Matrix Metallopeptidase-Gene Signature Predicts Stage I Lung Adenocarcinoma Survival Outcomes
Abstract
:1. Introduction
2. Results
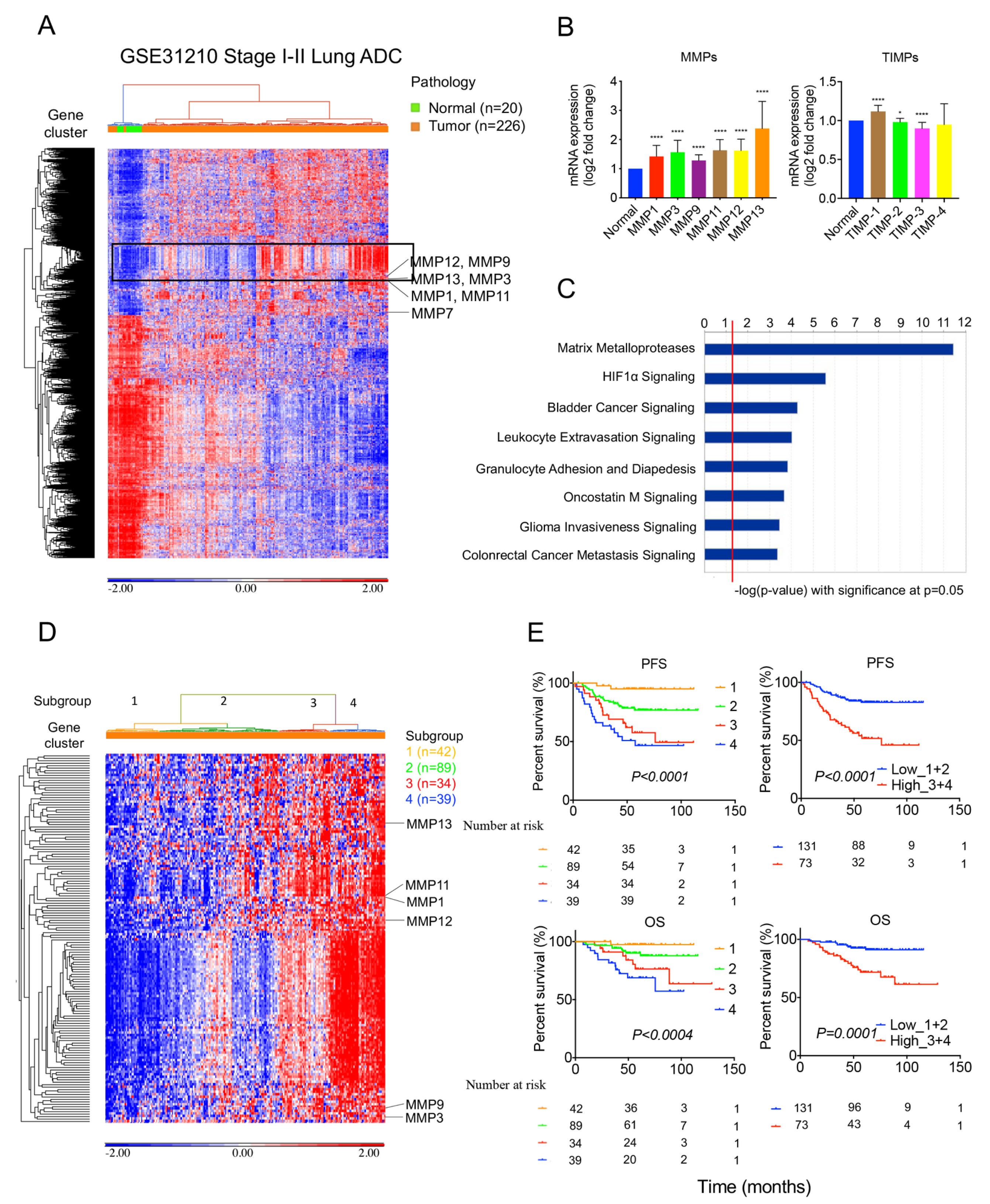
2.1. MMPs Overexpress in Lung Tumors and Correlate with Survival Outcomes in the GSE31210 Cohort
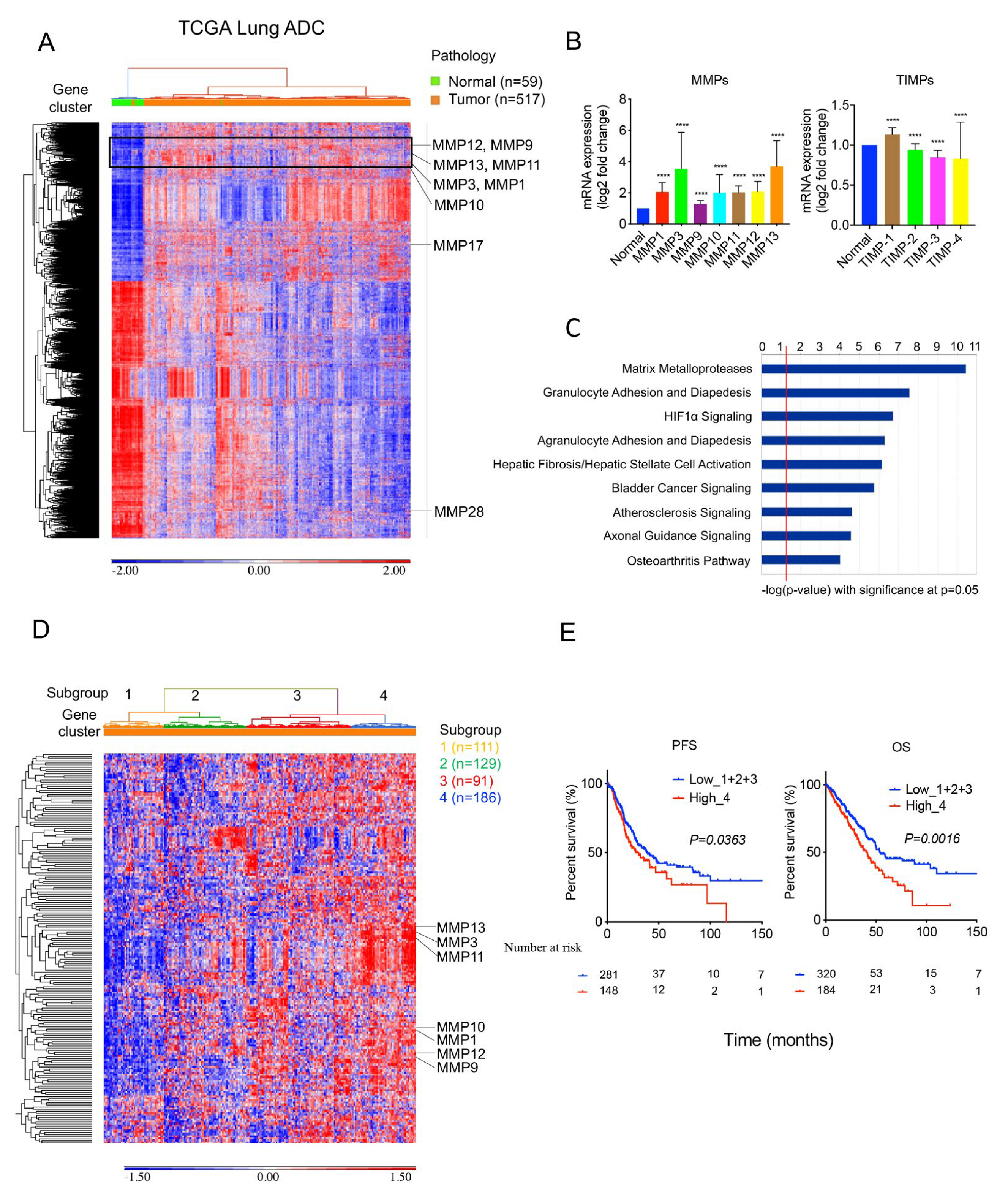
2.2. MMPs Overexpress in Lung Tumors and Correlate with Survival Outcomes in TCGA Cohort
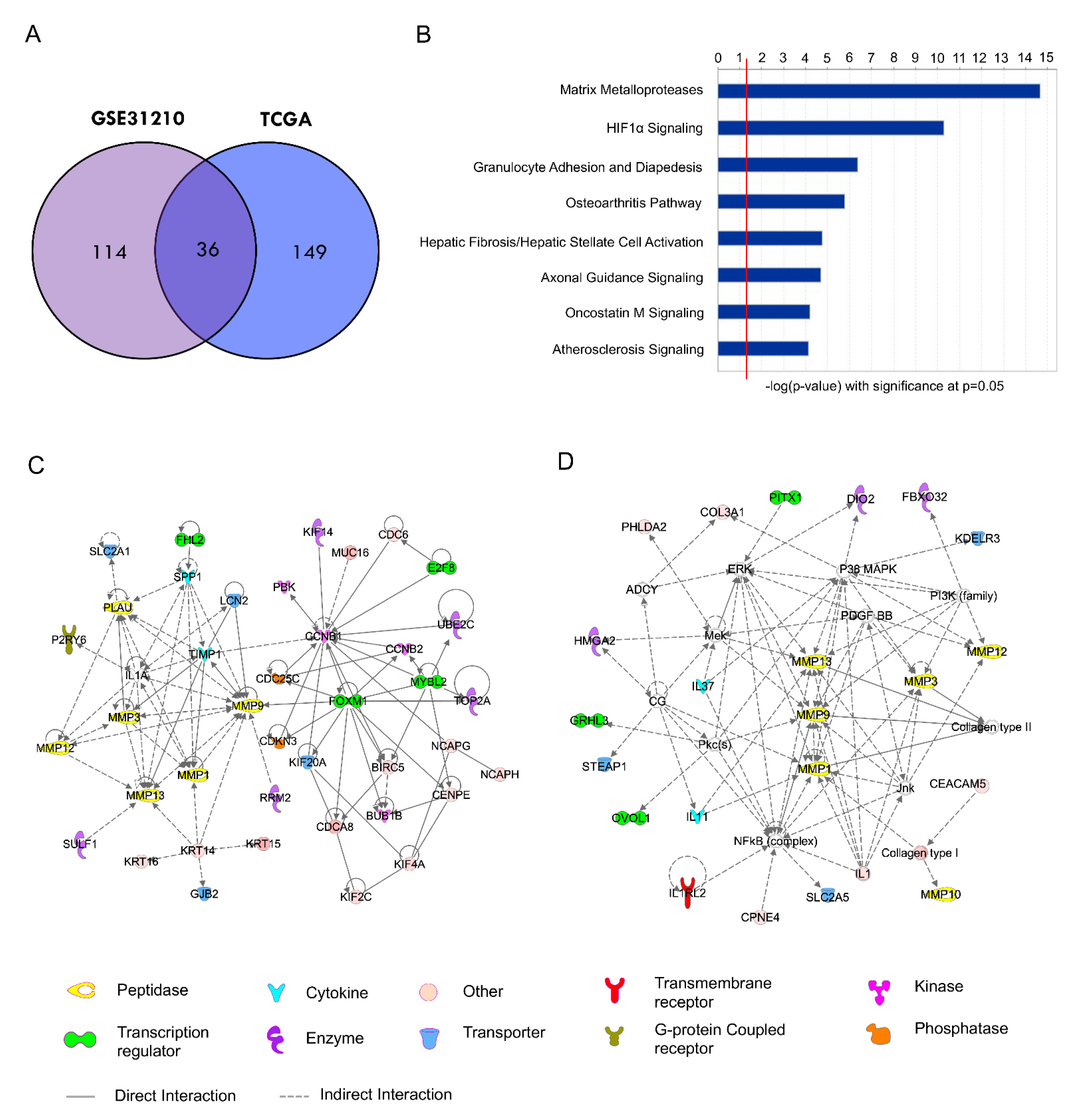
2.3. Development of a 36-Gene MMP Signature and Network Analysis
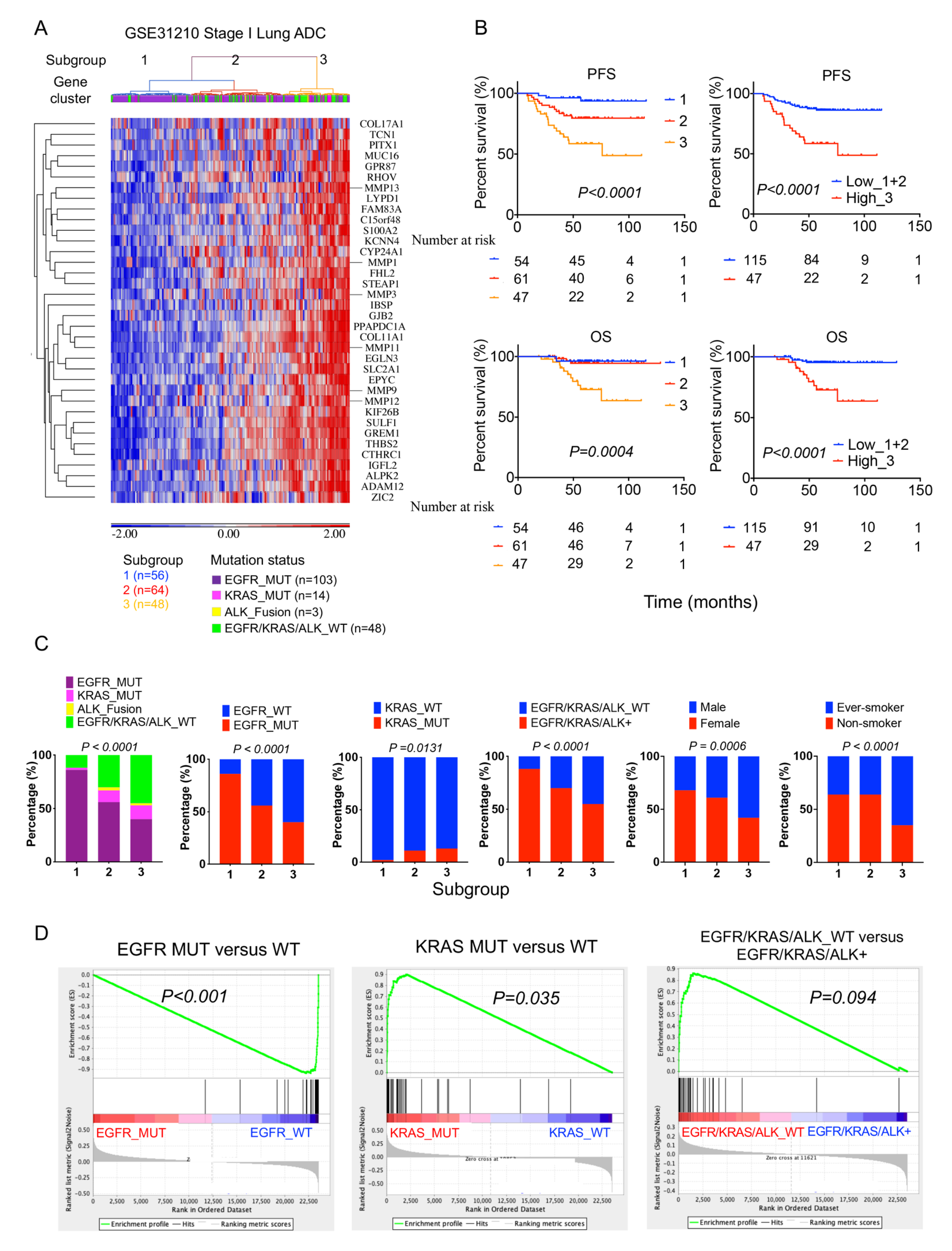
2.4. High 36-Gene MMP Signature Expression Predicts Poor Survival Outcomes in GSE3120 Stage I Lung Adenocarcinomas
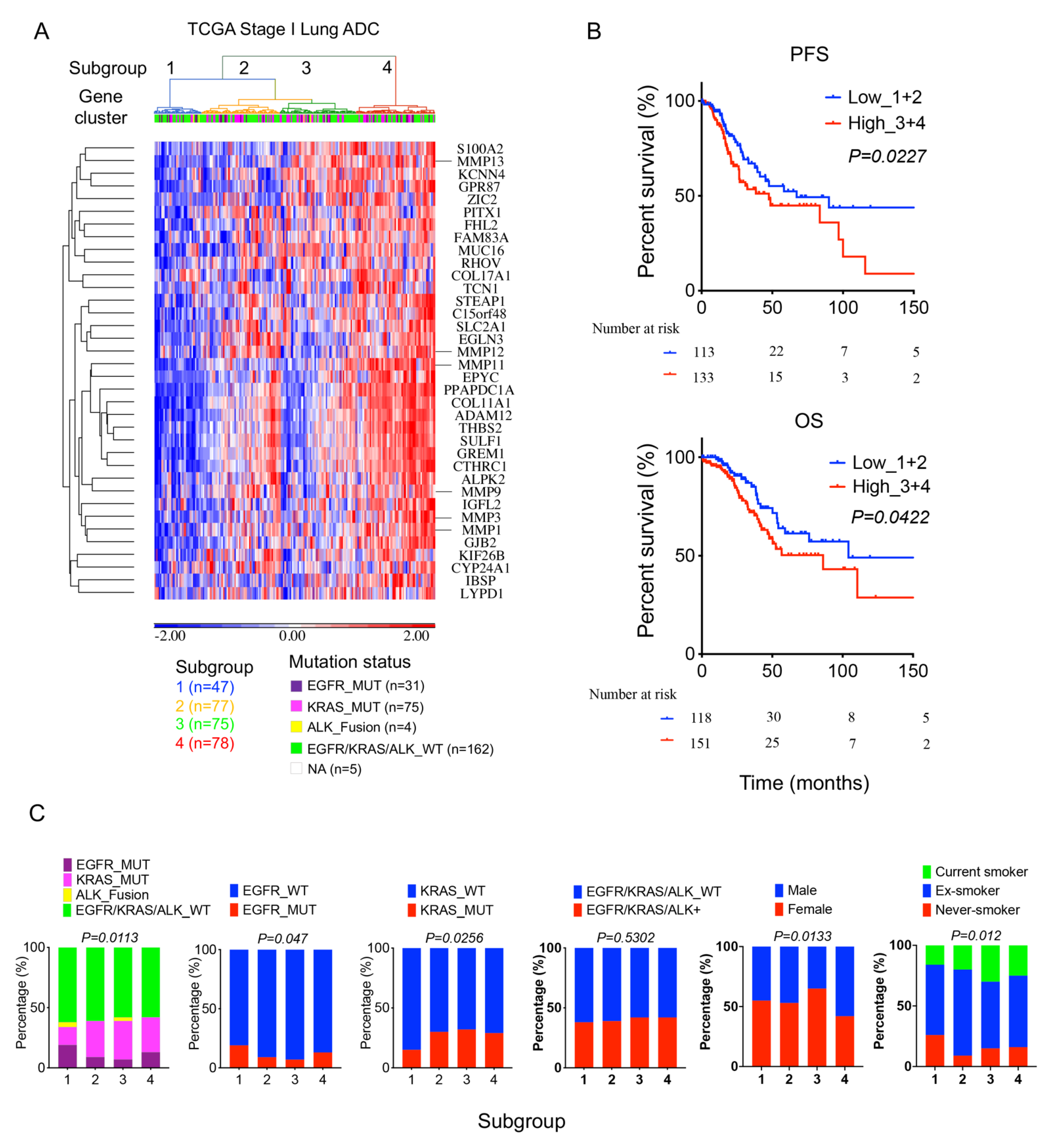
2.5. High 36-Gene MMP Signature Expression Predicts Poor Survival Outcomes in TCGA Stage I Lung Adenocarcinomas
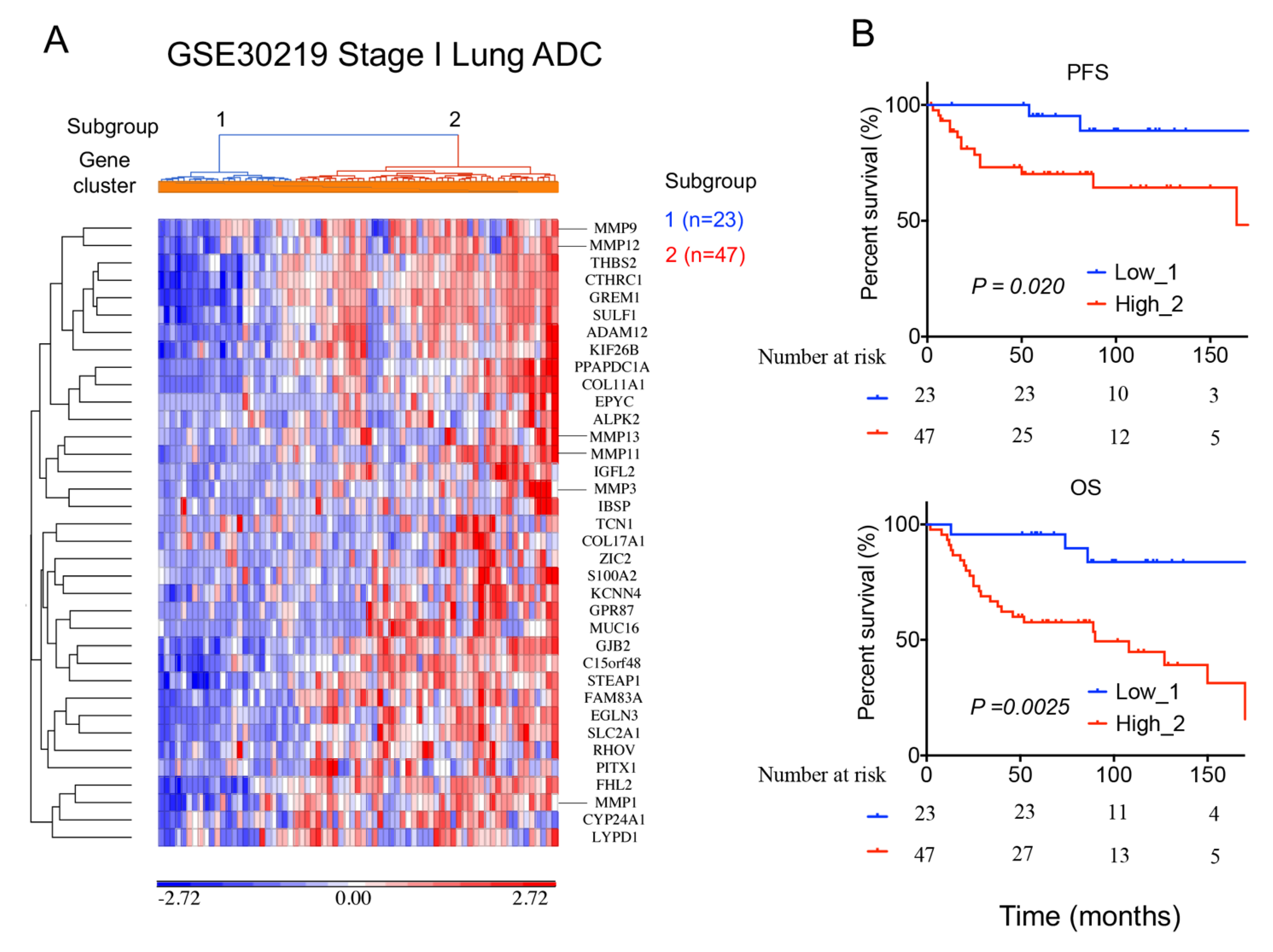
2.6. A 36-Gene MMPs Signature Is Validated in An Independent Lung Cancer Cohort
3. Discussion
4. Materials and Methods
4.1. Patient and Expression Data
4.1.1. GSE31210 Cohort
4.1.2. TCGA Cohort
4.1.3. GSE30219 Cohort
4.2. Ingenuity Pathway Analysis (IPA)
4.3. Gene Set Enrichment Analysis (GSEA)
4.4. Bioinformatic and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, N.; Bains, M.S.; Burt, M.E.; Zakowski, M.F.; McCormack, P.; Rusch, V.W.; Ginsberg, R.J. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J. Thorac. Cardiovasc. Surg. 1995, 109, 120–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harpole, D.H., Jr.; Herndon, J.E., 2nd; Young, W.G., Jr.; Wolfe, W.G.; Sabiston, D.C., Jr. Stage I nonsmall cell lung cancer. A multivariate analysis of treatment methods and patterns of recurrence. Cancer 1995, 76, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Al-Kattan, K.; Sepsas, E.; Fountain, S.W.; Townsend, E.R. Disease recurrence after resection for stage I lung cancer. Eur. J. Cardiothorac. Surg. 1997, 12, 380–384. [Google Scholar] [CrossRef]
- Nakagawa, T.; Okumura, N.; Ohata, K.; Igai, H.; Matsuoka, T.; Kameyama, K. Postrecurrence survival in patients with stage I non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2008, 34, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Hung, J.J.; Jeng, W.J.; Chou, T.Y.; Hsu, W.H.; Wu, K.J.; Huang, B.S.; Wu, Y.C. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann. Surg. 2013, 258, 1079–1086. [Google Scholar] [CrossRef]
- Tsutani, Y.; Suzuki, K.; Koike, T.; Wakabayashi, M.; Mizutani, T.; Aokage, K.; Saji, H.; Nakagawa, K.; Zenke, Y.; Takamochi, K.; et al. High-Risk Factors for Recurrence of Stage I Lung Adenocarcinoma: Follow-up Data From JCOG0201. Ann. Thorac. Surg. 2019, 108, 1484–1490. [Google Scholar] [CrossRef]
- Ujiie, H.; Kadota, K.; Chaft, J.E.; Buitrago, D.; Sima, C.S.; Lee, M.C.; Huang, J.; Travis, W.D.; Rizk, N.P.; Rudin, C.M.; et al. Solid Predominant Histologic Subtype in Resected Stage I Lung Adenocarcinoma Is an Independent Predictor of Early, Extrathoracic, Multisite Recurrence and of Poor Postrecurrence Survival. J. Clin. Oncol. 2015, 33, 2877–2884. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Chaft, J.E.; Shyr, Y.; Sepesi, B.; Forde, P.M. Preoperative and Postoperative Systemic Therapy for Operable Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 546–555. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef]
- Wu, Y.L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Strauss, G.M.; Herndon, J.E., 2nd; Maddaus, M.A.; Johnstone, D.W.; Johnson, E.A.; Harpole, D.H.; Gillenwater, H.H.; Watson, D.M.; Sugarbaker, D.J.; Schilsky, R.L.; et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J. Clin. Oncol. 2008, 26, 5043–5051. [Google Scholar] [CrossRef] [Green Version]
- Stamenkovic, I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 2000, 10, 415–433. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [Green Version]
- Shuman Moss, L.A.; Jensen-Taubman, S.; Stetler-Stevenson, W.G. Matrix metalloproteinases: Changing roles in tumor progression and metastasis. Am. J. Pathol. 2012, 181, 1895–1899. [Google Scholar] [CrossRef] [Green Version]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Passlick, B.; Sienel, W.; Seen-Hibler, R.; Wockel, W.; Thetter, O.; Mutschler, W.; Pantel, K. Overexpression of matrix metalloproteinase 2 predicts unfavorable outcome in early-stage non-small cell lung cancer. Clin. Cancer Res. 2000, 6, 3944–3948. [Google Scholar]
- Cai, M.; Onoda, K.; Takao, M.; Kyoko, I.Y.; Shimpo, H.; Yoshida, T.; Yada, I. Degradation of tenascin-C and activity of matrix metalloproteinase-2 are associated with tumor recurrence in early stage non-small cell lung cancer. Clin. Cancer Res. 2002, 8, 1152–1156. [Google Scholar] [PubMed]
- Sienel, W.; Hellers, J.; Morresi-Hauf, A.; Lichtinghagen, R.; Mutschler, W.; Jochum, M.; Klein, C.; Passlick, B.; Pantel, K. Prognostic impact of matrix metalloproteinase-9 in operable non-small cell lung cancer. Int. J. Cancer 2003, 103, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Hong, K.P.; Hong, S.H.; Kang, S.; Chung, K.Y.; Cho, S.H. MMP expression profiling in recurred stage IB lung cancer. Oncogene 2004, 23, 845–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, H.S.; Hansen, G.; Richter, G.; Taege, C.; Simm, A.; Silber, R.E.; Burdach, S. Matrix metalloproteinase-12 expression correlates with local recurrence and metastatic disease in non-small cell lung cancer patients. Clin. Cancer Res. 2005, 11, 1086–1092. [Google Scholar] [CrossRef]
- Hsu, C.P.; Shen, G.H.; Ko, J.L. Matrix metalloproteinase-13 expression is associated with bone marrow microinvolvement and prognosis in non-small cell lung cancer. Lung Cancer 2006, 52, 349–357. [Google Scholar] [CrossRef]
- Safranek, J.; Pesta, M.; Holubec, L.; Kulda, V.; Dreslerova, J.; Vrzalova, J.; Topolcan, O.; Pesek, M.; Finek, J.; Treska, V. Expression of MMP-7, MMP-9, TIMP-1 and TIMP-2 mRNA in lung tissue of patients with non-small cell lung cancer (NSCLC) and benign pulmonary disease. Anticancer Res. 2009, 29, 2513–2517. [Google Scholar]
- Li, M.; Xiao, T.; Zhang, Y.; Feng, L.; Lin, D.; Liu, Y.; Mao, Y.; Guo, S.; Han, N.; Di, X.; et al. Prognostic significance of matrix metalloproteinase-1 levels in peripheral plasma and tumour tissues of lung cancer patients. Lung Cancer 2010, 69, 341–347. [Google Scholar] [CrossRef]
- Lee, C.Y.; Shim, H.S.; Lee, S.; Lee, J.G.; Kim, D.J.; Chung, K.Y. Prognostic effect of matrix metalloproteinase-9 in patients with resected Non small cell lung cancer. J. Cardiothorac. Surg. 2015, 10, 44. [Google Scholar] [CrossRef] [Green Version]
- Greenlee, K.J.; Werb, Z.; Kheradmand, F. Matrix metalloproteinases in lung: Multiple, multifarious, and multifaceted. Physiol. Rev. 2007, 87, 69–98. [Google Scholar] [CrossRef] [Green Version]
- Hadler-Olsen, E.; Winberg, J.O.; Uhlin-Hansen, L. Matrix metalloproteinases in cancer: Their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biol. 2013, 34, 2041–2051. [Google Scholar] [CrossRef]
- Ylisirnio, S.; Hoyhtya, M.; Turpeenniemi-Hujanen, T. Serum matrix metalloproteinases -2, -9 and tissue inhibitors of metalloproteinases -1, -2 in lung cancer--TIMP-1 as a prognostic marker. Anticancer Res. 2000, 20, 1311–1316. [Google Scholar]
- Kim, S.J.; Rabbani, Z.N.; Dewhirst, M.W.; Vujaskovic, Z.; Vollmer, R.T.; Schreiber, E.G.; Oosterwijk, E.; Kelley, M.J. Expression of HIF-1alpha, CA IX, VEGF, and MMP-9 in surgically resected non-small cell lung cancer. Lung Cancer 2005, 49, 325–335. [Google Scholar] [CrossRef]
- Shimanuki, Y.; Takahashi, K.; Cui, R.; Hori, S.; Takahashi, F.; Miyamoto, H.; Fukurchi, Y. Role of serum vascular endothelial growth factor in the prediction of angiogenesis and prognosis for non-small cell lung cancer. Lung 2005, 183, 29–42. [Google Scholar] [CrossRef]
- Leinonen, T.; Pirinen, R.; Bohm, J.; Johansson, R.; Ropponen, K.; Kosma, V.M. Expression of matrix metalloproteinases 7 and 9 in non-small cell lung cancer. Relation to clinicopathological factors, beta-catenin and prognosis. Lung Cancer 2006, 51, 313–321. [Google Scholar] [CrossRef]
- Hoikkala, S.; Paakko, P.; Soini, Y.; Makitaro, R.; Kinnula, V.; Turpeenniemi-Hujanen, T. Tissue MMP-2 and MMP-9 [corrected] are better prognostic factors than serum MMP-2/TIMP-2--complex or TIMP-1 [corrected] in stage [corrected] I–III lung carcinoma. Cancer Lett 2006, 236, 125–132. [Google Scholar] [CrossRef]
- Takemoto, N.; Tada, M.; Hida, Y.; Asano, T.; Cheng, S.; Kuramae, T.; Hamada, J.; Miyamoto, M.; Kondo, S.; Moriuchi, T. Low expression of reversion-inducing cysteine-rich protein with Kazal motifs (RECK) indicates a shorter survival after resection in patients with adenocarcinoma of the lung. Lung Cancer 2007, 58, 376–383. [Google Scholar] [CrossRef]
- Liu, D.; Nakano, J.; Ishikawa, S.; Yokomise, H.; Ueno, M.; Kadota, K.; Urushihara, M.; Huang, C.L. Overexpression of matrix metalloproteinase-7 (MMP-7) correlates with tumor proliferation, and a poor prognosis in non-small cell lung cancer. Lung Cancer 2007, 58, 384–391. [Google Scholar] [CrossRef]
- Matsuyama, M.; Chijiwa, T.; Inoue, Y.; Abe, Y.; Nishi, M.; Miyazaki, N.; Furukawa, D.; Mukai, M.; Suemizu, H.; Sekido, Y.; et al. Alternative splicing variant of vascular endothelial growth factor-A is a critical prognostic factor in non-small cell lung cancer. Oncol. Rep. 2009, 22, 1407–1413. [Google Scholar]
- Grossi, F.; Spizzo, R.; Bordo, D.; Cacitti, V.; Valent, F.; Rossetto, C.; Follador, A.; Di Terlizzi, S.; Aita, M.; Morelli, A.; et al. Prognostic stratification of stage IIIA pN2 non-small cell lung cancer by hierarchical clustering analysis of tissue microarray immunostaining data: An Alpe Adria Thoracic Oncology Multidisciplinary Group study (ATOM 014). J. Thorac. Oncol. 2010, 5, 1354–1360. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Chang, Y.; Hodges, K.B.; Sun, Y.; Ma, X.; Xue, Y.; Williamson, S.R.; Lopez-Beltran, A.; Montironi, R.; Cheng, L. Expression of KISS1 and MMP-9 in non-small cell lung cancer and their relations to metastasis and survival. Anticancer Res. 2010, 30, 713–718. [Google Scholar]
- Peng, W.J.; Zhang, J.Q.; Wang, B.X.; Pan, H.F.; Lu, M.M.; Wang, J. Prognostic value of matrix metalloproteinase 9 expression in patients with non-small cell lung cancer. Clin. Chim. Acta 2012, 413, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Wang, C.Y.; Werb, Z. Matrix metalloproteinases in stem cell regulation and cancer. Matrix Biol. 2015, 44–46, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yu, H.; Liu, S.Y.; Xiao, X.S.; Dong, W.H.; Chen, Y.N.; Xu, W.; Zhu, T. Prognostic value of tissue inhibitor of metalloproteinase-2 expression in patients with non-small cell lung cancer: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0124230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvaraj, G.; Kaliamurthi, S.; Lin, S.; Gu, K.; Wei, D.Q. Prognostic Impact of Tissue Inhibitor of Metalloproteinase-1 in Non- Small Cell Lung Cancer: Systematic Review and Meta-Analysis. Curr. Med. Chem. 2019, 26, 7694–7713. [Google Scholar] [CrossRef]
- Mino, N.; Takenaka, K.; Sonobe, M.; Miyahara, R.; Yanagihara, K.; Otake, Y.; Wada, H.; Tanaka, F. Expression of tissue inhibitor of metalloproteinase-3 (TIMP-3) and its prognostic significance in resected non-small cell lung cancer. J. Surg. Oncol. 2007, 95, 250–257. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Shao, D.; Deng, Q.; Tang, H.; Liu, Z.; Chen, X.; Guo, F.; Lin, Y.; Mao, M.; et al. Comprehensive genomic profiling of lung cancer using a validated panel to explore therapeutic targets in East Asian patients. Cancer Sci. 2017, 108, 2487–2494. [Google Scholar] [CrossRef]
- Liu, S.Y.; Sun, H.; Zhou, J.Y.; Jie, G.L.; Xie, Z.; Shao, Y.; Zhang, X.; Ye, J.Y.; Chen, C.X.; Zhang, X.C.; et al. Clinical characteristics and prognostic value of the KRAS G12C mutation in Chinese non-small cell lung cancer patients. Biomark Res. 2020, 8, 22. [Google Scholar] [CrossRef]
- Woo, T.; Okudela, K.; Yazawa, T.; Wada, N.; Ogawa, N.; Ishiwa, N.; Tajiri, M.; Rino, Y.; Kitamura, H.; Masuda, M. Prognostic value of KRAS mutations and Ki-67 expression in stage I lung adenocarcinomas. Lung Cancer 2009, 65, 355–362. [Google Scholar] [CrossRef]
- Izar, B.; Zhou, H.; Heist, R.S.; Azzoli, C.G.; Muzikansky, A.; Scribner, E.E.; Bernardo, L.A.; Dias-Santagata, D.; Iafrate, A.J.; Lanuti, M. The prognostic impact of KRAS, its codon and amino acid specific mutations, on survival in resected stage I lung adenocarcinoma. J. Thorac. Oncol. 2014, 9, 1363–1369. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.M.; Zhu, Q.G.; Ding, X.X.; Lin, S.; Zhao, J.; Guan, L.; Li, T.; He, B.; Zhang, H.Q. Prognostic value of EGFR and KRAS in resected non-small cell lung cancer: A systematic review and meta-analysis. Cancer Manag. Res. 2018, 10, 3393–3404. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Au, J.S.; Thongprasert, S.; Srinivasan, S.; Tsai, C.M.; Khoa, M.T.; Heeroma, K.; Itoh, Y.; Cornelio, G.; Yang, P.C. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J. Thorac. Oncol. 2014, 9, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Hirte, H.; Vergote, I.B.; Jeffrey, J.R.; Grimshaw, R.N.; Coppieters, S.; Schwartz, B.; Tu, D.; Sadura, A.; Brundage, M.; Seymour, L. A phase III randomized trial of BAY 12-9566 (tanomastat) as maintenance therapy in patients with advanced ovarian cancer responsive to primary surgery and paclitaxel/platinum containing chemotherapy: A National Cancer Institute of Canada Clinical Trials Group Study. Gynecol. Oncol. 2006, 102, 300–308. [Google Scholar]
- Bramhall, S.R.; Schulz, J.; Nemunaitis, J.; Brown, P.D.; Baillet, M.; Buckels, J.A. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br. J. Cancer 2002, 87, 161–167. [Google Scholar] [CrossRef]
- Sparano, J.A.; Bernardo, P.; Stephenson, P.; Gradishar, W.J.; Ingle, J.N.; Zucker, S.; Davidson, N.E. Randomized phase III trial of marimastat versus placebo in patients with metastatic breast cancer who have responding or stable disease after first-line chemotherapy: Eastern Cooperative Oncology Group trial E2196. J. Clin. Oncol. 2004, 22, 4683–4690. [Google Scholar] [CrossRef]
- Coussens, L.M.; Fingleton, B.; Matrisian, L.M. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science 2002, 295, 2387–2392. [Google Scholar] [CrossRef]
- Winer, A.; Janosky, M.; Harrison, B.; Zhong, J.; Moussai, D.; Siyah, P.; Schatz-Siemers, N.; Zeng, J.; Adams, S.; Mignatti, P. Inhibition of Breast Cancer Metastasis by Presurgical Treatment with an Oral Matrix Metalloproteinase Inhibitor: A Preclinical Proof-of-Principle Study. Mol. Cancer Ther. 2016, 15, 2370–2377. [Google Scholar] [CrossRef]
- Overall, C.M.; Lopez-Otin, C. Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nat. Rev. Cancer 2002, 2, 657–672. [Google Scholar] [CrossRef]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef] [Green Version]
- Okayama, H.; Kohno, T.; Ishii, Y.; Shimada, Y.; Shiraishi, K.; Iwakawa, R.; Furuta, K.; Tsuta, K.; Shibata, T.; Yamamoto, S.; et al. Identification of genes upregulated in ALK-positive and EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res. 2012, 72, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304.e6. [Google Scholar] [CrossRef] [Green Version]
- Rousseaux, S.; Debernardi, A.; Jacquiau, B.; Vitte, A.L.; Vesin, A.; Nagy-Mignotte, H.; Moro-Sibilot, D.; Brichon, P.Y.; Lantuejoul, S.; Hainaut, P.; et al. Ectopic activation of germline and placental genes identifies aggressive metastasis-prone lung cancers. Sci. Transl. Med. 2013, 5, 186ra66. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-H.; Di, Y.P. Matrix Metallopeptidase-Gene Signature Predicts Stage I Lung Adenocarcinoma Survival Outcomes. Int. J. Mol. Sci. 2023, 24, 2382. https://doi.org/10.3390/ijms24032382
Liu C-H, Di YP. Matrix Metallopeptidase-Gene Signature Predicts Stage I Lung Adenocarcinoma Survival Outcomes. International Journal of Molecular Sciences. 2023; 24(3):2382. https://doi.org/10.3390/ijms24032382
Chicago/Turabian StyleLiu, Chia-Hsin, and Yuanpu Peter Di. 2023. "Matrix Metallopeptidase-Gene Signature Predicts Stage I Lung Adenocarcinoma Survival Outcomes" International Journal of Molecular Sciences 24, no. 3: 2382. https://doi.org/10.3390/ijms24032382





