Recent Developments in Polymer Nanocomposites for Bone Regeneration
Abstract
:1. Introduction
2. Regeneration of Bones Using Nanocomposite Scaffolds
3. Nanocomposite Ceramic’s Potential Role in Bone Regeneration
4. Contribution of Nanostructured Biomaterials to Bone Regeneration
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbasi, N.; Hamlet, S.M.; Love, R.M.; Nguyen, N. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.A.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.F.; Cengiz, I.F.; Silva, F.S.; Reis, R.L.; Oliveira, J.M. Scaffolds and coatings for bone regeneration. J. Mater. Sci. Mater. Med. 2020, 31, 27. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.; Kanjilal, D.; Teitelbaum, M.; Lin, S.; Cottrell, J. Zinc as a Therapeutic Agent in Bone Regeneration. Materials 2020, 13, 2211. [Google Scholar] [CrossRef]
- Huang, C.; Kang, M.; Lu, Y.D.; Shirazi, S.; Diaz, J.I.; Cooper, L.F.; Gajendrareddy, P.K.; Ravindran, S. Functionally Engineered Extracellular Vesicles Improve Bone Regeneration. Acta Biomater. 2020, 109, 182–194. [Google Scholar] [CrossRef]
- Yang, Q.; Yin, H.; Xu, T.; Zhu, D.; Yin, J.; Chen, Y.; Yu, X.; Gao, J.; Zhang, C.; Chen, Y.; et al. Engineering 2D Mesoporous Silica@MXene-Integrated three-dimentional-Printing Scaffolds for Combinatory Osteosarcoma Therapy and NO-Augmented Bone Regeneration. Small 2020, 16, e1906814. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Saito, N. Biodegradable Polymers as Drug Delivery Systems for Bone Regeneration. Pharmaceutics 2020, 12, 95. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C 2020, 107, 110195. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Hasani-Sadrabadi, M.M.; Sarrion, P.; Pouraghaei, S.; Chau, Y.; Ansari, S.; Li, S.; Aghaloo, T.; Moshaverinia, A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 2020, 12, eaay6853. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Huang, C.; Lu, Y.; Shirazi, S.; Gajendrareddy, P.K.; Ravindran, S.; Cooper, L.F. Bone Regeneration is Mediated by Macrophage Extracellular Vesicles. Bone 2020, 141, 115627. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Byun, H.; Madhurakkat Perikamana, S.K.; Lee, S.; Shin, H. Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv. Healthc. Mater. 2019, 8, e1801106. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Yin, C.; Tu, H.; Jiang, S.; Wang, Q.; Zhou, X.; Xing, X.; Xie, C.; Shi, X.; Du, Y.; et al. Controlled Co-delivery of Growth Factors through Layer-by-Layer Assembly of Core-Shell Nanofibers for Improving Bone Regeneration. ACS Nano 2019, 13, 6372–6382. [Google Scholar] [CrossRef]
- Jin, S.; He, D.; Luo, D.; Wang, Y.; Yu, M.; Guan, B.; Fu, Y.; Li, Z.; Zhang, T.; Zhou, Y.; et al. A Biomimetic Hierarchical Nanointerface Orchestrates Macrophage Polarization and Mesenchymal Stem Cell Recruitment to Promote Endogenous Bone Regeneration. ACS Nano 2019, 13, 6581–6595. [Google Scholar] [CrossRef]
- Yuan, Z.; Wei, P.; Huang, Y.; Zhang, W.; Chen, F.; Zhang, X.; Mao, J.; Chen, D.; Cai, Q.; Yang, X. Injectable PLGA microspheres with tunable magnesium ion release for promoting bone regeneration. Acta Biomater. 2019, 85, 294–309. [Google Scholar] [CrossRef]
- Sanz, M.; Dahlin, C.; Apatzidou, D.A.; Artzi, Z.; Božić, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 82–91. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The Application of Hyaluronic Acid in Bone Regeneration. Int. J. Biol. Macromol. 2019, 151, 1224–1239. [Google Scholar] [CrossRef]
- Tamburaci, S.; Tihminlioglu, F. Chitosan-hybrid poss nanocomposites for bone regeneration: The effect of poss nanocage on surface, morphology, structure and In Vitro bioactivity. Int. J. Biol. Macromol. 2019, 142, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Purohit, S.D.; Singh, H.; Bhaskar, R.; Yadav, I.; Chou, C.; Gupta, M.K.; Mishra, N.C. Gelatin-alginate-cerium oxide nanocomposite scaffold for bone regeneration. Mater. Sci. Eng. C 2020, 116, 111111. [Google Scholar] [CrossRef]
- Purohit, S.D.; Singh, H.; Bhaskar, R.; Yadav, I.; Bhushan, S.; Gupta, M.K.; Kumar, A.; Mishra, N.C. Fabrication of Graphene Oxide and Nanohydroxyapatite Reinforced Gelatin–Alginate Nanocomposite Scaffold for Bone Tissue Regeneration. Front. Mater. 2020, 7, 250. [Google Scholar] [CrossRef]
- Maghsoudlou, M.A.; Nassireslami, E.; Saber-Samandari, S.; Khandan, A. Bone Regeneration Using Bio-Nanocomposite Tissue Reinforced with Bioactive Nanoparticles for Femoral Defect Applications in Medicine. Avicenna J. Med. Biotechnol. 2020, 12, 68–76. [Google Scholar]
- Wu, M.; Chen, F.; Wu, P.; Yang, Z.; Zhang, S.; Xiao, L.; Deng, Z.; Zhang, C.; Chen, Y.; Cai, L. Nanoclay mineral-reinforced macroporous nanocomposite scaffolds for in situ bone regeneration: In Vitro and In Vivo studies. Mater. Des. 2021, 205, 109734. [Google Scholar] [CrossRef]
- Dulany, K.; Goins, A.; Kelley, A.; Allen, J.B. Fabrication of a Free Radical Scavenging Nanocomposite Scaffold for Bone Tissue Regeneration. Regen. Eng. Transl. Med. 2018, 4, 257–267. [Google Scholar] [CrossRef]
- Covarrubias, C.; Cádiz, M.; Maureira, M.; Celhay, I.; Cuadra, F.; von Marttens, A. Bionanocomposite scaffolds based on chitosan–gelatin and nanodimensional bioactive glass particles: In Vitro properties and In Vivo bone regeneration. J. Biomater. Appl. 2018, 32, 1155–1163. [Google Scholar] [CrossRef]
- Al-Arjan, W.S.; Aslam Khan, M.U.; Nazir, S.; Abd Razak, S.I.; Abdul Kadir, M.R. Development of Arabinoxylan-Reinforced Apple Pectin/Graphene Oxide/Nano-Hydroxyapatite Based Nanocomposite Scaffolds with Controlled Release of Drug for Bone Tissue Engineering: In-Vitro Evaluation of Biocompatibility and Cytotoxicity against MC3T3-E1. Coatings. 2020, 10, 1120. [Google Scholar] [CrossRef]
- Dulany, K.; Hepburn, K.; Goins, A.; Allen, J.B. In-Vitro and In-Vivo Biocompatibility Assessment of Free Radical Scavenging Nanocomposite Scaffolds for Bone Tissue Regeneration. J. Biomed. Mater. Res. Part A 2019, 108, 301–315. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Yekta, H.J.; Ahmadi, S.; Alamara, K. The role of titanium dioxide on the morphology, microstructure, and bioactivity of grafted cellulose/hydroxyapatite nanocomposites for a potential application in bone repair. Int. J. Biol. Macromol. 2018, 106, 481–488. [Google Scholar] [CrossRef]
- Zhang, N. Magnetic Nanocomposites and Fields for Bone and Cartilage Tissue Engineering Applications. Ph.D. Thesis, University of California, Riverside, CA, USA, 2017. [Google Scholar]
- Esmaeili, S.; Khandan, A.; Saber-Samandari, S. Mechanical performance of three-dimensional bio-nanocomposite scaffolds designed with digital light processing for biomedical applications. Iran. J. Med. Phys. 2018, 15, 328. [Google Scholar]
- Abdollahi, E.; Bakhsheshi-Rad, H.R. Evaluation of mechanical properties and apatite formation of synthesized fluorapatite-hardystonite nanocomposite scaffolds. Adv. Ceram. Prog. 2018, 4, 8–15. [Google Scholar]
- Boraei, S.B.; Nourmohammadi, J.; Bakhshandeh, B.; Dehghan, M.M.; Gonzalez, Z.; Ferrari, B. The Effect of Protelos Content on the Physicochemical, Mechanical and Biological Properties of Gelatin-Based Scaffolds. J. Appl. Biotechnol. Rep. 2020, 7, 41–47. [Google Scholar]
- Aslam Khan, M.U.; Al-Arjan, W.S.; Binkadem, M.S.; Mehboob, H.; Haider, A.; Raza, M.A.; Abd Razak, S.I.; Hasan, A.; Amin, R. Development of Biopolymeric Hybrid Scaffold-Based on AAc/GO/nHAp/TiO2 Nanocomposite for Bone Tissue Engineering: In-Vitro Analysis. Nanomaterials 2021, 11, 1319. [Google Scholar] [CrossRef] [PubMed]
- Mandakhbayar, N.; El-Fiqi, A.; Dashnyam, K.; Kim, H.W. Feasibility of Defect Tunable Bone Engineering Using Electroblown Bioactive Fibrous Scaffolds with Dental Stem Cells. ACS Biomater. Sci. Eng. 2018, 4, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Liu, S.; Ma, Y.; Xu, W.; Meng, W.; Lin, X.; Wang, W.; Wang, S.; Zhang, J. Innovative biodegradable poly(L-lactide)/collagen/hydroxyapatite composite fibrous scaffolds promote osteoblastic proliferation and differentiation. Int. J. Nanomed. 2017, 12, 7577–7588. [Google Scholar] [CrossRef]
- Mahmood, S.K.; Razak, I.S.; Ghaji, M.S.; Yusof, L.M.; Mahmood, Z.K.; Rameli, M.A.; Zakaria, Z.A. In Vivo evaluation of a novel nanocomposite porous three-dimentional scaffold in a rabbit model: Histological analysis. Int. J. Nanomed. 2017, 12, 8587–8598. [Google Scholar] [CrossRef]
- Khandan, A.; Nassireslami, E.; Saber-Samandari, S.; Arabi, N. Fabrication and Characterization of Porous Bioceramic-Magnetite Biocomposite for Maxillofacial Fractures Application. Dent. Hypotheses 2020, 11, 74–85. [Google Scholar]
- Apicella, A.; Aversa, R.; Petrescu, F.I. Hybrid Ceramo-Polymeric Nano-Diamond Composites. Am. J. Eng. Appl. Sci. 2018, 11, 766–782. [Google Scholar] [CrossRef]
- Kolathupalayam Shanmugam, B.; Rangaraj, S.; Subramani, K.; Srinivasan, S.; Aicher, W.K.; Venkatachalam, R. Biomimetic TiO2-chitosan/sodium alginate blended nanocomposite scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2020, 110, 110710. [Google Scholar] [CrossRef]
- Alam, F.; Varadarajan, K.M.; Kumar, S. Three-dimentional printed polylactic acid nanocomposite scaffolds for tissue engineering applications. Polym. Test. 2020, 81, 106203. [Google Scholar] [CrossRef]
- Khan, M.U.; al-Thebaiti, M.A.; Hashmi, M.U.; Aftab, S.; Abd Razak, S.I.; Abu Hassan, S.; Abdul Kadir, M.R.; Amin, R. Synthesis of Silver-Coated Bioactive Nanocomposite Scaffolds Based on Grafted Beta-Glucan/Hydroxyapatite via Freeze-Drying Method: Anti-Microbial and Biocompatibility Evaluation for Bone Tissue Engineering. Materials 2020, 13, 971. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Shukla, V.R.; Varadarajan, K.M.; Kumar, S. Microarchitected three-dimentional printed polylactic acid (PLA) nanocomposite scaffolds for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020, 103, 103576. [Google Scholar] [CrossRef] [PubMed]
- Christy, P.N.; Basha, S.K.; Kumari, V.S.; Bashir, A.; Maaza, M.; Kaviyarasu, K.; Arasu, M.V.; Al-Dhabi, N.A.; Ignacimuthu, S. Biopolymeric nanocomposite scaffolds for bone tissue engineering applications—A review. J. Drug Deliv. Sci. Technol. 2020, 55, 101452. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kaith, B.S.; Shanker, U.; Gupta, B. γ-radiation induced synthesis of antibacterial silver nanocomposite scaffolds derived from natural gum Boswellia serrata. J. Drug Deliv. Sci. Technol. 2020, 56, 101550. [Google Scholar] [CrossRef]
- Rostami, F.; Tamjid, E.; Behmanesh, M. Drug-eluting PCL/graphene oxide nanocomposite scaffolds for enhanced osteogenic differentiation of mesenchymal stem cells. Mater. Sci. Eng. C 2020, 115, 111102. [Google Scholar] [CrossRef]
- Pathmanapan, S.; Periyathambi, P.; Ananda Sadagopan, S.K. Fibrin Hydrogel incorporated with graphene oxide functionalized nanocomposite scaffolds for bone repair—In Vitro and In Vivo study. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102251. [Google Scholar] [CrossRef]
- Gao, W.; Sun, L.; Zhang, Z.; Li, Z. Cellulose nanocrystals reinforced gelatin/bioactive glass nanocomposite scaffolds for potential application in bone regeneration. J. Biomater. Sci. Polym. Ed. 2020, 31, 984–998. [Google Scholar] [CrossRef]
- Raval, J.; Joshi, P.; Chejara, D.; Disher, I.A. Fabrication and applications of hydroxyapatite-based nanocomposites coating for bone tissue engineering. In Applications of Nanocomposite Materials in Orthopedics; Woodhead Publishing: Cambridge, UK, 2019. [Google Scholar]
- Xu, H.; Zou, X.; Xia, P.; Huang, H.; Liu, F.; Ramesh, T. Osteoblast cell viability over ultra-long tricalcium phosphate nanocrystal-based methacrylate chitosan composite for bone regeneration. Biomed. Mater. 2021, 16, 045006. [Google Scholar] [CrossRef]
- Maheshwari, S.; Govindan, K.; Raja, M.M.; Raja, A.A.; Pravin, M.B.; Kumar, S.V. Synthesis and Characterization of Calcium Phosphate Ceramic/(Poly(vinyl alcohol)–Polycaprolactone) Bilayer Nanocomposites—A Bone Tissue Regeneration Scaffold. J. Nanosci. Nanotechnol. 2018, 18, 1548–1556. [Google Scholar] [CrossRef]
- Parai, R.; Bandyopadhyay-Ghosh, S. Engineered bio-nanocomposite magnesium scaffold for bone tissue regeneration. J. Mech. Behav. Biomed. Mater. 2019, 96, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Bramhill, J.; Ross, S.; Ross, G.M. Bioactive Nanocomposites for Tissue Repair and Regeneration: A Review. Int. J. Environ. Res. Public Health 2017, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S. Biodegradable Polymer-Bioactive Ceramic Composites for Guided Bone Regeneration. Master’s Thesis, Iowa State University, Ames, IA, USA, 2017. [Google Scholar]
- Lei, L.; Wei, Y.; Wang, Z.; Han, J.; Sun, J.; Chen, Y.; Yang, X.; Wu, Y.; Chen, L.; Gou, Z. Core-Shell Bioactive Ceramic Robocasting: Tuning Component Distribution Beneficial for Highly Efficient Alveolar Bone Regeneration and Repair. ACS Biomater. Sci. Eng. 2020, 6, 2376–2387. [Google Scholar] [CrossRef] [PubMed]
- Maciel, P.P.; Pessôa, J.A.; Medeiros, E.L.; Batista, A.U.; Figueiredo, L.R.; Medeiros, E.S.; Duarte, D.F.; Alves, A.F.; Sousa, F.B.; Vieira, B.R.; et al. Use of strontium doping glass-ceramic material for bone regeneration in critical defect: In Vitro and In Vivo analyses. Ceram. Int. 2020, 46, 24940–24954. [Google Scholar] [CrossRef]
- Li, X.; Song, T.; Chen, X.; Wang, M.; Yang, X.; Xiao, Y.M.; Zhang, X. Osteoinductivity of Porous Biphasic Calcium Phosphate Ceramic Spheres with Nanocrystalline and Their Efficacy in Guiding Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 3722–3736. [Google Scholar] [CrossRef]
- Popescu, R.A.; Flaviu, T.; Bogdan, S.; Farcasanu, A.S.; Purdoiu, R.; Magyari, K.; Vulpoi, A.; Dreanca, A.I.; Sevastre, B.; Şimon, S.; et al. Bone regeneration response in an experimental long bone defect orthotopically implanted with alginate-pullulan-glass-ceramic composite scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 108, 1129–1140. [Google Scholar] [CrossRef]
- Dascălu, C.; Maidaniuc, A.; Pandele, A.M.; Voicu, Ș.I.; Machedon-Pisu, T.; Stan, G.E.; Cîmpean, A.; Mitran, V.; Antoniac, I.V.; Miculescu, F. Synthesis and characterization of biocompatible polymer-ceramic film structures as favorable interface in guided bone regeneration. Appl. Surf. Sci. 2019, 494, 335–352. [Google Scholar] [CrossRef]
- Li, L.; Hu, H.; Zhu, Y.; Zhu, M.; Liu, Z. three-dimentional-printed ternary SiO2CaO P2O5 bioglass-ceramic scaffolds with tunable compositions and properties for bone regeneration. Ceram. Int. 2019, 45, 10997–11005. [Google Scholar] [CrossRef]
- Zhang, M.; Pu, X.; Chen, X.; Yin, G. In-Vivo performance of plasma-sprayed CaO–MgO–SiO2-based bioactive glass-ceramic coating on Ti–6Al–4V alloy for bone regeneration. Heliyon 2019, 5, e02824. [Google Scholar] [CrossRef]
- Gómez-Lizárraga, K.K.; Flores-Morales, C.; Del Prado-Audelo, M.L.; Álvarez-Pérez, M.A.; Piña-Barba, M.C.; Escobedo, C. Polycaprolactone- and polycaprolactone/ceramic-based three-dimentional-bioplotted porous scaffolds for bone regeneration: A comparative study. Mater. Sci. Eng. C 2017, 79, 326–335. [Google Scholar] [CrossRef]
- Nikpour, P.; Salimi-Kenari, H.; Fahimipour, F.; Rabiee, S.M.; Imani, M.; Dashtimoghadam, E.; Tayebi, L. Dextran hydrogels incorporated with bioactive glass-ceramic: Nanocomposite scaffolds for bone tissue engineering. Carbohydr. Polym. 2018, 190, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Raju, R.S.; Ghosh, N.; Chaudhury, K.; Ghosh, S.; Banerjee, I.; Pramanik, N. Development and physicochemical characterization of doxorubicin-encapsulated hydroxyapatite–polyvinyl alcohol nanocomposite for repair of osteosarcoma-affected bone tissues. Rep. Chim. 2019, 22, 46–57. [Google Scholar] [CrossRef]
- López-Píriz, R.; Fernández, A.; Goyos-Ball, L.; Rivera, S.; Díaz, L.A.; Fernández-Domínguez, M.; Prado, C.; Moya, J.S.; Torrecillas, R. Performance of a New Al2O3/Ce–TZP Ceramic Nanocomposite Dental Implant: A Pilot Study in Dogs. Materials 2017, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Iwanami-Kadowaki, K.; Uchikoshi, T.; Uezono, M.; Kikuchi, M.; Moriyama, K. Development of novel bone-like nanocomposite coating of hydroxyapatite/collagen on titanium by modified electrophoretic deposition. J. Biomed. Mater. Res. Part A 2021, 109, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, S.V.; Mony, U.; Jayachandran, P.; Reshma, S.; Kumar, R.A.; Arzate, H.; Nair, S.V.; Jayakumar, R. Tri-Layered Nanocomposite Hydrogel Scaffold for the Concurrent Regeneration of Cementum, Periodontal Ligament, and Alveolar Bone. Adv. Healthc. Mater. 2017, 6, 1601251. [Google Scholar] [CrossRef] [PubMed]
- Stīpniece, L.; Narkevica, I.; Sokolova, M.D.; Locs, J.; Ozoliņš, J. Novel scaffolds based on hydroxyapatite/poly(vinyl alcohol) nanocomposite coated porous TiO2 ceramics for bone tissue engineering. Ceram. Int. 2016, 42, 1530–1537. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Mokhtarpour, M.; Shekaari, H.; Sharifi, S. Hydroxyapatite-gelatin nanocomposite films; Production and evaluation of the physicochemical properties. J. Adv. Chem. Pharm. Mater. 2019, 2, 111–115. [Google Scholar]
- Molaei, A.; Yousefpour, M. Preparation of Chitosan-based nanocomposites and biomedical investigations in bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 701–713. [Google Scholar] [CrossRef]
- Bakir, M.; Meyer, J.L.; Sutrisno, A.; Economy, J.; Jasiuk, I.M. Aromatic thermosetting copolyester bionanocomposites as reconfigurable bone substitute materials: Interfacial interactions between reinforcement particles and polymer network. Sci. Rep. 2018, 8, 14869. [Google Scholar] [CrossRef]
- Sukhodub, L.; Dyadyura, K.; Prokopovich, I.; Oborskyi, H. The Modeling of the Apatite Nanocrystals of Bone, Illustrating its Physicochemical Evolution and Surface Reactivity. In Proceedings of the 2018 IEEE 8th International Conference Nanomaterials: Application & Properties (NAP), Zatoka, Ukraine, 9–14 September 2018; pp. 1–7. [Google Scholar]
- Pansini, M.; Dell’Agli, G.; Marocco, A.; Netti, P.A.; Battista, E.; Lettera, V.; Vergara, P.; Allia, P.; Bonelli, B.; Tiberto, P.; et al. Preparation and Characterization of Magnetic and Porous Metal-Ceramic Nanocomposites from a Zeolite Precursor and Their Application for DNA Separation. J. Biomed. Nanotechnol. 2017, 13, 337–348. [Google Scholar] [CrossRef]
- Biloshytsky, V.; Nakhaba, A.; Shmeleva, A.; Robak, O.; Dubok, V. Analysis of the effectiveness of delayed cranioplasty of skull defects using nanocomposite metalloceramic in the experiment. Ukr. Neurosurg. J. 2016, 4, 50–54. [Google Scholar] [CrossRef]
- Esmaeili, S.; Akbari Aghdam, H.; Motififard, M.; Saber-Samandari, S.; Montazeran, A.H.; Bigonah, M.; Sheikhbahaei, E.; Khandan, A. A porous polymeric–hydroxyapatite scaffold used for femur fractures treatment: Fabrication, analysis, and simulation. Eur. J. Orthop. Surg. Traumatol. 2019, 30, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Rad, M.M.; Saber-Samandari, S.; Sadighi, M.; Tayebi, L.; Aghdam, M.M.; Khandan, A. Macro-and micromechanical modelling of HA-Elastin scaffold fabricated using freeze drying technique. J. Nanoanal. 2020, 8, 17–31. [Google Scholar]
- Barrera, G.; Tiberto, P.; Allia, P.; Bonelli, B.; Esposito, S.; Marocco, A.; Pansini, M.; Leterrier, Y. Magnetic Properties of Nanocomposites. Appl. Sci. 2019, 9, 212. [Google Scholar] [CrossRef]
- Rizvi, H.R. Bioinspired and Biocompatible Coatings of Poly(butylene Adipate-co-terephthalate) and Layer Double Hydroxide Composites for Corrosion Resistance. Ph.D. Thesis, University of North Texas, Denton, TX, USA, 2016. [Google Scholar]
- Toledano, M.; Toledano-Osorio, M.; Osorio, R.; Carrasco-Carmona, Á.; Gutierrez-Perez, J.; Gutiérrez-Corrales, A.; Serrera-Figallo, M.; Lynch, C.D.; Torres-Lagares, D. Doxycycline and Zinc Loaded Silica-Nanofibrous Polymers as Biomaterials for Bone Regeneration. Polymers 2020, 12, 1201. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Ahmad, S.A.; Chaudhary, N.; Hoda, M.N.; Nayak, A.K. Biodegradable polymer matrix nanocomposites for bone tissue engineering. In Applications of Nanocomposite Materials in Orthopedics; Woodhead Publishing: Cambridge, UK, 2019. [Google Scholar]
- Felgueiras, H.P.; Amorim, M.T. Production of polymer–bioactive glass nanocomposites for bone repair and substitution. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Campos-Cruz, J.; Rangel-Vázquez, N.; Rangel-Vázquez, R. Molecular study of simulated body fluid and temperature on polyurethane/graphene polymeric nanocomposites: Calcium carbonate and polymethyl methacrylate using dynamics modeling by Monte Carlo for applications in bone regeneration. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Kuang, L.; Ma, X.; Ma, Y.; Yao, Y.; Tariq, M.; Yuan, Y.; Liu, C. Self-Assembled Injectable Nanocomposite Hydrogels Coordinated by in Situ Generated CaP Nanoparticles for Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 17234–17246. [Google Scholar] [CrossRef]
- Zhang, J.V.; Jia, J.; Kim, J.P.; Shen, H.; Yang, F.; Zhang, Q.; Xu, M.; Bi, W.; Wang, X.; Yang, J.; et al. Ionic Colloidal Molding as a Biomimetic Scaffolding Strategy for Uniform Bone Tissue Regeneration. Adv. Mater. 2017, 29, 17. [Google Scholar] [CrossRef]
- Liu, X.; George, M.N.; Li, L.; Gamble, D.; Miller, A.L.; Gaihre, B.; Waletzki, B.E.; Lu, L. Injectable Electrical Conductive and Phosphate Releasing Gel with Two-Dimensional Black Phosphorus and Carbon Nanotubes for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 4653–4665. [Google Scholar] [CrossRef]
- Gantar, A.; Drnovšek, N.; Casuso, P.; Vicente, A.P.; Rodríguez, J.; Dupin, D.; Novak, S.; Loinaz, I. Injectable and self-healing dynamic hydrogel containing bioactive glass nanoparticles as a potential biomaterial for bone regeneration. RSC Adv. 2016, 6, 69156–69166. [Google Scholar] [CrossRef]
- Acevedo, C.A.; Olguín, Y.; Briceño, M.; Forero, J.C.; Osses, N.; Díaz-Calderón, P.; Jaques, A.V.; Ortiz, R. Design of a biodegradable UV-irradiated gelatin-chitosan/nanocomposed membrane with osteogenic ability for application in bone regeneration. Mater. Sci. Eng. C 2019, 99, 875–886. [Google Scholar] [CrossRef]
- Dai, Z.; Dang, M.; Zhang, W.; Murugan, S.; Teh, S.W.; Pan, H. Biomimetic hydroxyapatite/poly xylitol sebacic adibate/vitamin K nanocomposite for enhancing bone regeneration. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Acri, T.; Geary, S.; Salem, A.K. Biomimetic Mineralization of Biomaterials Using Simulated Body Fluids for Bone Tissue Engineering and Regenerative Medicine. Tissue Eng. Part A 2017, 23, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.A.; Prema, D.; Venkataprasanna, K.S.; Balagangadharan, K.; Selvamurugan, N.; Venkatasubbu, G.D. Nanocomposite chitosan film containing graphene oxide/hydroxyapatite/gold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 154, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Tamburaci, S.; Tihminlioglu, F. Development of Si doped nano hydroxyapatite reinforced bilayer chitosan nanocomposite barrier membranes for guided bone regeneration. Mater. Sci. Eng. C 2021, 128, 112298. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Jayakumar, R.; Anil, S.; Kim, S. 7—Chitosan–Nanohydroxyapatite Nanocomposite for Bone-Tissue Regeneration. In Nanocomposites for Musculoskeletal Tissue Regeneration; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Wang, P.; Hao, L.; Wang, Z.; Wang, Y.; Guo, M.; Zhang, P. Gadolinium-Doped BTO-Functionalized Nanocomposites with Enhanced MRI and X-ray Dual Imaging to Simulate the Electrical Properties of Bone. ACS Appl. Mater. Interfaces 2020, 12, 49464–49479. [Google Scholar] [CrossRef] [PubMed]
- Beig, B.; Liaqat, U.; Niazi, M.F.; Douna, I.; Zahoor, M.; Niazi, M.F. Current Challenges and Innovative Developments in Hydroxyapatite-Based Coatings on Metallic Materials for Bone Implantation: A Review. Coatings 2020, 10, 1249. [Google Scholar] [CrossRef]
- Xing, H.; Wang, X.; Xiao, G.; Zhao, Z.; Zou, S.; Li, M.; Richardson, J.J.; Tardy, B.L.; Xie, L.; Komasa, S.; et al. Hierarchical assembly of nanostructured coating for siRNA-based dual therapy of bone regeneration and revascularization. Biomaterials 2020, 235, 119784. [Google Scholar] [CrossRef]
- Schneider Werner Vianna, T.; Sartoretto, S.C.; Neves Novellino Alves, A.T.; Figueiredo de Brito Resende, R.; de Almeida Barros Mourão, C.F.; de Albuquerque Calasans-Maia, J.; Martinez-Zelaya, V.R.; Malta Rossi, A.; Granjeiro, J.M.; Calasans-Maia, M.D.; et al. Nanostructured Carbonated Hydroxyapatite Associated to rhBMP-2 Improves Bone Repair in Rat Calvaria. J. Funct. Biomater. 2020, 11, 87. [Google Scholar] [CrossRef]
- Targonska, S.; Sikora, M.; Marycz, K.; Śmieszek, A.; Wiglusz, R.J. Theranostic Applications of Nanostructured Silicate-Substituted Hydroxyapatite Codoped with Eu3+ and Bi3+ Ions-A Novel Strategy for Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 6148–6160. [Google Scholar] [CrossRef]
- Huang, Y.; Deng, H.; Fan, Y.; Zheng, L.; Che, J.; Li, X.; Aifantis, K.E. Conductive nanostructured Si biomaterials enhance osteogeneration through electrical stimulation. Mater. Sci. Eng. C 2019, 103, 109748. [Google Scholar] [CrossRef]
- Madeira, S.; Souza, J.C.; Fredel, M.C.; Henriques, B.; Silva, F.S.; Zhang, Y. Functionally graded nanostructured biomaterials (FGNB). In Nanostructured Biomaterials for Cranio-Maxillofacial and Oral Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Ma, Q.; Fang, L.; Jiang, N.; Zhang, L.; Wang, Y.; Zhang, Y.; Chen, L. Bone mesenchymal stem cell secretion of sRANKL/OPG/M-CSF in response to macrophage-mediated inflammatory response influences osteogenesis on nanostructured Ti surfaces. Biomaterials 2018, 154, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Milan, P.B.; Baino, F.; Mozafari, M. Nanoengineered biomaterials for bone/dental regeneration. In Nanoengineered Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Wang, Q.; Huang, Y.; Qian, Z. Nanostructured Surface Modification to Bone Implants for Bone Regeneration. J. Biomed. Nanotechnol. 2019, 14, 628–648. [Google Scholar] [CrossRef] [PubMed]
- Andrés, N.C.; Sieben, J.M.; Baldini, M.D.; Rodríguez, C.H.; Famiglietti, Á.; Messina, P.V. Electroactive Mg2+-Hydroxyapatite Nanostructured Networks against Drug-Resistant Bone Infection Strains. ACS Appl. Mater. Interfaces 2018, 10, 19534–19544. [Google Scholar] [CrossRef] [PubMed]
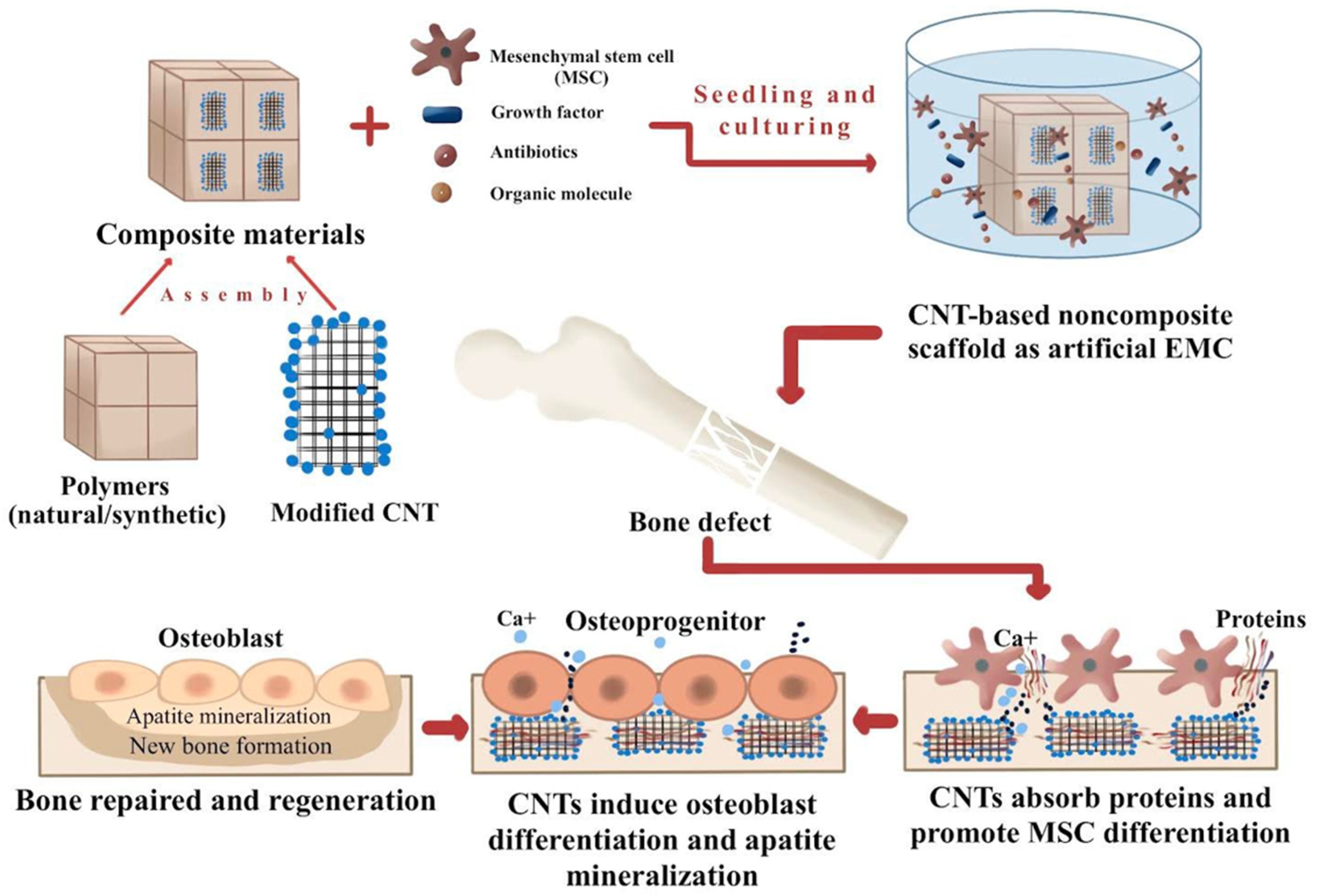

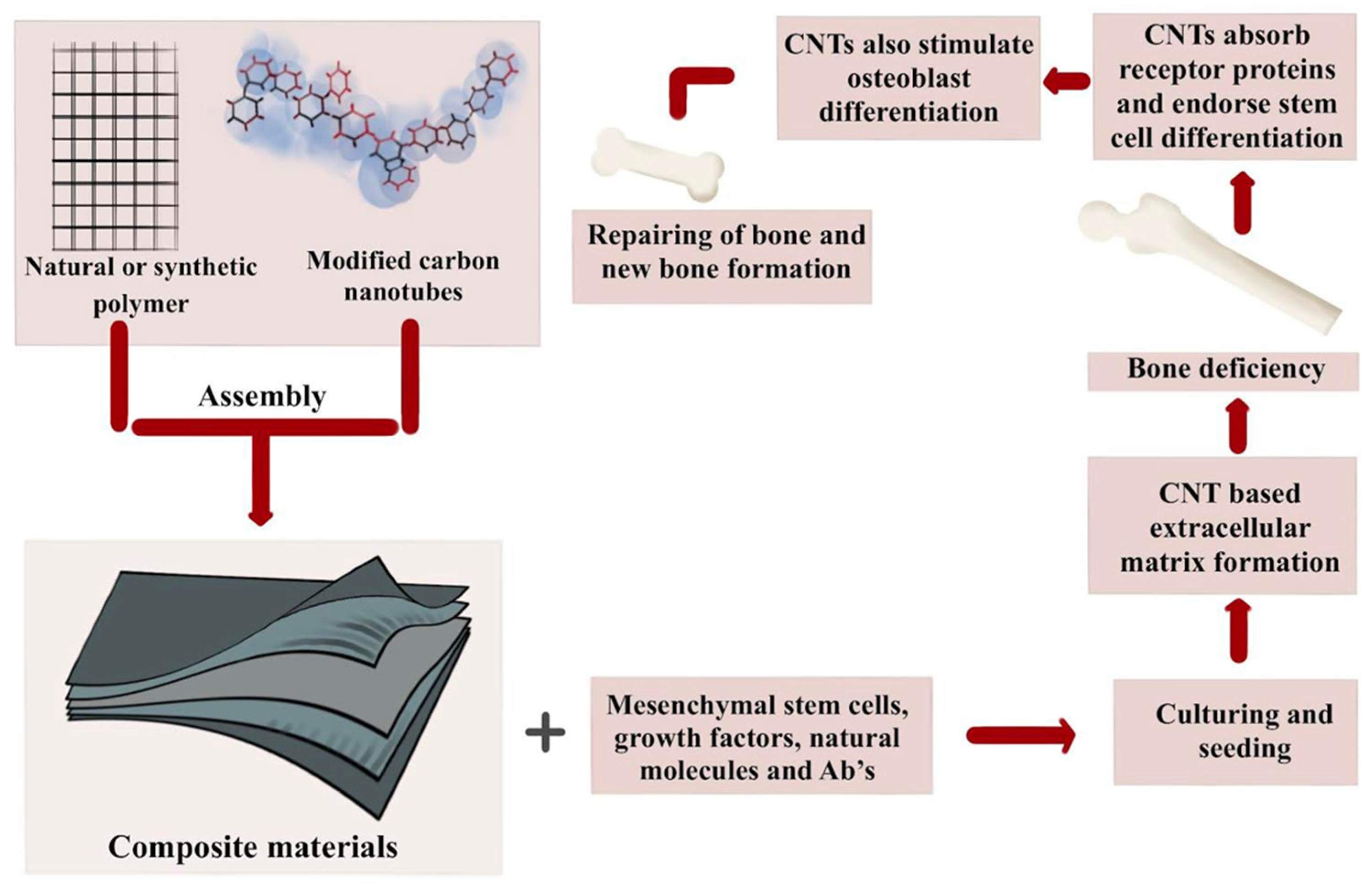
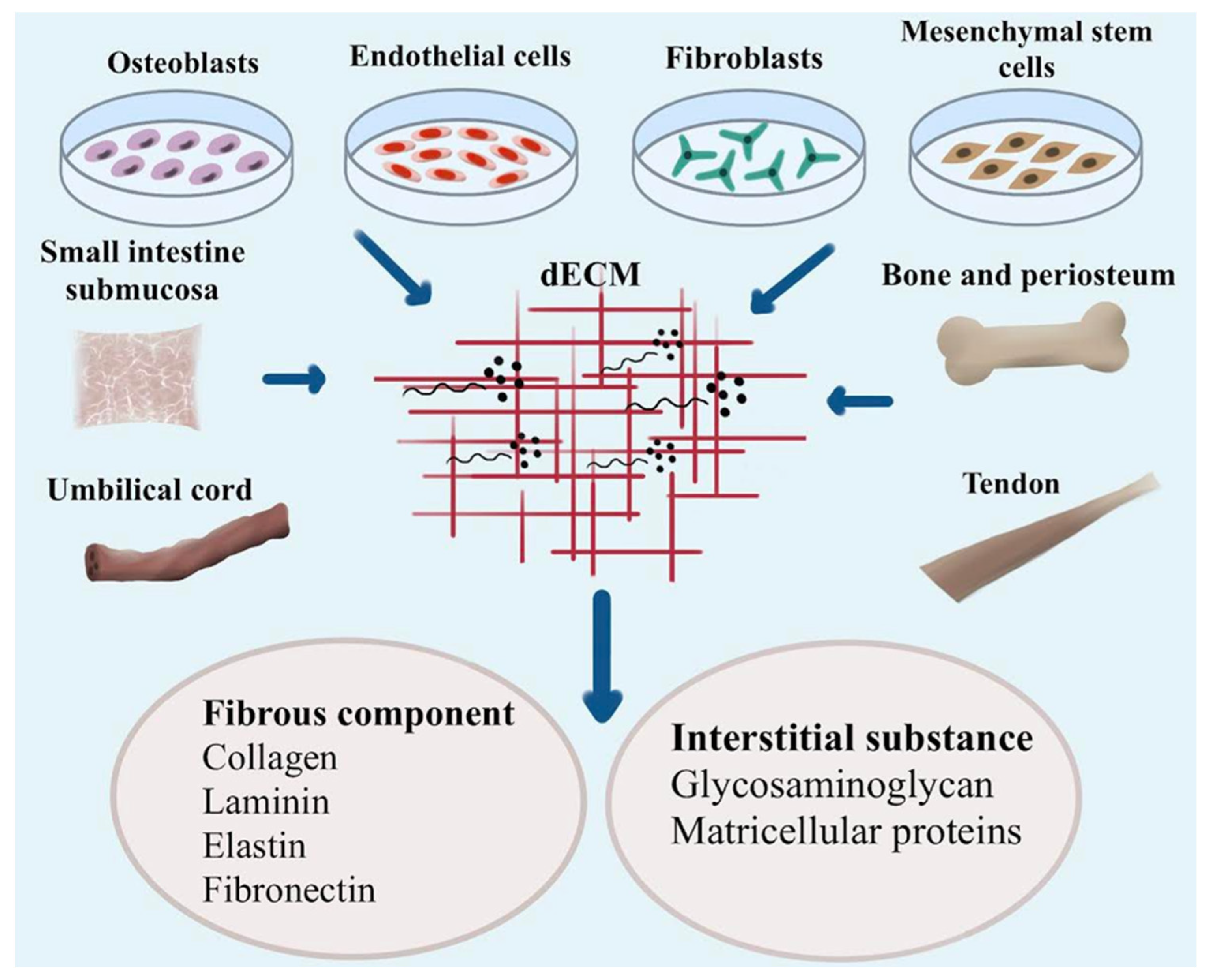

| Nanocomposites | Function | Advantages | Limitations |
|---|---|---|---|
| Scaffolds | A scaffold is a unique vehicle for transporting cells and drugs. In the field of regenerative medicine, scaffolds serve as biological mediums that promote cell proliferation and differentiation. | Scaffolds aid in the healing of cutaneous wounds by encouraging the differentiation of endothelial and epithelial cells and the production of angiogenic growth factors. Their biocompatibility ensures that they will not trigger any sort of immune response. | To spin the compositions, it is necessary to find the optimum solvent ratio. Fail to contain functional groups essential for protein binding or cellular adhesion. |
| Ceramic | Many metalloid solids, such as oxides, carbides, carbonates, and phosphates, can be produced by heating to a high temperature and then rapidly cooling. In addition, they contain both metallic and nonmetallic elements, as well as oxides, carbides, and nitrides. | In the fight against cancer and other diseases, including bacterial infections and glaucoma, they have been used as drug delivery systems. Even at nanoscale thicknesses, the greater phase stability and fracture toughness of transition metal oxide coatings make them far superior to conventional metallic or organic oxide coatings. | Since powders can become contaminated with the milling media used to grind them, especially when long and repetitive milling cycles are done, it is difficult to generate discrete nanoparticles in the lowest size range. |
| Biomaterials | They are designed to have some sort of interaction with the body to aid, enhance, or replace natural functions. They aim to heal damaged tissue in the body by tapping into the body’s regenerative capacity through the merging of materials engineering and biological science. | These composites are lighter than conventional ones because high levels of stiffness and strength may be achieved with considerably less high-density materials. The barrier properties of these modified polymers are improved above those of the unmodified counterpart. Their biocompatibility and performance are significantly higher than those of conventional or microstructure materials. | Some of the problems that need to be fixed include the unknown cytotoxicity, the structural integrity, the mechanical characteristics, the corrosion properties, and the long-term stability and service of the components. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, M.; Alqahtani, M.S.; Alhifzi, R. Recent Developments in Polymer Nanocomposites for Bone Regeneration. Int. J. Mol. Sci. 2023, 24, 3312. https://doi.org/10.3390/ijms24043312
Abbas M, Alqahtani MS, Alhifzi R. Recent Developments in Polymer Nanocomposites for Bone Regeneration. International Journal of Molecular Sciences. 2023; 24(4):3312. https://doi.org/10.3390/ijms24043312
Chicago/Turabian StyleAbbas, Mohamed, Mohammed S. Alqahtani, and Roaa Alhifzi. 2023. "Recent Developments in Polymer Nanocomposites for Bone Regeneration" International Journal of Molecular Sciences 24, no. 4: 3312. https://doi.org/10.3390/ijms24043312





