Novel Silver Complexes Based on Phosphanes and Ester Derivatives of Bis(pyrazol-1-yl)acetate Ligands Targeting TrxR: New Promising Chemotherapeutic Tools Relevant to SCLC Management
Abstract
:1. Introduction
2. Results and Discussion
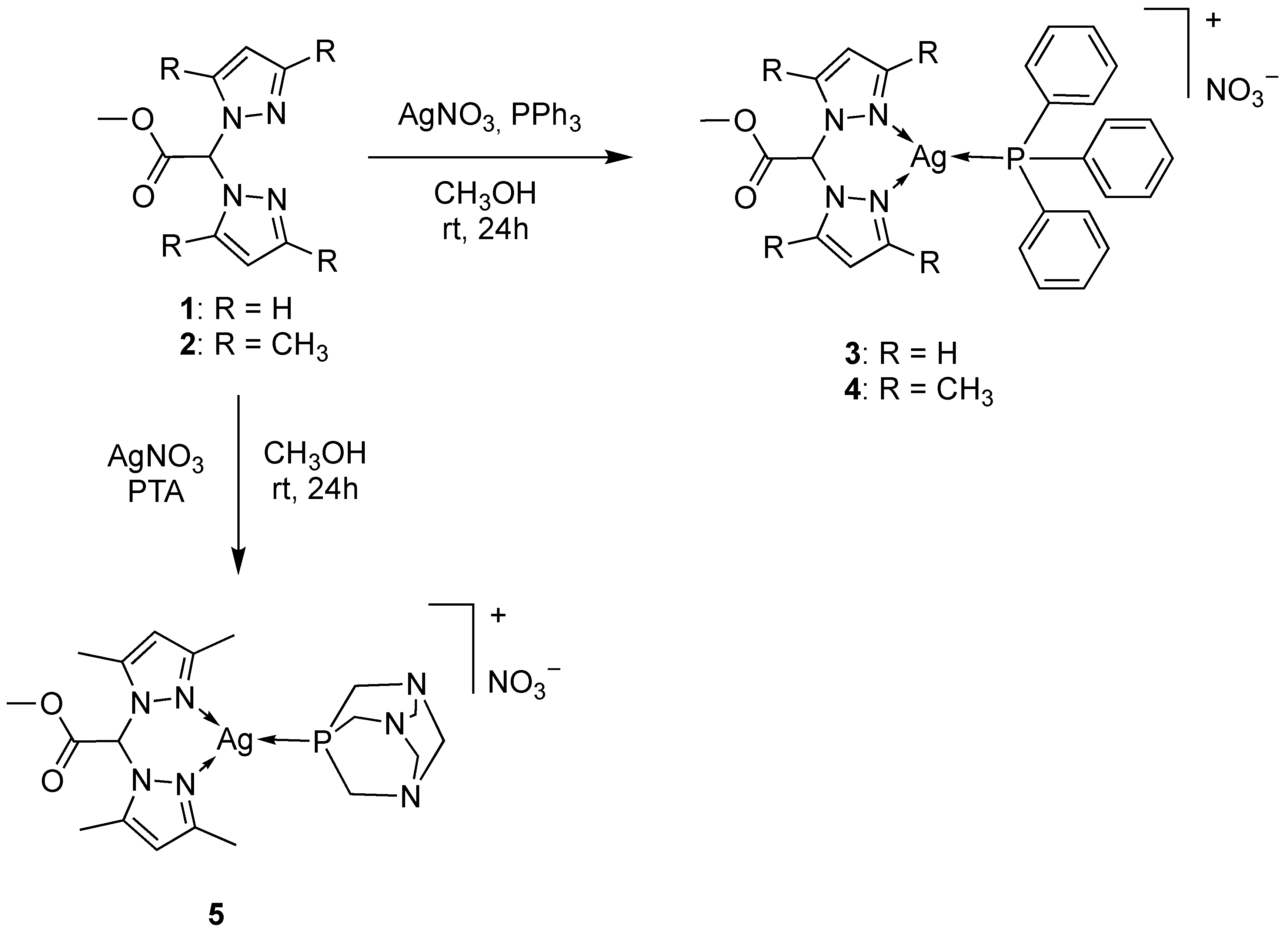
2.1. Synthesis and Characterization
2.2. Cytotoxicity Studies
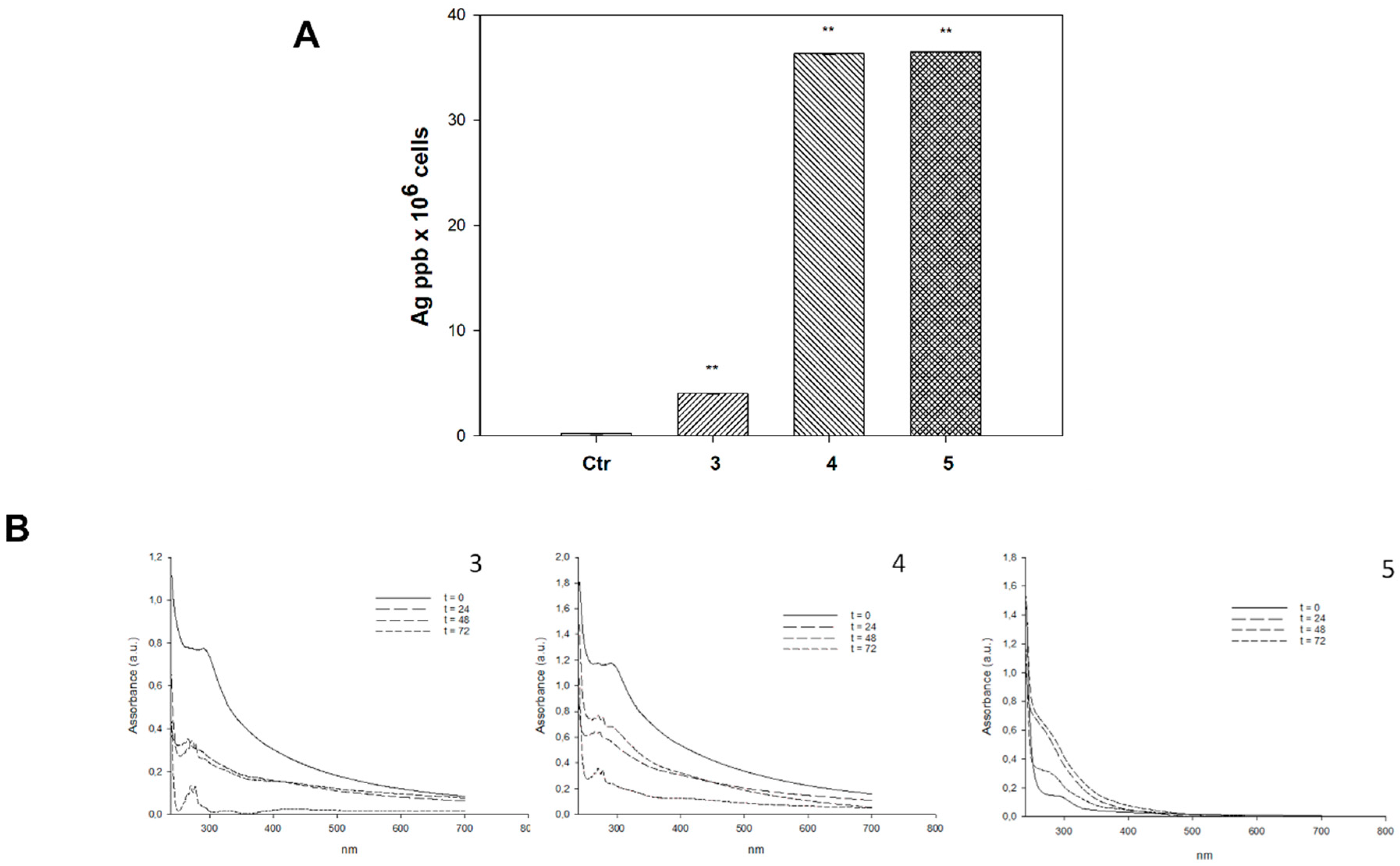
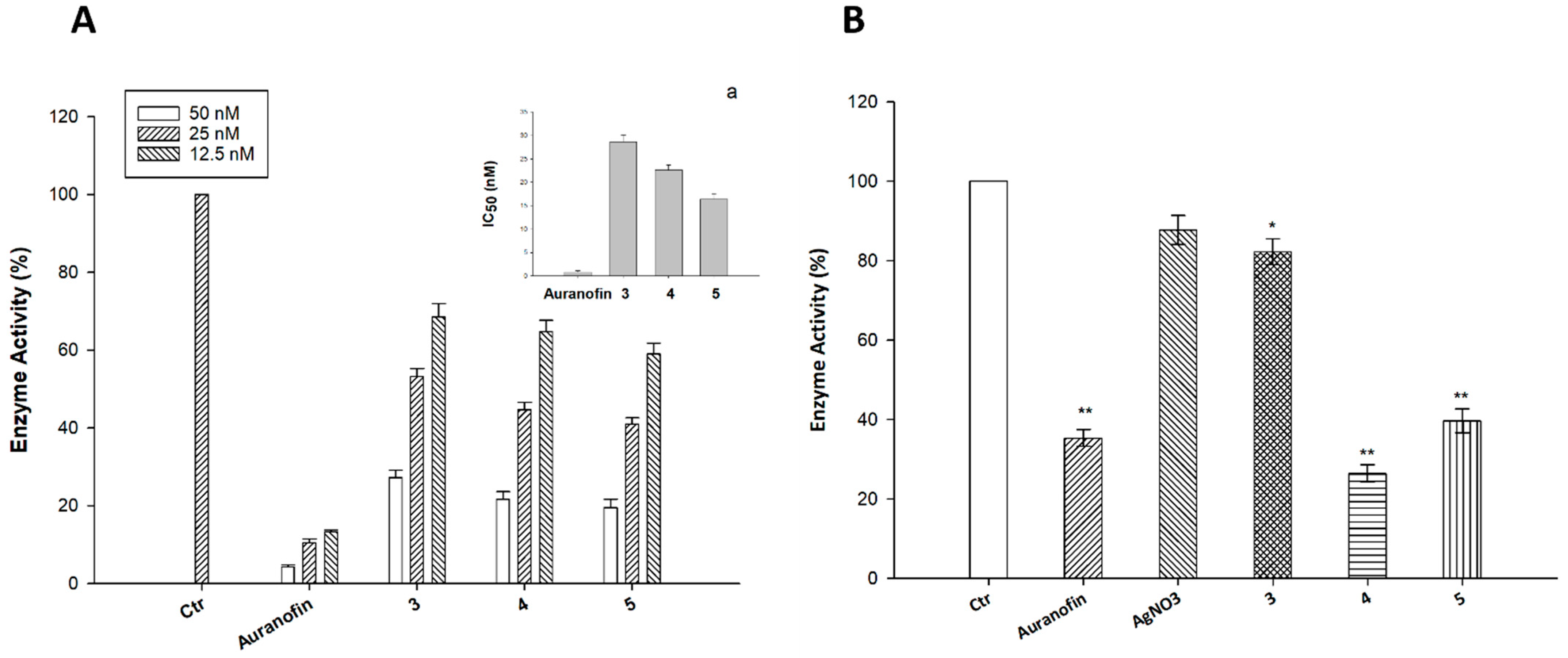
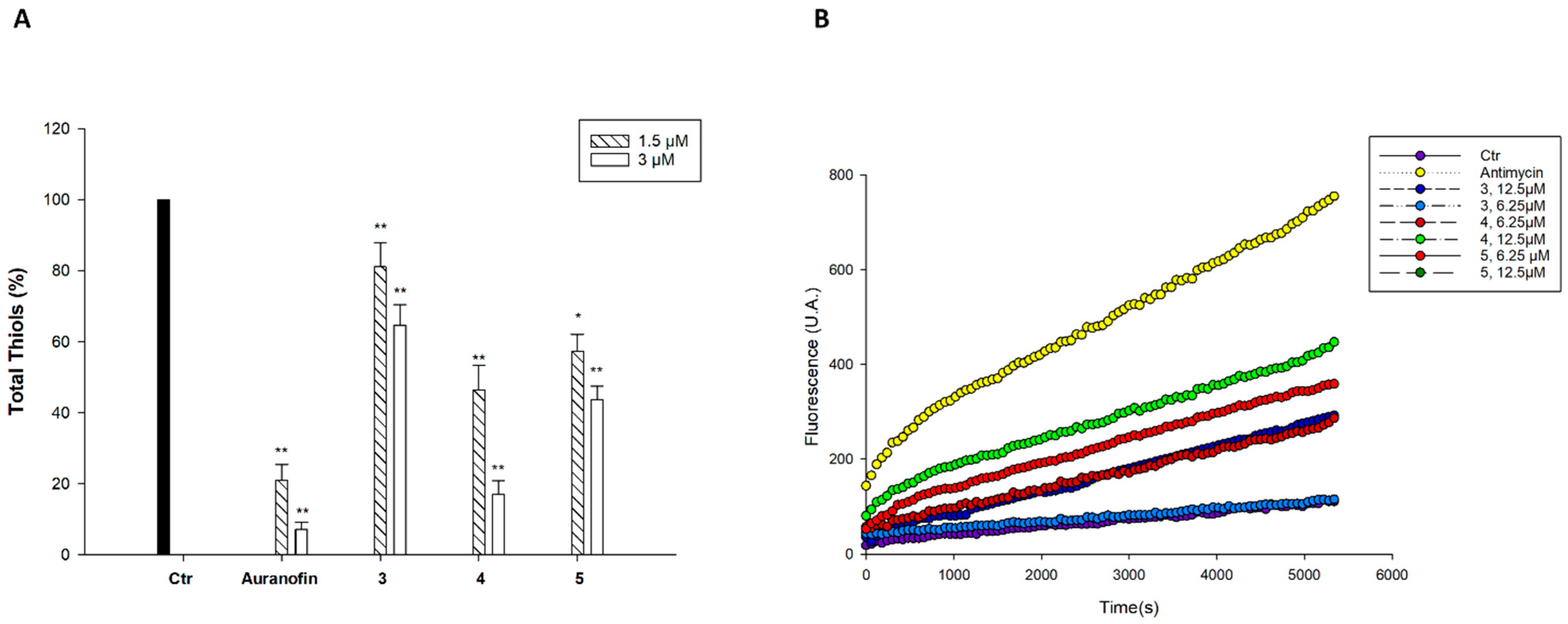
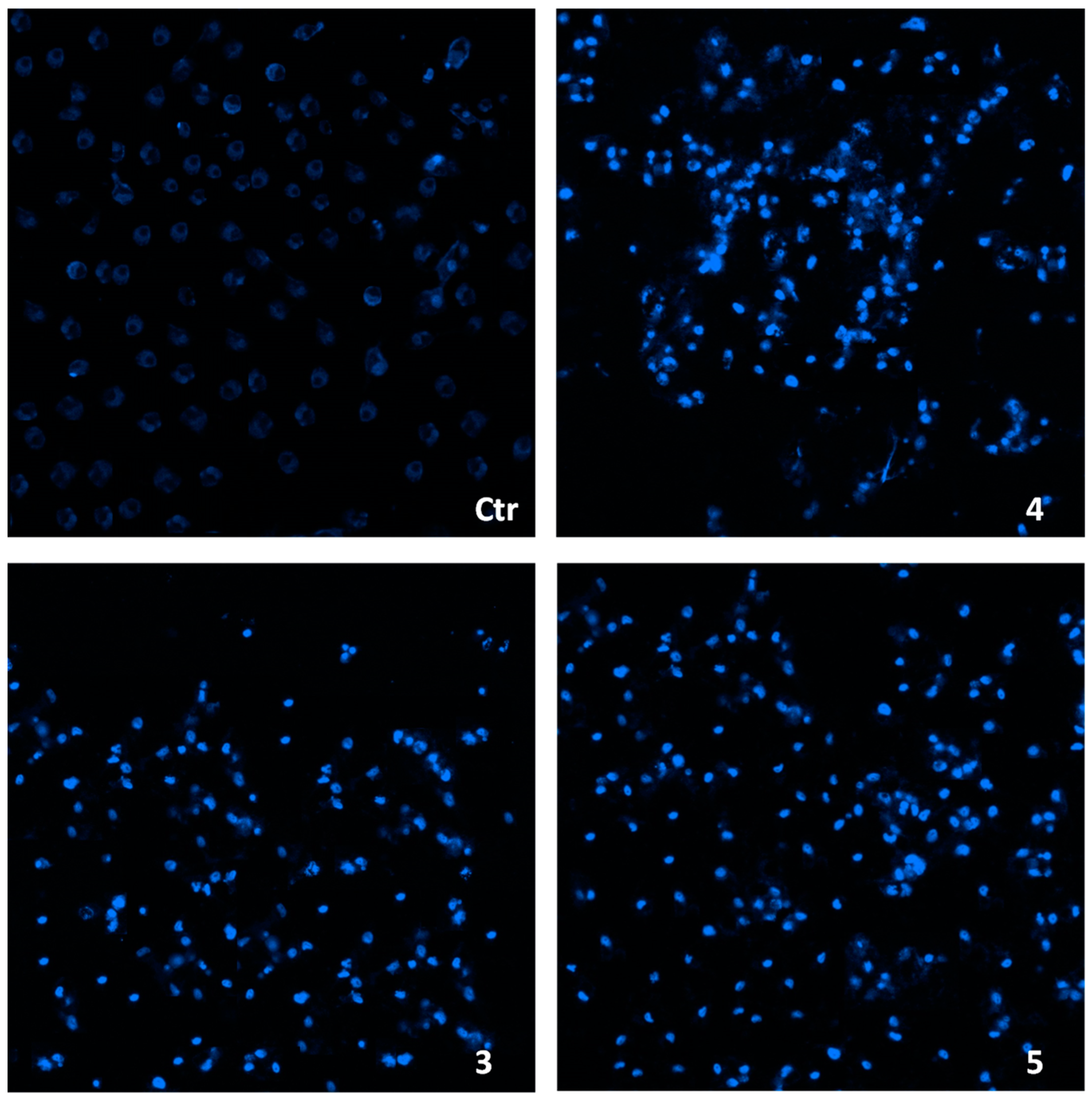
2.3. Cellular Uptake and Mechanistic Studies
3. Materials and Methods
3.1. Chemistry
3.1.1. Materials and General Methods
3.1.2. Synthesis of [Ag(PPh3)(LOMe)]NO3 (3)
3.1.3. Synthesis of [Ag(PPh3)(L2OMe)]NO3 (4)
3.1.4. Synthesis of [Ag(PTA)(L2OMe)]NO3 (5)
3.1.5. Stability Studies in DMSO/RPMI
3.2. Experiments with Cultured Human Cancer Cells
3.2.1. Cell Cultures
3.2.2. MTT Assay
3.2.3. Spheroid Cultures
3.2.4. Acid Phosphatase (APH) Assay
3.2.5. Cellular Uptake
3.2.6. TrxR Inhibition
3.2.7. Quantification of Thiols
3.2.8. Reactive Oxygen Species Production
3.2.9. Mitochondrial Membrane Potential (ΔΨ)
3.2.10. TEM Analysis
3.2.11. Cell Death Induction
3.2.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.; Bravin, A.; Cavaletti, G.; Pellei, M.; Santini, C. The combined therapeutical effect of metal-based drugs and radiation therapy: The present status of research. Curr. Med. Chem. 2014, 21, 2237–2265. [Google Scholar] [CrossRef] [PubMed]
- Barry, N.P.E.; Sadler, P.J. Exploration of the medical periodic table: Towards new targets. Chem. Commun. 2013, 49, 5106–5131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medici, S.; Peana, M.; Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Remelli, M.; Zoroddu, M.A. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 2016, 327–328, 349–359. [Google Scholar] [CrossRef]
- Porchia, M.; Pellei, M.; Marinelli, M.; Tisato, F.; Del Bello, F.; Santini, C. New insights in Au-NHCs complexes as anticancer agents. Eur. J. Med. Chem. 2018, 146, 709–746. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in copper complexes as anticancer agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef]
- Nobili, S.; Mini, E.; Landini, I.; Gabbiani, C.; Casini, A.; Messori, L. Gold compounds as anticancer agents: Chemistry, cellular pharmacology, and preclinical studies. Med. Res. Rev. 2010, 30, 550–580. [Google Scholar] [CrossRef]
- Tisato, F.; Marzano, C.; Porchia, M.; Pellei, M.; Santini, C. Copper in diseases and treatments, and copper-based anticancer strategies. Med. Res. Rev. 2010, 30, 708–749. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical uses of silver: History, myths, and scientific evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble metals in medicine: Latest advances. Coord. Chem. Rev. 2015, 284 (Suppl. C), 329–350. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Paralikar, P.; Gupta, I.; Medici, S.; Santos, C.A. Recent advances in use of silver nanoparticles as antimalarial agents. Int. J. Pharm. 2017, 526, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Luan, S.; Yin, Z.; He, M.; He, C.; Yin, L.; Zou, Y.; Yuan, Z.; Li, L.; Song, X.; et al. Recent advances in the medical use of silver complex. Eur. J. Med. Chem. 2018, 157, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, M.; Santini, C.; Pellei, M. Recent advances in medicinal applications of coinage-metal (Cu and Ag) N-Heterocyclic carbene complexes. Curr. Trends Med. Chem. 2016, 16, 2995–3017. [Google Scholar] [CrossRef] [PubMed]
- McKeage, M.J.; Papathanasiou, P.; Salem, G.; Sjaarda, A.; Swiegers, G.F.; Waring, P.; Wild, S.B. Antitumor activity of gold(I), silver(I) and copper(I) complexes containing chiral tertiary phosphines. Met. Based Drugs 1998, 5, 217–223. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Papini, G.; Morresi, B.; Galassi, R.; Ricci, S.; Tisato, F.; Porchia, M.; Rigobello, M.P.; Gandin, V.; et al. In vitro antitumour activity of water soluble Cu(I), Ag(I) and Au(I) complexes supported by hydrophilic alkyl phosphine ligands. J. Inorg. Biochem. 2011, 105, 232–240. [Google Scholar] [CrossRef]
- Berners-Price, S.J.; Bowen, R.J.; Galettis, P.; Healy, P.C.; McKeage, M.J. Structural and solution chemistry of gold(I) and silver(I) complexes of bidentate pyridyl phosphines: Selective antitumour agents. Coord. Chem. Rev. 1999, 185–186, 823–836. [Google Scholar] [CrossRef]
- Papini, G.; Bandoli, G.; Dolmella, A.; Lobbia, G.G.; Pellei, M.; Santini, C. New homoleptic carbene transfer ligands and related coinage metal complexes. Inorg. Chem. Commun. 2008, 11, 1103–1106. [Google Scholar] [CrossRef]
- Banti, C.N.; Hadjikakou, S.K. Anti-proliferative and anti-tumor activity of silver(i) compounds. Metallomics 2013, 5, 569–596. [Google Scholar] [CrossRef]
- Shi, T.; Sun, X.; He, Q.Y. Cytotoxicity of silver nanoparticles against bacteria and tumor cells. Curr. Protein Pept. Sci. 2018, 19, 525. [Google Scholar] [CrossRef]
- Jin, X.; Tan, X.; Zhang, X.; Han, M.; Zhao, Y. In vitro and in vivo anticancer effects of singly protonated dehydronorcantharidin silver coordination polymer in CT-26 murine colon carcinoma model. Bioorg. Med. Chem. Lett. 2015, 25, 4477. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Q.; Chen, J.; Zhang, P.; Huang, Q.; Zhang, X.; Yang, L.; Xu, D.; Zhao, C.; Wang, X.; et al. Inhibition of proteasomal deubiquitinase by silver complex induces apoptosis in non-small cell lung cancer cells. Cell. Physiol. Biochem. 2018, 49, 780. [Google Scholar] [CrossRef] [PubMed]
- Medvetz, D.A.; Hindi, K.M.; Panzner, M.J.; Ditto, A.J.; Yun, Y.H.; Youngs, W.J. Anticancer activity of Ag(I) N-heterocyclic carbene complexes derived from 4,5-dichloro-1H-imidazole. Met. Based Drugs 2008, 2008, 384010. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, M.; Pellei, M.; Cimarelli, C.; Dias, H.V.R.; Marzano, C.; Tisato, F.; Porchia, M.; Gandin, V.; Santini, C. Novel multicharged silver(I)-NHC complexes derived from zwitterionic 1,3-symmetrically and 1,3-unsymmetrically substituted imidazoles and benzimidazoles: Synthesis and cytotoxic properties. J. Organomet. Chem. 2016, 806, 45–53. [Google Scholar] [CrossRef]
- Pellei, M.; Gandin, V.; Marinelli, M.; Orsetti, A.; Del Bello, F.; Santini, C.; Marzano, C. Novel triazolium based 11th group NHCs: Synthesis, characterization and cellular response mechanisms. Dalton Trans. 2015, 44, 21041–21052. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595. [Google Scholar] [CrossRef] [Green Version]
- Beck, A.; Weibert, B.; Burzlaff, N. Monoanionic N,N,O-scorpionate ligands and their iron(II) and zinc(II) complexes: Models for mononuclear active sites of non-heme iron oxidases and zinc enzymes. Eur. J. Inorg. Chem. 2001, 2, 521–527. [Google Scholar] [CrossRef]
- Burzlaff, N.; Hegelmann, I.; Weibert, B. Bis(pyrazol-1-yl)acetates as tripodal “scorpionate” ligands in transition metal carbonyl chemistry: Syntheses, structures and reactivity of manganese and rhenium carbonyl complexes of the type [LM(CO)3] (L. = bpza, bdmpza). J. Organomet. Chem. 2001, 626, 16–23. [Google Scholar] [CrossRef]
- Otero, A.; Fernandez-Baeza, J.; Tejeda, J.; Antinolo, A.; Carrillo-Hermosilla, F.; Diez-Barra, E.; Lara-Sanchez, A.; Fernandez-Lopez, M.; Lanfranchi, M.; Pellinghelli, M.A. Syntheses and crystal structures of lithium and niobium complexes containing a new type of monoanionic “scorpionate” ligand. J. Chem. Soc. Dalton Trans. 1999, 20, 3537–3539. [Google Scholar] [CrossRef]
- Alkorta, I.; Claramunt, R.M.; Díez-Barra, E.; Elguero, J.; de la Hoz, A.; López, C. The organic chemistry of poly(1H-pyrazol-1-yl)methanes. Coord. Chem. Rev. 2017, 339, 153–182. [Google Scholar] [CrossRef]
- Otero, A.; Fernández-Baeza, J.; Lara-Sánchez, A.; Sánchez-Barba, L.F. Metal complexes with heteroscorpionate ligands based on the bis(pyrazol-1-yl)methane moiety: Catalytic chemistry. Coord. Chem. Rev. 2013, 257, 1806–1868. [Google Scholar] [CrossRef]
- Paul, T.; Rodehutskors, P.M.; Schmidt, J.; Burzlaff, N. Oxygen atom transfer catalysis with homogenous and polymer-supported N,N- and N,N,O-heteroscorpionate dioxidomolybdenum(VI) complexes. Eur. J. Inorg. Chem. 2016, 2016, 2595–2602. [Google Scholar] [CrossRef]
- Fischer, N.V.; Türkoglu, G.; Burzlaff, N. Scorpionate complexes suitable for enzyme inhibitor studies. Curr. Bioact. Compd. 2009, 5, 277–295. [Google Scholar] [CrossRef]
- Costas, M.; Mehn, M.P.; Jensen, M.P.; Que Jr, L. Dioxygen activation at mononuclear nonheme iron active sites: Enzymes, models, and intermediates. Chem. Rev. 2004, 104, 939–986. [Google Scholar] [CrossRef] [PubMed]
- Parkin, G. Synthetic analogues relevant to the structure and function of zinc enzymes. Chem. Rev. 2004, 104, 699–767. [Google Scholar] [CrossRef] [PubMed]
- Burzlaff, N. Tripodal N,N,O-ligands for metalloenzyme models and organometallics. In Advances in Inorganic Chemistry; van Eldik, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 60, pp. 101–165. [Google Scholar]
- Giorgetti, M.; Tonelli, S.; Zanelli, A.; Aquilanti, G.; Pellei, M.; Santini, C. Synchrotron radiation X-ray absorption spectroscopic studies in solution and electrochemistry of a nitroimidazole conjugated heteroscorpionate copper(II) complex. Polyhedron 2012, 48, 174–180. [Google Scholar] [CrossRef]
- Pellei, M.; Papini, G.; Trasatti, A.; Giorgetti, M.; Tonelli, D.; Minicucci, M.; Marzano, C.; Gandin, V.; Aquilanti, G.; Dolmella, A.; et al. Nitroimidazole and glucosamine conjugated heteroscorpionate ligands and related copper(II) complexes. Syntheses, biological activity and XAS studies. Dalton Trans. 2011, 40, 9877–9888. [Google Scholar] [CrossRef] [PubMed]
- Pellei, M.; Bagnarelli, L.; Luciani, L.; Del Bello, F.; Giorgioni, G.; Piergentili, A.; Quaglia, W.; De Franco, M.; Gandin, V.; Marzano, C.; et al. Synthesis and cytotoxic activity evaluation of new Cu(I) complexes of Bis(pyrazol-1-yl) acetate ligands functionalized with an NMDA receptor antagonist. Int. J. Mol. Sci. 2020, 21, 2616. [Google Scholar] [CrossRef] [Green Version]
- Morelli, M.B.; Amantini, C.; Santoni, G.; Pellei, M.; Santini, C.; Cimarelli, C.; Marcantoni, E.; Petrini, M.; Del Bello, F.; Giorgioni, G.; et al. Novel antitumor copper(ii) complexes designed to act through synergistic mechanisms of action, due to the presence of an NMDA receptor ligand and copper in the same chemical entity. New J. Chem. 2018, 42, 11878–11887. [Google Scholar] [CrossRef]
- Pellei, M.; Gandin, V.; Cimarelli, C.; Quaglia, W.; Mosca, N.; Bagnarelli, L.; Marzano, C.; Santini, C. Syntheses and biological studies of nitroimidazole conjugated heteroscorpionate ligands and related Cu(I) and Cu(II) complexes. J. Inorg. Biochem. 2018, 187, 33–40. [Google Scholar] [CrossRef]
- Del Bello, F.; Pellei, M.; Bagnarelli, L.; Santini, C.; Giorgioni, G.; Piergentili, A.; Quaglia, W.; Battocchio, C.; Iucci, G.; Schiesaro, I.; et al. Cu(I) and Cu(II) complexes based on lonidamine-conjugated ligands designed to promote synergistic antitumor effects. Inorg. Chem. 2022, 61, 4919–4937. [Google Scholar] [CrossRef]
- Pellei, M.; Santini, C.; Bagnarelli, L.; Battocchio, C.; Iucci, G.; Venditti, I.; Meneghini, C.; Amatori, S.; Sgarbossa, P.; Marzano, C.; et al. Exploring the antitumor potential of copper complexes based on ester derivatives of Bis(pyrazol-1-yl)acetate ligands. Int. J. Mol. Sci. 2022, 23, 9397. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, S.; Pellei, M.; Venditti, I.; Fratoddi, I.; Battocchio, C.; Iucci, G.; Schiesaro, I.; Meneghini, C.; Palmieri, A.; Marcantoni, E.; et al. Development of new and efficient copper(II) complexes of hexyl bis(pyrazolyl)acetate ligands as catalysts for allylic oxidation. Dalton Trans. 2020, 49, 15622–15632. [Google Scholar] [CrossRef] [PubMed]
- Bagnarelli, L.; Dolmella, A.; Santini, C.; Vallesi, R.; Giacomantonio, R.; Gabrielli, S.; Pellei, M. A new dimeric Copper(II) complex of hexyl bis(pyrazolyl)acetate ligand as an efficient catalyst for allylic oxidations. Molecules 2021, 26, 6271. [Google Scholar] [CrossRef] [PubMed]
- Pellei, M.; Bagnarelli, L.; Gabrielli, S.; Lupidi, G.; Cimarelli, C.; Stella, F.; Dolmella, A.; Santini, C. Copper(II) complexes based on isopropyl ester derivatives of bis(pyrazol-1-yl)acetate ligands with catalytic potency in organic macro(molecules) synthesis. Inorg. Chim. Acta 2023, 544, 121234. [Google Scholar] [CrossRef]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Gill, D.S.; Rana, D. Preparation of some novel copper(I) complexes and their molar conductances in organic solvents. Z. Naturforsch. 2009, 64, 269–272. [Google Scholar] [CrossRef]
- Effendy; Gioia Lobbia, G.; Marchetti, F.; Pellei, M.; Pettinari, C.; Pettinari, R.; Santini, C.; Skelton, B.W.; White, A.H. Syntheses and spectroscopic and structural characterization of silver(I) complexes containing tris(isobutyl)phosphine and poly(azol-1-yl)borates. Inorg. Chim. Acta 2004, 357, 4247–4256. [Google Scholar] [CrossRef]
- Pellei, M.; Alidori, S.; Papini, G.; Lobbia, G.G.; Gorden, J.D.; Dias, H.V.R.; Santini, C. Silver(i)-organophosphane complexes of electron withdrawing CF3- or NO2-substituted scorpionate ligands. Dalton Trans. 2007, 42, 4845–4853. [Google Scholar] [CrossRef]
- Dias, H.V.R.; Flores, J.A.; Pellei, M.; Morresi, B.; Lobbia, G.G.; Singh, S.; Kobayashi, Y.; Yousufuddin, M.; Santini, C. Silver(I) and copper(I) complexes supported by fully fluorinated 1,3,5-triazapentadienyl ligands. Dalton Trans. 2011, 40, 8569–8580. [Google Scholar] [CrossRef]
- Marverti, G.; Andrews, P.A.; Piccinini, G.; Ghiaroni, S.; Barbieri, D.; Moruzzi, M.S. Modulation of cis-diamminedichloroplatinum (II) accumulation and cytotoxicity by spermine in sensitive and resistant human ovarian carcinoma cells. Eur. J. Cancer 1997, 33, 669–675. [Google Scholar] [CrossRef]
- Andrews, P.A.; Murphy, M.P.; Howell, S.B. Differential potentiation of alkylating and platinating agent cytotoxicity in human ovarian carcinoma cells by glutathione depletion1. Cancer Res. 1985, 45, 6250–6253. [Google Scholar] [PubMed]
- Scanlon, K.J.; Kashani-Sabet, M.; Tone, T.; Funato, T. Cisplatin resistance in human cancers. Pharmacol. Ther. 1991, 52, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Marzano, C.; Gandin, V.; Folda, A.; Scutari, G.; Bindoli, A.; Rigobello, M.P. Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free Radic. Biol. Med. 2007, 42, 872–881. [Google Scholar] [CrossRef]
- Kunz-Schughart, L.A.; Freyer, J.P.; Hofstaedter, F.; Ebner, R. The use of 3-D cultures for high-throughput screening: The multicellular spheroid model. J. Biomol. Screen. 2004, 9, 273–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandin, V.; Fernandes, A.P. Metal- and semimetal-containing inhibitors of thioredoxin reductase as anticancer agents. Molecules 2015, 20, 12732–12756. [Google Scholar] [CrossRef] [PubMed]
- De Franco, M.; Saab, M.; Porchia, M.; Marzano, C.; Nolan, S.P.; Nahra, F.; Van Hecke, K.; Gandin, V. Unveiling the potential of innovative gold(I) and silver(I) selenourea complexes as anticancer agents targeting TrxR and cellular redox homeostasis. Chem. Eur. J. 2022, 28, e202201898. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Qi, Y. Current progresses in metal-based anticancer complexes as mammalian TrxR inhibitors. Anti-Cancer Agents Med. Chem. 2017, 17, 1046–1069. [Google Scholar] [CrossRef]
- Björkhem-Bergman, L.; Jönsson-Videsäter, K.; Paul, C.; Björnstedt, M.; Andersson, M. Mammalian thioredoxin reductase alters cytolytic activity of an antibacterial peptide. Peptides 2004, 25, 1849–1855. [Google Scholar] [CrossRef]
- Bebber, C.M.; von Karstedt, S. Non-neuroendocrine differentiation generates a ferroptosis-prone lipidome in small cell lung cancer (SCLC). Mol. Cell. Oncol. 2021, 8, 1933871. [Google Scholar] [CrossRef]
- Galassi, R.; Burini, A.; Ricci, S.; Pellei, M.; Rigobello, M.P.; Citta, A.; Dolmella, A.; Gandin, V.; Marzano, C. Synthesis and characterization of azolate gold(i) phosphane complexes as thioredoxin reductase inhibiting antitumor agents. Dalton Trans. 2012, 41, 5307–5318. [Google Scholar] [CrossRef]
- Arnér, E.S.J. Focus on mammalian thioredoxin reductases—Important selenoproteins with versatile functions. Biochim. Biophys. Acta. Gen. Subj. 2009, 1790, 495–526. [Google Scholar] [CrossRef] [PubMed]
- Scalcon, V.; Bindoli, A.; Rigobello, M.P. Significance of the mitochondrial thioredoxin reductase in cancer cells: An update on role, targets and inhibitors. Free Radic. Biol. Med. 2018, 127, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Suski, J.; Lebiedzinska, M.; Bonora, M.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Relation between mitochondrial membrane potential and ROS formation. In Mitochondrial Bioenergetics: Methods and Protocols; Palmeira, C.M., Moreno, A.J., Eds.; Springer: New York, NY, USA, 2018; pp. 357–381. [Google Scholar]
- Porchia, M.; Papini, G.; Santini, C.; Lobbia, G.G.; Pellei, M.; Tisato, F.; Bandoll, G.; Dolmella, A. Novel rhenium(V) oxo complexes containing bis(pyrazol-1-yl)acetate and bis(pyrazol-1-yl) sulfonate as tripodal N,N,O-heteroscorplonate ligands. Inorg. Chem. 2005, 44, 4045–4054. [Google Scholar] [CrossRef] [PubMed]
- Hübner, E.; Haas, T.; Burzlaff, N. Synthesis and transition metal complexes of novel N,N,O scorpionate ligands suitable for solid phase immobilisation. Eur. J. Inorg. Chem. 2006, 24, 4989–4997. [Google Scholar] [CrossRef]
- Zheng, Z.; Groaz, E.; Snoeck, R.; De Jonghe, S.; Herdewijn, P.; Andrei, G. Influence of 4′-substitution on the activity of gemcitabine and its ProTide against VZV and SARS-CoV-2. ACS Med. Chem. Lett. 2020, 12, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Rigobello, M.P.; Gandin, V.; Folda, A.; Rundlöf, A.-K.; Fernandes, A.P.; Bindoli, A.; Marzano, C.; Björnstedt, M. Treatment of human cancer cells with selenite or tellurite in combination with auranofin enhances cell death due to redox shift. Free Radic. Biol. Med. 2009, 47, 710–721. [Google Scholar] [CrossRef]

| IC50 (µM) ± S.D. | ||||||||
|---|---|---|---|---|---|---|---|---|
| HCT-15 | PSN-1 | A431 | U1285 | MDA-MB-231 | 2008 | C13* (R.F.) | HEK293 | |
| 1 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| 2 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| 3 | 6.5 ± 0.5 | 24.6 ± 0.9 | 4.8 ± 1.8 | 2.1 ± 0.3 | 5.4 ± 0.9 | 6.9 ± 1.2 | 5.7 ± 0.2 (0.8) | 17.5 ± 1.3 |
| 4 | 9.2 ± 2.9 | 1.2 ± 0.4 | 0.9 ± 0.2 | 0.7 ± 0.1 | 10.6 ± 1.9 | 1.3 ± 0.2 | 0.20 ± 0.02 (0.2) | 15.7 ± 1.8 |
| 5 | 7.4 ± 0.9 | 3.7 ± 0.4 | 1.4 ± 0.4 | 1.2 ± 0.2 | 2.8 ± 0.4 | 1.4 ± 0.3 | 0.5 ± 0.1 (0.4) | 13.8 ± 2.1 |
| PPh3 | >50 | >50 | 47.5 ± 1.1 | >50 | >50 | >50 | >50 | >100 |
| PTA | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| cisplatin | 15.3 ± 2.6 | 18.3 ± 3.1 | 2.1 ± 0.9 | 9.6 ± 1.2 | 30.5 ± 2.6 | 2.2 ± 1.0 | 30.5 ± 2.6 (13.9) | 21.6 ± 3.5 |
| IC50 (µM) ± S.D. | |
|---|---|
| 1 | >100 |
| 2 | >100 |
| 3 | 63.8 ± 4.4 |
| 4 | 27.9 ± 1.8 |
| 5 | 22.0 ± 2.1 |
| cisplatin | 65.4 ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellei, M.; Santini, C.; Bagnarelli, L.; Caviglia, M.; Sgarbossa, P.; De Franco, M.; Zancato, M.; Marzano, C.; Gandin, V. Novel Silver Complexes Based on Phosphanes and Ester Derivatives of Bis(pyrazol-1-yl)acetate Ligands Targeting TrxR: New Promising Chemotherapeutic Tools Relevant to SCLC Management. Int. J. Mol. Sci. 2023, 24, 4091. https://doi.org/10.3390/ijms24044091
Pellei M, Santini C, Bagnarelli L, Caviglia M, Sgarbossa P, De Franco M, Zancato M, Marzano C, Gandin V. Novel Silver Complexes Based on Phosphanes and Ester Derivatives of Bis(pyrazol-1-yl)acetate Ligands Targeting TrxR: New Promising Chemotherapeutic Tools Relevant to SCLC Management. International Journal of Molecular Sciences. 2023; 24(4):4091. https://doi.org/10.3390/ijms24044091
Chicago/Turabian StylePellei, Maura, Carlo Santini, Luca Bagnarelli, Miriam Caviglia, Paolo Sgarbossa, Michele De Franco, Mirella Zancato, Cristina Marzano, and Valentina Gandin. 2023. "Novel Silver Complexes Based on Phosphanes and Ester Derivatives of Bis(pyrazol-1-yl)acetate Ligands Targeting TrxR: New Promising Chemotherapeutic Tools Relevant to SCLC Management" International Journal of Molecular Sciences 24, no. 4: 4091. https://doi.org/10.3390/ijms24044091





