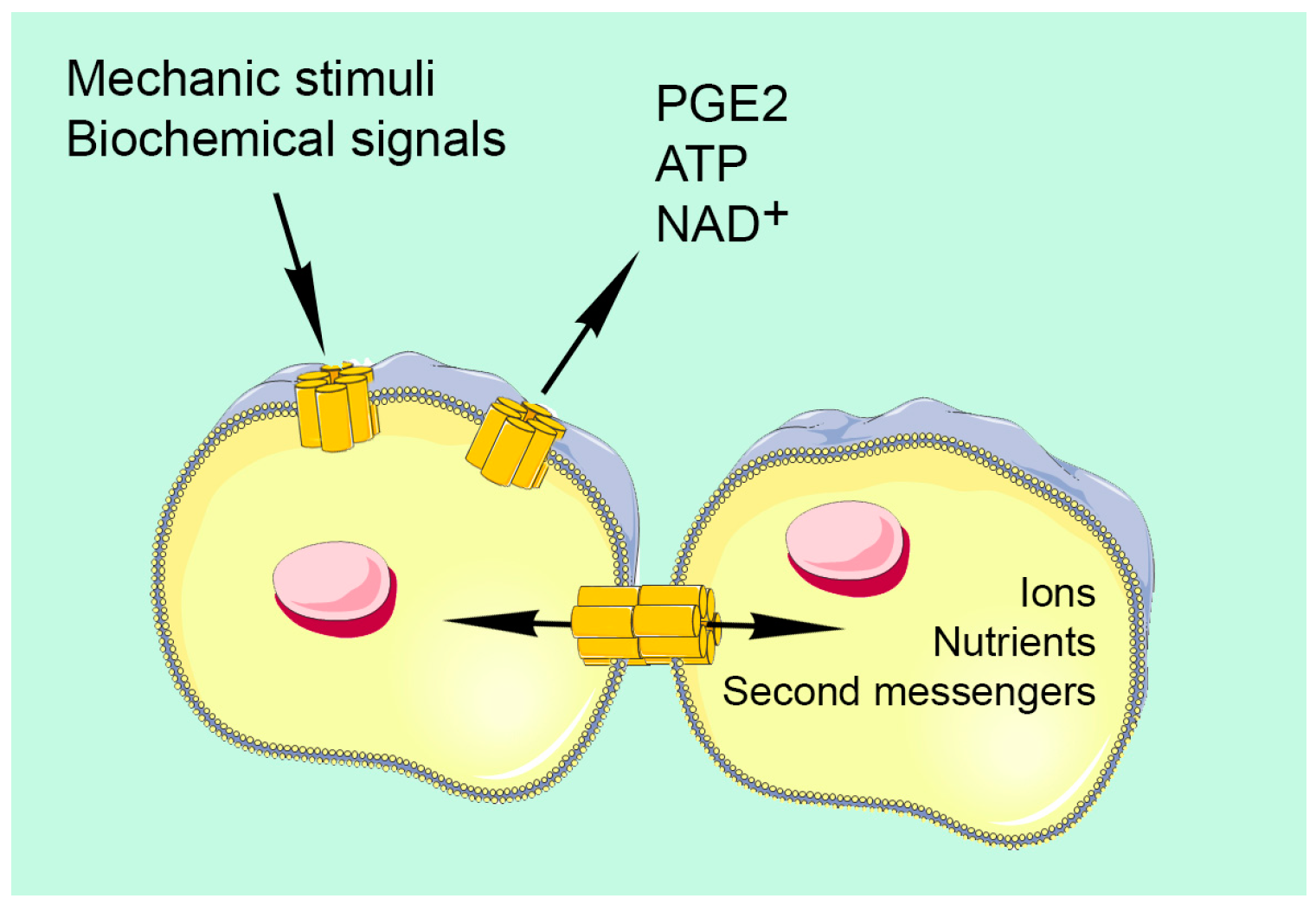
:1. Introduction
2. Bone Tissue
2.1. Osteoblasts
2.2. Osteocytes
2.3. Osteoclasts
2.4. Connexin-Mediated Bone Tissue Plasticity
3. Cartilage
4. Bone Marrow Mesenchymal Stem Cells (BMSC)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Akt serine/threonine kinase |
| BMP | Bone morphogenetic protein |
| BMSCs | Bone marrow stromal cells (103) |
| Cx | Connexin |
| CXCL12 | C-X-C motif chemokine ligand 12 |
| ECM | Extracellular matrix |
| ERK | Extracellular signal-regulated kinase |
| GJs | Gap junctions |
| HC | Hemichannel |
| IL-1 | Interleukin-1 |
| IP3 | Inositol trisphosphate |
| PGE2 | Prostaglandin E2 |
| PKC | Protein kinase C |
| PTH | Parathyroid hormone |
| RANKL | Receptor activator of nuclear factor kappaΒ ligand |
| TGF | Transforming growth factor |
References
- Carpintero-Fernandez, P.; Gago-Fuentes, R.; Wang, H.Z.; Fonseca, E.; Caeiro, J.R.; Valiunas, V.; Brink, P.R.; Mayan, M.D. Intercellular communication via gap junction channels between chondrocytes and bone cells. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2499–2505. [Google Scholar] [CrossRef] [PubMed]
- Sohl, G.; Willecke, K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004, 62, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Magnotti, L.M.; Goodenough, D.A.; Paul, D.L. Functional heterotypic interactions between astrocyte and oligodendrocyte connexins. Glia 2011, 59, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nualart-Marti, A.; Solsona, C.; Fields, R.D. Gap junction communication in myelinating glia. Biochim. Biophys. Acta 2013, 1828, 69–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamoto, T.; Nakamura, T.; Doyle, A.; Ishikawa, M.; de Vega, S.; Fukumoto, S.; Yamada, Y. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J. Biol. Chem. 2010, 285, 18948–18958. [Google Scholar] [CrossRef] [Green Version]
- Bruzzone, R.; Hormuzdi, S.G.; Barbe, M.T.; Herb, A.; Monyer, H. Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13644–13649. [Google Scholar] [CrossRef] [Green Version]
- Barbe, M.T.; Monyer, H.; Bruzzone, R. Cell-cell communication beyond connexins: The pannexin channels. Physiology (Bethesda) 2006, 21, 103–114. [Google Scholar] [CrossRef]
- Willecke, K.; Eiberger, J.; Degen, J.; Eckardt, D.; Romualdi, A.; Guldenagel, M.; Deutsch, U.; Sohl, G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002, 383, 725–737. [Google Scholar] [CrossRef]
- Vicario, N.; Zappala, A.; Calabrese, G.; Gulino, R.; Parenti, C.; Gulisano, M.; Parenti, R. Connexins in the Central Nervous System: Physiological Traits and Neuroprotective Targets. Front. Physiol. 2017, 8, 1060. [Google Scholar] [CrossRef] [Green Version]
- Parenti, R.; Gulisano, M.; Zappala, A.; Cicirata, F. Expression of connexin36 mRNA in adult rodent brain. Neuroreport 2000, 11, 1497–1502. [Google Scholar] [CrossRef]
- Batra, N.; Kar, R.; Jiang, J.X. Gap junctions and hemichannels in signal transmission, function and development of bone. Biochim. Biophys. Acta 2012, 1818, 1909–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyer, E.C.; Berthoud, V.M. Gap junction gene and protein families: Connexins, innexins, and pannexins. Biochim. Biophys. Acta Biomembr. 2018, 1860, 5–8. [Google Scholar] [CrossRef]
- Stains, J.P.; Civitelli, R. Gap junctions in skeletal development and function. Biochim. Biophys. Acta 2005, 1719, 69–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buo, A.M.; Stains, J.P. Gap junctional regulation of signal transduction in bone cells. FEBS Lett. 2014, 588, 1315–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanaporis, G.; Brink, P.R.; Valiunas, V. Gap junction permeability: Selectivity for anionic and cationic probes. Am. J. Physiol. Cell Physiol. 2011, 300, C600–C609. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Costa, R.; Kadakia, J.R.; Atkinson, E.G.; Wallace, J.M.; Plotkin, L.I.; Reginato, R.D. Connexin37 deficiency alters organic bone matrix, cortical bone geometry, and increases Wnt/beta-catenin signaling. Bone 2017, 97, 105–113. [Google Scholar] [CrossRef]
- Pizard, A.; Burgon, P.G.; Paul, D.L.; Bruneau, B.G.; Seidman, C.E.; Seidman, J.G. Connexin 40, a target of transcription factor Tbx5, patterns wrist, digits, and sternum. Mol. Cell Biol. 2005, 25, 5073–5083. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, T.H.; Civitelli, R.; Geist, S.T.; Robertson, A.J.; Hick, E.; Veenstra, R.D.; Wang, H.Z.; Warlow, P.M.; Westphale, E.M.; Laing, J.G.; et al. Connexin43 and connexin45 form gap junctions with different molecular permeabilities in osteoblastic cells. EMBO J. 1994, 13, 744–750. [Google Scholar] [CrossRef]
- Martinez, A.D.; Hayrapetyan, V.; Moreno, A.P.; Beyer, E.C. Connexin43 and connexin45 form heteromeric gap junction channels in which individual components determine permeability and regulation. Circ. Res. 2002, 90, 1100–1107. [Google Scholar] [CrossRef] [Green Version]
- Koval, M.; Harley, J.E.; Hick, E.; Steinberg, T.H. Connexin46 is retained as monomers in a trans-Golgi compartment of osteoblastic cells. J. Cell Biol. 1997, 137, 847–857. [Google Scholar] [CrossRef] [Green Version]
- Sanches, D.S.; Pires, C.G.; Fukumasu, H.; Cogliati, B.; Matsuzaki, P.; Chaible, L.M.; Torres, L.N.; Ferrigno, C.R.; Dagli, M.L. Expression of connexins in normal and neoplastic canine bone tissue. Vet. Pathol. 2009, 46, 846–859. [Google Scholar] [CrossRef]
- Makarenkova, H.; Becker, D.L.; Tickle, C.; Warner, A.E. Fibroblast growth factor 4 directs gap junction expression in the mesenchyme of the vertebrate limb Bud. J. Cell. Biol. 1997, 138, 1125–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiller, P.C.; D’Ippolito, G.; Balkan, W.; Roos, B.A.; Howard, G.A. Gap-junctional communication is required for the maturation process of osteoblastic cells in culture. Bone 2001, 28, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Lecanda, F.; Towler, D.A.; Ziambaras, K.; Cheng, S.L.; Koval, M.; Steinberg, T.H.; Civitelli, R. Gap junctional communication modulates gene expression in osteoblastic cells. Mol. Biol. Cell 1998, 9, 2249–2258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecanda, F.; Warlow, P.M.; Sheikh, S.; Furlan, F.; Steinberg, T.H.; Civitelli, R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J. Cell Biol. 2000, 151, 931–944. [Google Scholar] [CrossRef] [Green Version]
- Inose, H.; Ochi, H.; Kimura, A.; Fujita, K.; Xu, R.; Sato, S.; Iwasaki, M.; Sunamura, S.; Takeuchi, Y.; Fukumoto, S.; et al. A microRNA regulatory mechanism of osteoblast differentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 20794–20799. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Huo, S.; Cen, X.; Pan, X.; Huang, X.; Zhao, Z. circAKT3 positively regulates osteogenic differentiation of human dental pulp stromal cells via miR-206/CX43 axis. Stem Cell Res. Ther. 2020, 11, 531. [Google Scholar] [CrossRef]
- Loiselle, A.E.; Paul, E.M.; Lewis, G.S.; Donahue, H.J. Osteoblast and osteocyte-specific loss of Connexin43 results in delayed bone formation and healing during murine fracture healing. J. Orthop. Res. 2013, 31, 147–154. [Google Scholar] [CrossRef]
- Palumbo, C.; Ferretti, M. The Osteocyte: From “Prisoner” to “Orchestrator”. J. Funct. Morphol. Kinesiol. 2021, 6, 28. [Google Scholar] [CrossRef]
- Riquelme, M.A.; Cardenas, E.R.; Xu, H.; Jiang, J.X. The Role of Connexin Channels in the Response of Mechanical Loading and Unloading of Bone. Int. J. Mol. Sci. 2020, 21, 1146. [Google Scholar] [CrossRef] [Green Version]
- Cherian, P.P.; Siller-Jackson, A.J.; Gu, S.; Wang, X.; Bonewald, L.F.; Sprague, E.; Jiang, J.X. Mechanical strain opens connexin 43 hemichannels in osteocytes: A novel mechanism for the release of prostaglandin. Mol. Biol. Cell 2005, 16, 3100–3106. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, S.A.; Loiselle, A.E.; Zhang, Y.; Donahue, H.J. Connexin 43 deficiency desensitizes bone to the effects of mechanical unloading through modulation of both arms of bone remodeling. Bone 2013, 57, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimston, S.K.; Watkins, M.P.; Brodt, M.D.; Silva, M.J.; Civitelli, R. Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional connexin43 deficient mice. PLoS ONE 2012, 7, e44222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanello, M.; D’Andrea, P. Dual mechanism of intercellular communication in HOBIT osteoblastic cells: A role for gap-junctional hemichannels. J. Bone Miner. Res. 2001, 16, 1465–1476. [Google Scholar] [CrossRef]
- Civitelli, R.; Ziambaras, K.; Warlow, P.M.; Lecanda, F.; Nelson, T.; Harley, J.; Atal, N.; Beyer, E.C.; Steinberg, T.H. Regulation of connexin43 expression and function by prostaglandin E2 (PGE2) and parathyroid hormone (PTH) in osteoblastic cells. J. Cell Biochem. 1998, 68, 8–21. [Google Scholar] [CrossRef]
- Ponsioen, B.; van Zeijl, L.; Moolenaar, W.H.; Jalink, K. Direct measurement of cyclic AMP diffusion and signaling through connexin43 gap junctional channels. Exp. Cell Res. 2007, 313, 415–423. [Google Scholar] [CrossRef]
- Ilvesaro, J.; Vaananen, K.; Tuukkanen, J. Bone-resorbing osteoclasts contain gap-junctional connexin-43. J. Bone Miner. Res. 2000, 15, 919–926. [Google Scholar] [CrossRef]
- Schilling, A.F.; Filke, S.; Lange, T.; Gebauer, M.; Brink, S.; Baranowsky, A.; Zustin, J.; Amling, M. Gap junctional communication in human osteoclasts in vitro and in vivo. J. Cell Mol. Med. 2008, 12, 2497–2504. [Google Scholar] [CrossRef] [Green Version]
- Ransjo, M.; Sahli, J.; Lie, A. Expression of connexin 43 mRNA in microisolated murine osteoclasts and regulation of bone resorption in vitro by gap junction inhibitors. Biochem. Biophys. Res. Commun. 2003, 303, 1179–1185. [Google Scholar] [CrossRef]
- Matemba, S.F.; Lie, A.; Ransjo, M. Regulation of osteoclastogenesis by gap junction communication. J. Cell. Biochem. 2006, 99, 528–537. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Manolagas, S.C.; Bellido, T. Transduction of cell survival signals by connexin-43 hemichannels. J. Biol. Chem. 2002, 277, 8648–8657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, E.J.; Lee, S.Y.; Jung, J.C.; Bang, O.S.; Kang, S.S. TGF-beta3 inhibits chondrogenesis of cultured chick leg bud mesenchymal cells via downregulation of connexin 43 and integrin beta4. J. Cell. Physiol. 2008, 214, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Findlay, D.M.; Kuliwaba, J.S. Bone-cartilage crosstalk: A conversation for understanding osteoarthritis. Bone Res. 2016, 4, 16028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donsante, S.; Palmisano, B.; Serafini, M.; Robey, P.G.; Corsi, A.; Riminucci, M. From Stem Cells to Bone-Forming Cells. Int. J. Mol. Sci. 2021, 22, 3989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Green, C.; Stott, N.S. Bone morphogenetic protein-2 modulation of chondrogenic differentiation in vitro involves gap junction-mediated intercellular communication. J. Cell. Physiol. 2002, 193, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Gago-Fuentes, R.; Carpintero-Fernandez, P.; Goldring, M.B.; Brink, P.R.; Mayan, M.D.; Blanco, F.J. Biochemical evidence for gap junctions and Cx43 expression in immortalized human chondrocyte cell line: A potential model in the study of cell communication in human chondrocytes. Osteoarthr. Cartil. 2014, 22, 586–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayan, M.D.; Gago-Fuentes, R.; Carpintero-Fernandez, P.; Fernandez-Puente, P.; Filgueira-Fernandez, P.; Goyanes, N.; Valiunas, V.; Brink, P.R.; Goldberg, G.S.; Blanco, F.J. Articular chondrocyte network mediated by gap junctions: Role in metabolic cartilage homeostasis. Ann. Rheum. Dis. 2015, 74, 275–284. [Google Scholar] [CrossRef] [Green Version]
- D’Andrea, P.; Calabrese, A.; Capozzi, I.; Grandolfo, M.; Tonon, R.; Vittur, F. Intercellular Ca2+ waves in mechanically stimulated articular chondrocytes. Biorheology 2000, 37, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Guilak, F.; Zell, R.A.; Erickson, G.R.; Grande, D.A.; Rubin, C.T.; McLeod, K.J.; Donahue, H.J. Mechanically induced calcium waves in articular chondrocytes are inhibited by gadolinium and amiloride. J. Orthop. Res. 1999, 17, 421–429. [Google Scholar] [CrossRef]
- Tonon, R.; D’Andrea, P. Interleukin-1beta increases the functional expression of connexin 43 in articular chondrocytes: Evidence for a Ca2+-dependent mechanism. J. Bone Miner. Res. 2000, 15, 1669–1677. [Google Scholar] [CrossRef]
- Tonon, R.; D’Andrea, P. The functional expression of connexin 43 in articular chondrocytes is increased by interleukin 1beta: Evidence for a Ca2+-dependent mechanism. Biorheology 2002, 39, 153–160. [Google Scholar] [PubMed]
- Varela-Eirin, M.; Varela-Vazquez, A.; Guitian-Caamano, A.; Paino, C.L.; Mato, V.; Largo, R.; Aasen, T.; Tabernero, A.; Fonseca, E.; Kandouz, M.; et al. Targeting of chondrocyte plasticity via connexin43 modulation attenuates cellular senescence and fosters a pro-regenerative environment in osteoarthritis. Cell Death Dis. 2018, 9, 1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela-Eirin, M.; Loureiro, J.; Fonseca, E.; Corrochano, S.; Caeiro, J.R.; Collado, M.; Mayan, M.D. Cartilage regeneration and ageing: Targeting cellular plasticity in osteoarthritis. Ageing Res. Rev. 2018, 42, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.X.; Zheng, G.Z.; Chang, B.; Chen, R.C.; Zhang, Q.H.; Xie, P.; Xie, D.; Yu, G.Y.; Hu, Q.X.; Liu, D.Z.; et al. Connexin 43 Modulates Osteogenic Differentiation of Bone Marrow Stromal Cells Through GSK-3beta/Beta-Catenin Signaling Pathways. Cell. Physiol. Biochem. 2018, 47, 161–175. [Google Scholar] [CrossRef]
- Wagner, A.S.; Glenske, K.; Wolf, V.; Fietz, D.; Mazurek, S.; Hanke, T.; Moritz, A.; Arnhold, S.; Wenisch, S. Osteogenic differentiation capacity of human mesenchymal stromal cells in response to extracellular calcium with special regard to connexin 43. Ann. Anat. 2017, 209, 18–24. [Google Scholar] [CrossRef]
- Talbot, J.; Brion, R.; Lamora, A.; Mullard, M.; Morice, S.; Heymann, D.; Verrecchia, F. Connexin43 intercellular communication drives the early differentiation of human bone marrow stromal cells into osteoblasts. J. Cell. Physiol. 2018, 233, 946–957. [Google Scholar] [CrossRef]
- Rossello, R.A.; Wang, Z.; Kizana, E.; Krebsbach, P.H.; Kohn, D.H. Connexin 43 as a signaling platform for increasing the volume and spatial distribution of regenerated tissue. Proc. Natl. Acad. Sci. USA 2009, 106, 13219–13224. [Google Scholar] [CrossRef] [Green Version]
- Li, X.D.; Chang, B.; Chen, B.; Liu, Z.Y.; Liu, D.X.; Wang, J.S.; Hou, G.Q.; Huang, D.Y.; Du, S.X. Panax notoginseng saponins potentiate osteogenesis of bone marrow stromal cells by modulating gap junction intercellular communication activities. Cell. Physiol. Biochem. 2010, 26, 1081–1092. [Google Scholar] [CrossRef]
- Martin, T.; Gooi, J.H.; Sims, N.A. Molecular mechanisms in coupling of bone formation to resorption. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 73–88. [Google Scholar] [CrossRef]
- Lo Furno, D.; Graziano, A.C.; Avola, R.; Giuffrida, R.; Perciavalle, V.; Bonina, F.; Mannino, G.; Cardile, V. A Citrus bergamia Extract Decreases Adipogenesis and Increases Lipolysis by Modulating PPAR Levels in Mesenchymal Stem Cells from Human Adipose Tissue. PPAR Res. 2016, 2016, 4563815. [Google Scholar] [CrossRef] [Green Version]
- Mannino, G.; Vicario, N.; Parenti, R.; Giuffrida, R.; Lo Furno, D. Connexin expression decreases during adipogenic differentiation of human adipose-derived mesenchymal stem cells. Mol. Biol. Rep. 2020, 47, 9951–9958. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.K.; Miyake, T. The membranous skeleton: The role of cell condensations in vertebrate skeletogenesis. Anat. Embryol. 1992, 186, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.K.; Miyake, T. Divide, accumulate, differentiate: Cell condensation in skeletal development revisited. Int. J. Dev. Biol. 1995, 39, 881–893. [Google Scholar]
- Bonewald, L.F. Osteocytes as dynamic multifunctional cells. Ann. NY Acad. Sci. 2007, 1116, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Ilvesaro, J.; Tavi, P.; Tuukkanen, J. Connexin-mimetic peptide Gap 27 decreases osteoclastic activity. BMC Musculoskelet Disord 2001, 2, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosendaal, M.; Green, C.R.; Rahman, A.; Morgan, D. Up-regulation of the connexin43+ gap junction network in haemopoietic tissue before the growth of stem cells. J. Cell. Sci. 1994, 107, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Green, C.R.; Bowles, L.; Crawley, A.; Tickle, C. Expression of the connexin43 gap junctional protein in tissues at the tip of the chick limb bud is related to the epithelial-mesenchymal interactions that mediate morphogenesis. Dev. Biol. 1994, 161, 12–21. [Google Scholar] [CrossRef]
- Becker, D.L.; McGonnell, I.; Makarenkova, H.P.; Patel, K.; Tickle, C.; Lorimer, J.; Green, C.R. Roles for alpha 1 connexin in morphogenesis of chick embryos revealed using a novel antisense approach. Dev. Genet. 1999, 24, 33–42. [Google Scholar] [CrossRef]
- McGonnell, I.M.; Green, C.R.; Tickle, C.; Becker, D.L. Connexin43 gap junction protein plays an essential role in morphogenesis of the embryonic chick face. Dev. Dyn. 2001, 222, 420–438. [Google Scholar] [CrossRef]
- Litzenberger, J.B.; Kim, J.B.; Tummala, P.; Jacobs, C.R. Beta1 integrins mediate mechanosensitive signaling pathways in osteocytes. Calcif. Tissue Int. 2010, 86, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Geoghegan, I.P.; Hoey, D.A.; McNamara, L.M. Integrins in Osteocyte Biology and Mechanotransduction. Curr. Osteoporos. Rep. 2019, 17, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Genetos, D.C.; Kephart, C.J.; Zhang, Y.; Yellowley, C.E.; Donahue, H.J. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J. Cell. Physiol. 2007, 212, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein-Nulend, J.; Semeins, C.M.; Ajubi, N.E.; Nijweide, P.J.; Burger, E.H. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts--correlation with prostaglandin upregulation. Biochem. Biophys. Res. Commun. 1995, 217, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Huo, B.; Lu, X.L.; Hung, C.T.; Costa, K.D.; Xu, Q.; Whitesides, G.M.; Guo, X.E. Fluid Flow Induced Calcium Response in Bone Cell Network. Cell. Mol. Bioeng. 2008, 1, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.L.; Huo, B.; Park, M.; Guo, X.E. Calcium response in osteocytic networks under steady and oscillatory fluid flow. Bone 2012, 51, 466–473. [Google Scholar] [CrossRef] [Green Version]
- Liedert, A.; Kaspar, D.; Blakytny, R.; Claes, L.; Ignatius, A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem. Biophys. Res. Commun. 2006, 349, 1–5. [Google Scholar] [CrossRef]
- Jahani, M.; Genever, P.G.; Patton, R.J.; Ahwal, F.; Fagan, M.J. The effect of osteocyte apoptosis on signalling in the osteocyte and bone lining cell network: A computer simulation. J. Biomech. 2012, 45, 2876–2883. [Google Scholar] [CrossRef]
- Mayan, M.D.; Carpintero-Fernandez, P.; Gago-Fuentes, R.; Martinez-de-Ilarduya, O.; Wang, H.Z.; Valiunas, V.; Brink, P.; Blanco, F.J. Human articular chondrocytes express multiple gap junction proteins: Differential expression of connexins in normal and osteoarthritic cartilage. Am. J. Pathol. 2013, 182, 1337–1346. [Google Scholar] [CrossRef] [Green Version]
- Schwab, W.; Hofer, A.; Kasper, M. Immunohistochemical distribution of connexin 43 in the cartilage of rats and mice. Histochem. J. 1998, 30, 413–419. [Google Scholar] [CrossRef]
- Zhang, M.; Pritchard, M.R.; Middleton, F.A.; Horton, J.A.; Damron, T.A. Microarray analysis of perichondral and reserve growth plate zones identifies differential gene expressions and signal pathways. Bone 2008, 43, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Donahue, H.J.; Guilak, F.; Vander Molen, M.A.; McLeod, K.J.; Rubin, C.T.; Grande, D.A.; Brink, P.R. Chondrocytes isolated from mature articular cartilage retain the capacity to form functional gap junctions. J. Bone Miner. Res. 1995, 10, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.S.; Rattner, J.B.; Matyas, J.R. Communication between paired chondrocytes in the superficial zone of articular cartilage. J. Anat. 2004, 205, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Knight, M.M. Cyclic loading opens hemichannels to release ATP as part of a chondrocyte mechanotransduction pathway. J. Orthop. Res. 2010, 28, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.M.; McGlashan, S.R.; Garcia, M.; Jensen, C.G.; Poole, C.A. Articular chondrocytes express connexin 43 hemichannels and P2 receptors—A putative mechanoreceptor complex involving the primary cilium? J. Anat. 2009, 214, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Larranaga-Vera, A.; Marco-Bonilla, M.; Largo, R.; Herrero-Beaumont, G.; Mediero, A.; Cronstein, B. ATP transporters in the joints. Purinergic. Signal. 2021, 17, 591–605. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, P.; Vittur, F. Propagation of intercellular Ca2+ waves in mechanically stimulated articular chondrocytes. FEBS Lett. 1997, 400, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, P.; Calabrese, A.; Grandolfo, M. Intercellular calcium signalling between chondrocytes and synovial cells in co-culture. Biochem. J. 1998, 329, 681–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.L.; Meng, H.Y.; Wang, Y.C.; Peng, J.; Guo, Q.Y.; Wang, A.Y.; Lu, S.B. Bone-cartilage interface crosstalk in osteoarthritis: Potential pathways and future therapeutic strategies. Osteoarthr. Cartil. 2014, 22, 1077–1089. [Google Scholar] [CrossRef] [Green Version]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [CrossRef] [Green Version]
- Marino, A.A.; Waddell, D.D.; Kolomytkin, O.V.; Meek, W.D.; Wolf, R.; Sadasivan, K.K.; Albright, J.A. Increased intercellular communication through gap junctions may contribute to progression of osteoarthritis. Clin. Orthop. Relat. Res. 2004, 422, 224–232. [Google Scholar] [CrossRef]
- Kolomytkin, O.V.; Marino, A.A.; Waddell, D.D.; Mathis, J.M.; Wolf, R.E.; Sadasivan, K.K.; Albright, J.A. IL-1beta-induced production of metalloproteinases by synovial cells depends on gap junction conductance. Am. J. Physiol. Cell Physiol. 2002, 282, C1254–C1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannino, G.; Russo, C.; Maugeri, G.; Musumeci, G.; Vicario, N.; Tibullo, D.; Giuffrida, R.; Parenti, R.; Lo Furno, D. Adult stem cell niches for tissue homeostasis. J. Cell. Physiol. 2022, 237, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Godavarthy, P.S.; Krause, D.S. The bone marrow microenvironment in health and disease at a glance. J. Cell. Sci. 2018, 131, jcs201707. [Google Scholar] [CrossRef] [Green Version]
- Dorshkind, K.; Green, L.; Godwin, A.; Fletcher, W.H. Connexin-43-type gap junctions mediate communication between bone marrow stromal cells. Blood 1993, 82, 38–45. [Google Scholar] [CrossRef]
- Schajnovitz, A.; Itkin, T.; D’Uva, G.; Kalinkovich, A.; Golan, K.; Ludin, A.; Cohen, D.; Shulman, Z.; Avigdor, A.; Nagler, A.; et al. CXCL12 secretion by bone marrow stromal cells is dependent on cell contact and mediated by connexin-43 and connexin-45 gap junctions. Nat. Immunol. 2011, 12, 391–398. [Google Scholar] [CrossRef]
- Bodi, E.; Hurtado, S.P.; Carvalho, M.A.; Borojevic, R.; Carvalho, A.C. Gap junctions in hematopoietic stroma control proliferation and differentiation of blood cell precursors. An. Acad. Bras. Cienc. 2004, 76, 743–756. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, T.; Nagasawa, T. Bone marrow niches for hematopoietic stem cells and immune cells. Inflamm. Allergy Drug Targets 2012, 11, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Arthur, A.; Gronthos, S. Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue. Int. J. Mol. Sci. 2020, 21, 9759. [Google Scholar] [CrossRef]
- Calabrese, G.; Giuffrida, R.; Lo Furno, D.; Parrinello, N.L.; Forte, S.; Gulino, R.; Colarossi, C.; Schinocca, L.R.; Giuffrida, R.; Cardile, V.; et al. Potential Effect of CD271 on Human Mesenchymal Stromal Cell Proliferation and Differentiation. Int. J. Mol. Sci. 2015, 16, 15609–15624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, G.; Giuffrida, R.; Fabbi, C.; Figallo, E.; Lo Furno, D.; Gulino, R.; Colarossi, C.; Fullone, F.; Giuffrida, R.; Parenti, R.; et al. Collagen-Hydroxyapatite Scaffolds Induce Human Adipose Derived Stem Cells Osteogenic Differentiation In Vitro. PLoS ONE 2016, 11, e0151181. [Google Scholar] [CrossRef] [Green Version]
- Lo Furno, D.; Tamburino, S.; Mannino, G.; Gili, E.; Lombardo, G.; Tarico, M.S.; Vancheri, C.; Giuffrida, R.; Perrotta, R.E. Nanofat 2.0: Experimental evidence for a fat grafting rich in mesenchymal stem cells. Physiol. Res. 2017, 66, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Gennuso, F.; Giurdanella, G.; Conti, F.; Drago, F.; Salomone, S.; Furno, D.L.; Bucolo, C.; Giuffrida, R. Pericyte-like differentiation of human adipose-derived mesenchymal stem cells: An in vitro study. World J. Stem Cells 2020, 12, 1152–1170. [Google Scholar] [CrossRef]
- Mannino, G.; Longo, A.; Gennuso, F.; Anfuso, C.D.; Lupo, G.; Giurdanella, G.; Giuffrida, R.; Lo Furno, D. Effects of High Glucose Concentration on Pericyte-Like Differentiated Human Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 4604. [Google Scholar] [CrossRef]
- Lupo, G.; Agafonova, A.; Cosentino, A.; Giurdanella, G.; Mannino, G.; Lo Furno, D.; Romano, I.R.; Giuffrida, R.; D’Angeli, F.; Anfuso, C.D. Protective Effects of Human Pericyte-like Adipose-Derived Mesenchymal Stem Cells on Human Retinal Endothelial Cells in an In Vitro Model of Diabetic Retinopathy: Evidence for Autologous Cell Therapy. Int. J. Mol. Sci. 2023, 24, 913. [Google Scholar] [CrossRef]
- Lo Furno, D.; Pellitteri, R.; Graziano, A.C.; Giuffrida, R.; Vancheri, C.; Gili, E.; Cardile, V. Differentiation of human adipose stem cells into neural phenotype by neuroblastoma- or olfactory ensheathing cells-conditioned medium. J. Cell. Physiol. 2013, 228, 2109–2118. [Google Scholar] [CrossRef]
- Lo Furno, D.; Mannino, G.; Giuffrida, R.; Gili, E.; Vancheri, C.; Tarico, M.S.; Perrotta, R.E.; Pellitteri, R. Neural differentiation of human adipose-derived mesenchymal stem cells induced by glial cell conditioned media. J. Cell. Physiol. 2018, 233, 7091–7100. [Google Scholar] [CrossRef]
- Lo Furno, D.; Mannino, G.; Cardile, V.; Parenti, R.; Giuffrida, R. Potential Therapeutic Applications of Adipose-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2016, 25, 1615–1628. [Google Scholar] [CrossRef]
- Lo Furno, D.; Mannino, G.; Giuffrida, R. Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases. J. Cell. Physiol. 2018, 233, 3982–3999. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Russo, C.; Longo, A.; Anfuso, C.D.; Lupo, G.; Lo Furno, D.; Giuffrida, R.; Giurdanella, G. Potential therapeutic applications of mesenchymal stem cells for the treatment of eye diseases. World J. Stem Cells 2021, 13, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Lo Furno, D.; Mannino, G.; Pellitteri, R.; Zappala, A.; Parenti, R.; Gili, E.; Vancheri, C.; Giuffrida, R. Conditioned Media From Glial Cells Promote a Neural-Like Connexin Expression in Human Adipose-Derived Mesenchymal Stem Cells. Front. Physiol. 2018, 9, 1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Connexin | Human Gene | Mouse Gene | Cells | Functions | References |
|---|---|---|---|---|---|
| Cx26 | GJB2 | Gjb2 | Bone cells | It restricts the diffusion of anionic solutes and facilitates the transfer of positively charged molecules | [15] |
| Cx32 | GJB1 | Gjb1 | Chondrocytes, mesenchyme cells | Normal limb bud development | [22] |
| Cx37 | GJA4 | Gja4 | Bone cells | Osteoclast differentiation | [16] |
| Cx40 | GJA5 | Gja5 | Bone cells, chondrocytes | It participates in the development of the sternum and forelimb bones, although its expression in adults has not yet been demonstrated | [17] |
| Cx43 | GJA1 | Gja1 | Bone cells, chondrocytes | It is permeable to relatively large molecules, with a weak preference for negatively charged particles | [15] |
| Osteoblast differentiation and extracellular matrix mineralization | [23,24,25,26,27,28] | ||||
| Interconnections between osteocytes and between osteocytes with osteoblasts and osteoclasts, arranging a “functional syncytium” within bone tissue | [11,29] | ||||
| Transduction of mechanical into biochemical signals that are propagated through bone tissue by the syncytial network | [30,31,32,33] | ||||
| Cell response to biochemical signals from the external medium | [28,34,35,36] | ||||
| Osteoclastogenesis. Osteoclast reabsorption activity | [16,37,38,39,40] | ||||
| Anti-apoptotic effects | [13,14,41,42] | ||||
| Interactions between articular cartilage and subchondral bone | [43] | ||||
| Chondrocyte differentiation | [42,44,45] | ||||
| Arrangement of the articular chondrocyte network | [1,46,47] | ||||
| Intercellular propagation of Ca2+ waves following mechanical stimulation of articular chondrocytes | [48,49] | ||||
| Implicated in the etiology of osteoarthritis | [13,50,51,52,53] | ||||
| Bone marrow stromal cells | Osteogenic differentiation | [54,55,56,57,58] | |||
| Cx45 | GJC1 | Gjc1 | Osteoblasts, chondrocytes | Because of its small pore, it is mainly responsible for intercellular electrical coupling | [18,19] |
| Cx46 | GJA3 | Gja3 | Osteoblasts, chondrocytes | Localized in the cytoplasmic trans Golgi network, it cannot participate in channels at the plasma membrane level. It is possibly involved in osteoblast secretory pathways | [20,21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zappalà, A.; Romano, I.R.; D’Angeli, F.; Musumeci, G.; Lo Furno, D.; Giuffrida, R.; Mannino, G. Functional Roles of Connexins and Gap Junctions in Osteo-Chondral Cellular Components. Int. J. Mol. Sci. 2023, 24, 4156. https://doi.org/10.3390/ijms24044156
Zappalà A, Romano IR, D’Angeli F, Musumeci G, Lo Furno D, Giuffrida R, Mannino G. Functional Roles of Connexins and Gap Junctions in Osteo-Chondral Cellular Components. International Journal of Molecular Sciences. 2023; 24(4):4156. https://doi.org/10.3390/ijms24044156
Chicago/Turabian StyleZappalà, Agata, Ivana Roberta Romano, Floriana D’Angeli, Giuseppe Musumeci, Debora Lo Furno, Rosario Giuffrida, and Giuliana Mannino. 2023. "Functional Roles of Connexins and Gap Junctions in Osteo-Chondral Cellular Components" International Journal of Molecular Sciences 24, no. 4: 4156. https://doi.org/10.3390/ijms24044156






