Effect of Disulfiram on the Reproductive Capacity of Female Mice
Abstract
:1. Introduction
2. Results
2.1. Observation of Vulva
2.2. Vaginal Smears of Mice at Different Stages of the Estrous Cycle
2.3. Effect of DSF on Estrus Cycle of Mice
2.4. Effect of DSF on Reproductive Hormone Levels in Mice
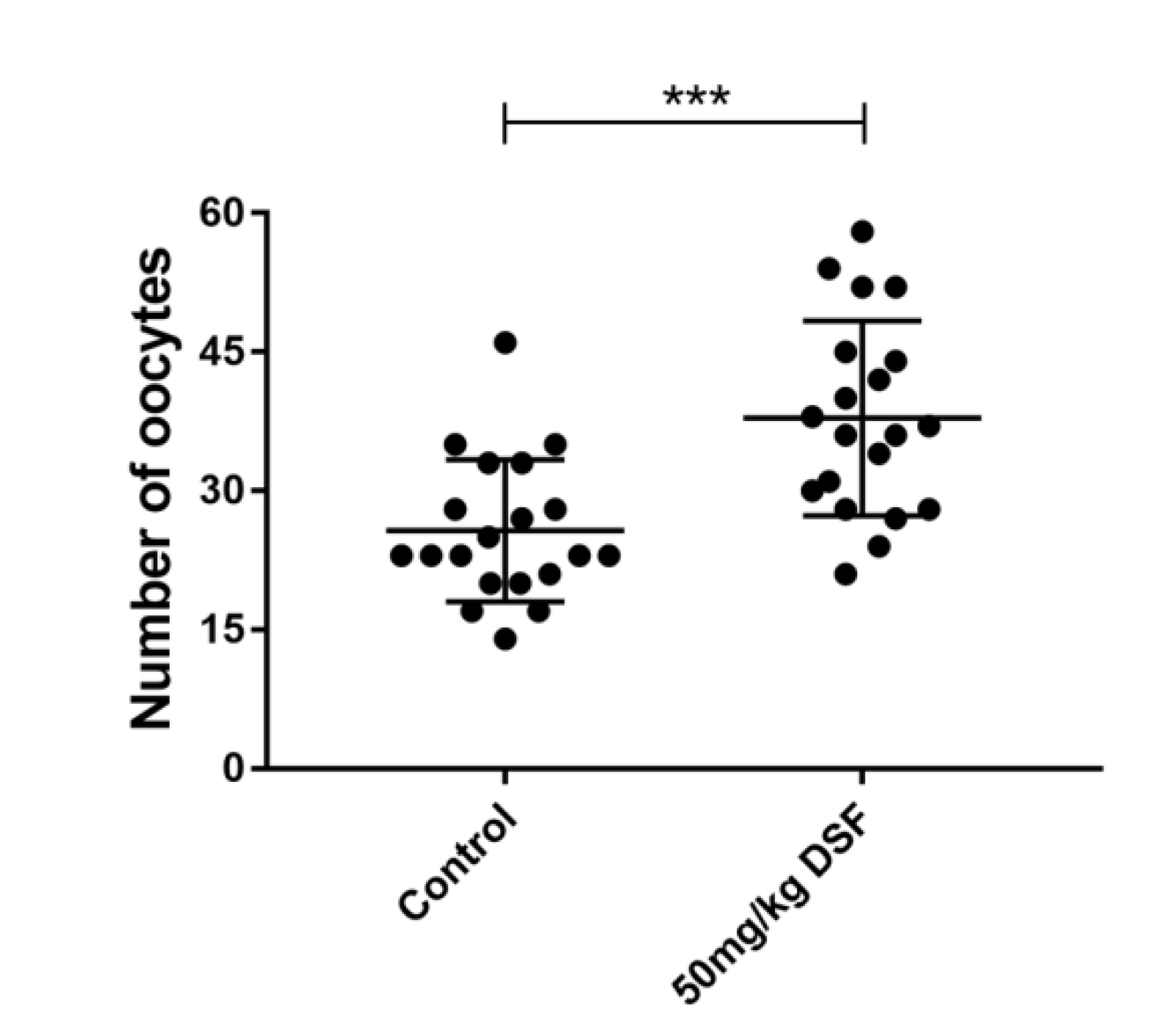
2.5. Effect of DSF on Ovulation Rate in Mice
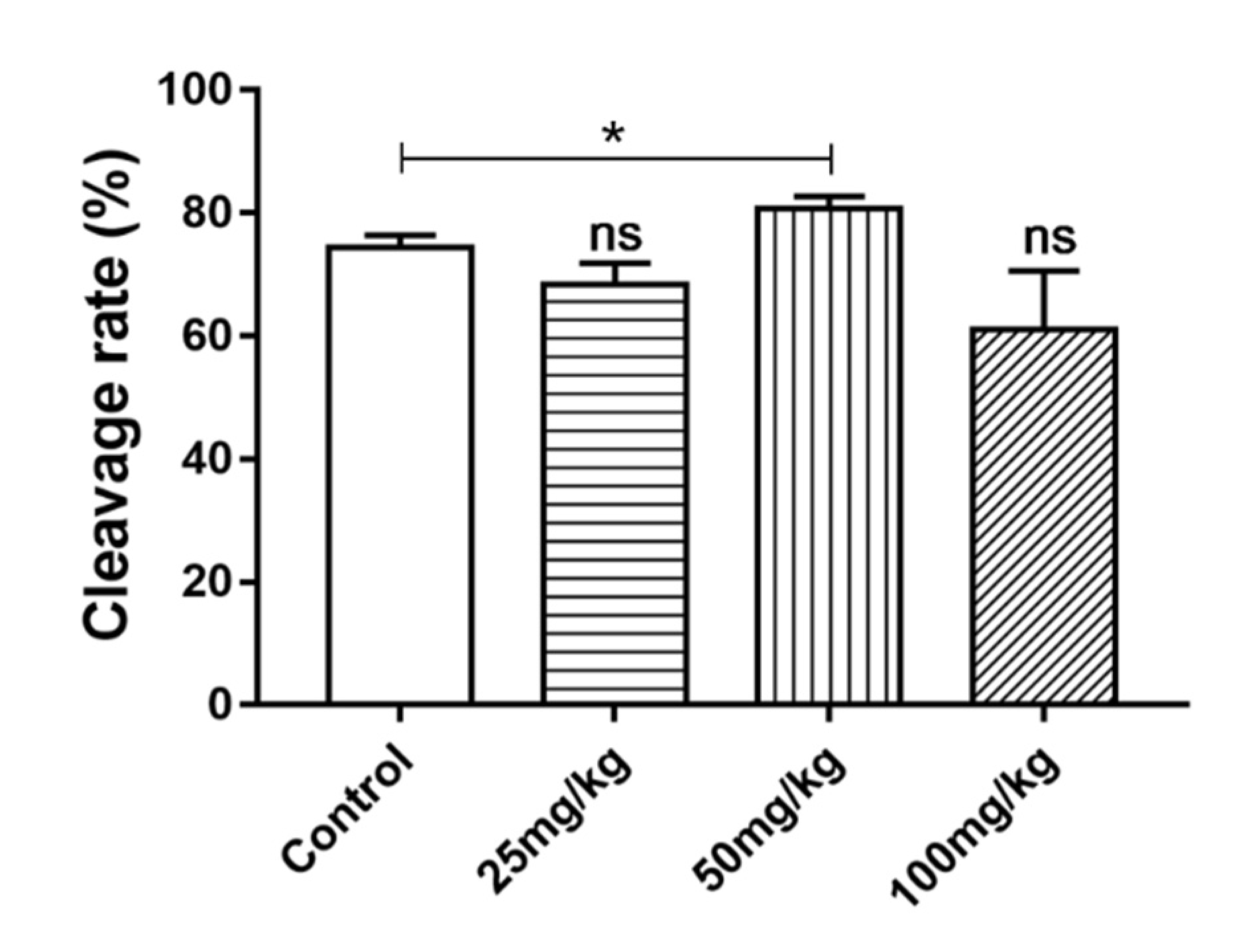
2.6. Effect of DSF on Cleavage Rate of Mice Oocytes In Vitro
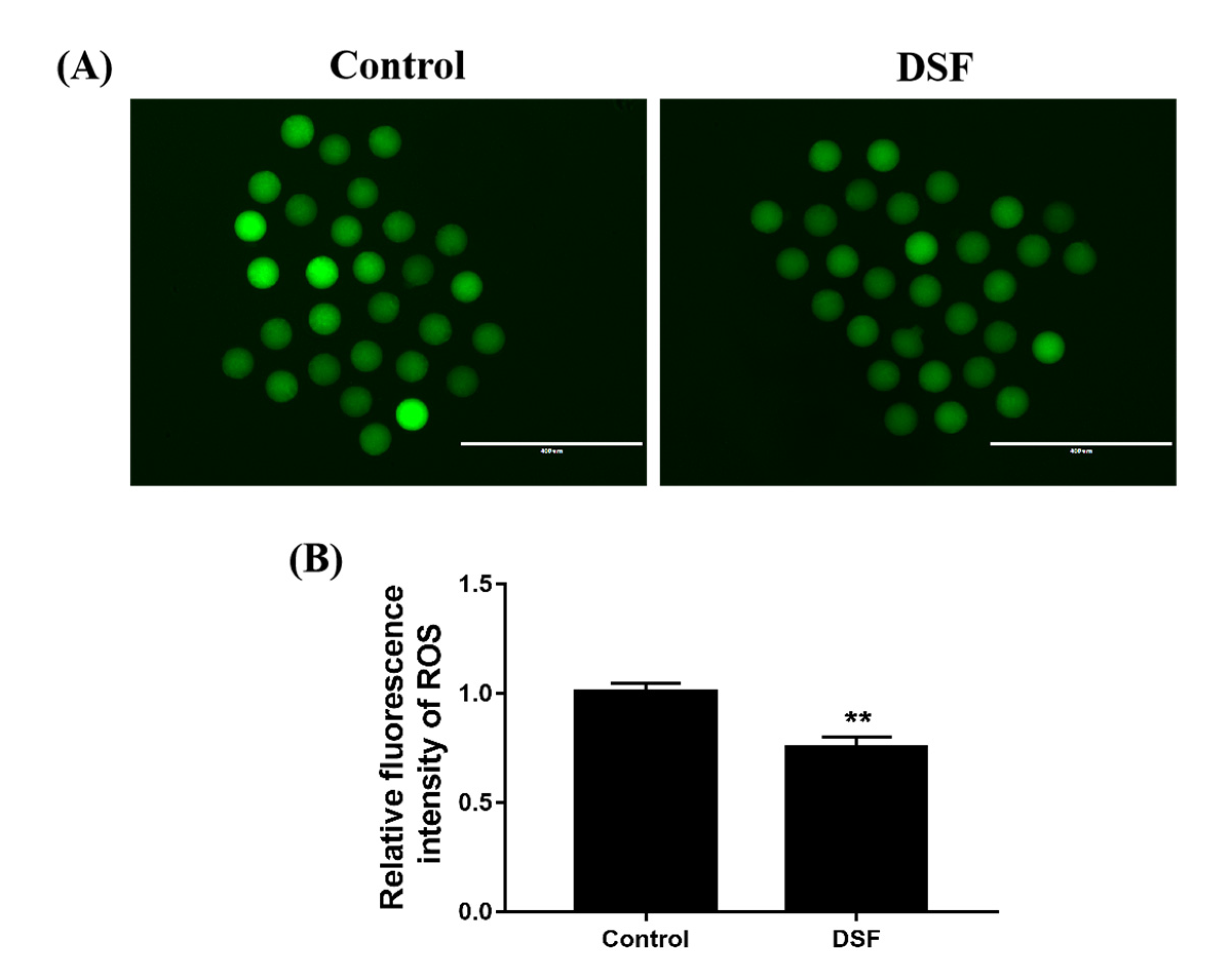
2.7. Effect of DSF on ROS Level in Mice Oocytes
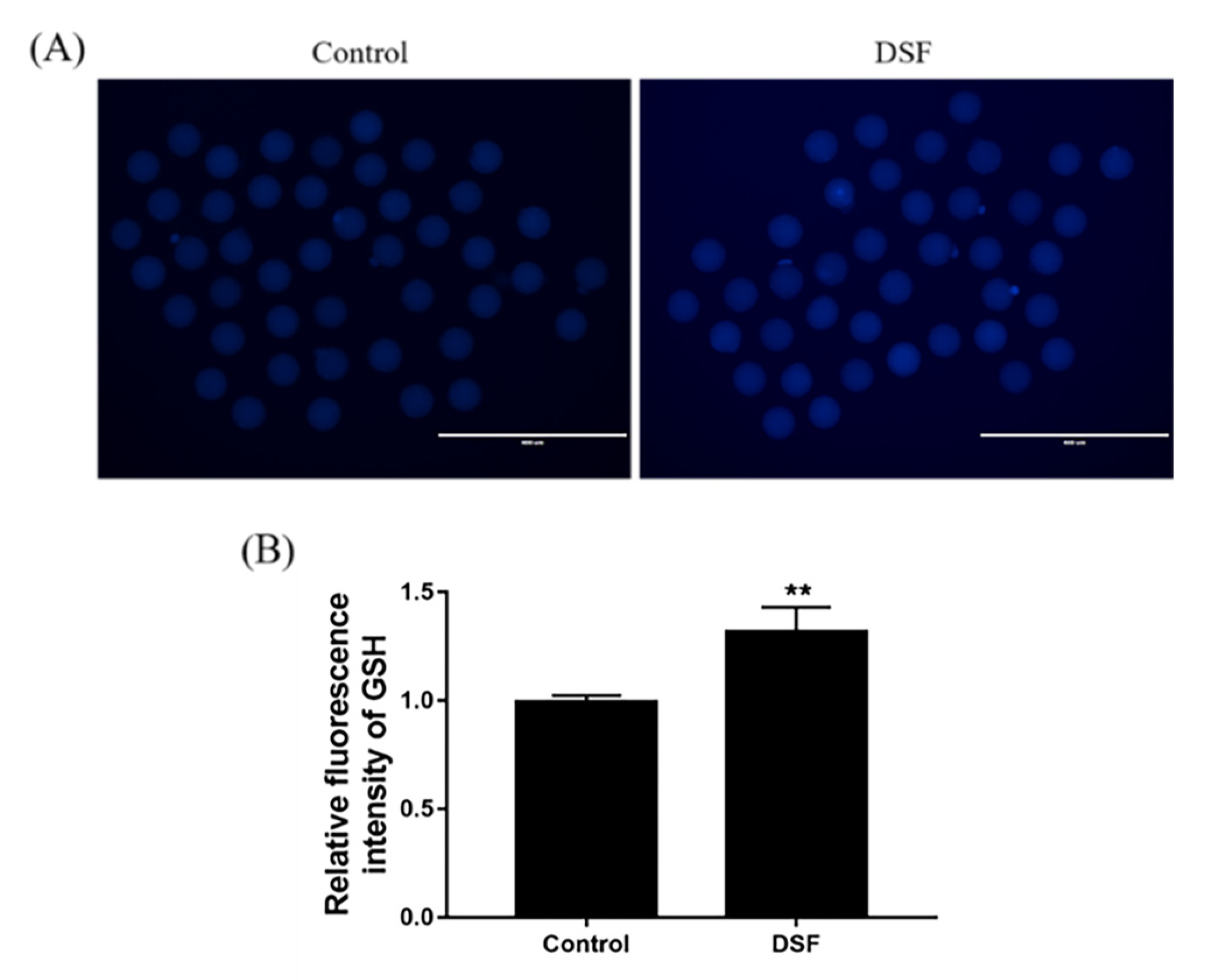
2.8. Effect of DSF on GSH Level in Mice Oocytes
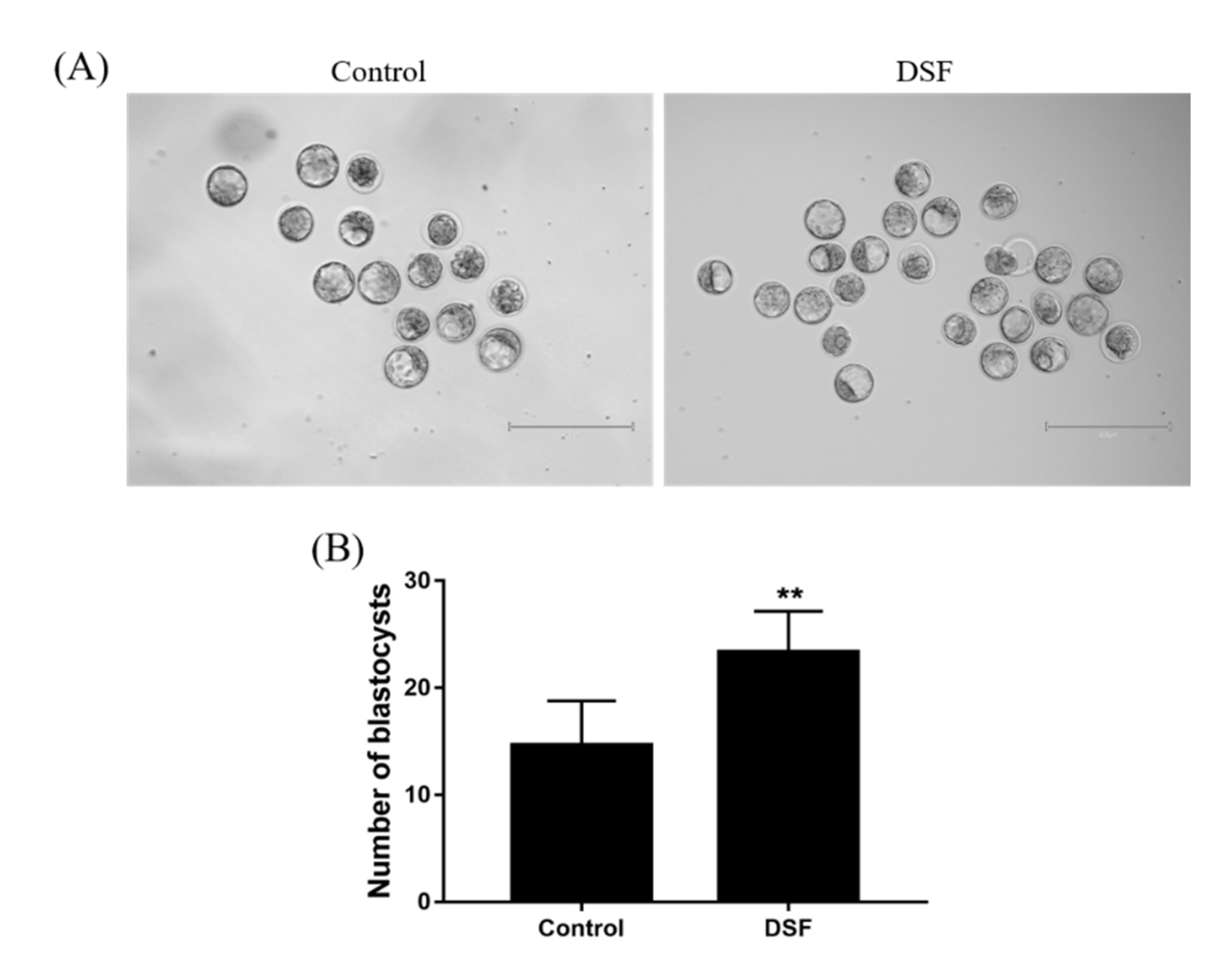
2.9. Effect of DSF on Mice Embryo Development
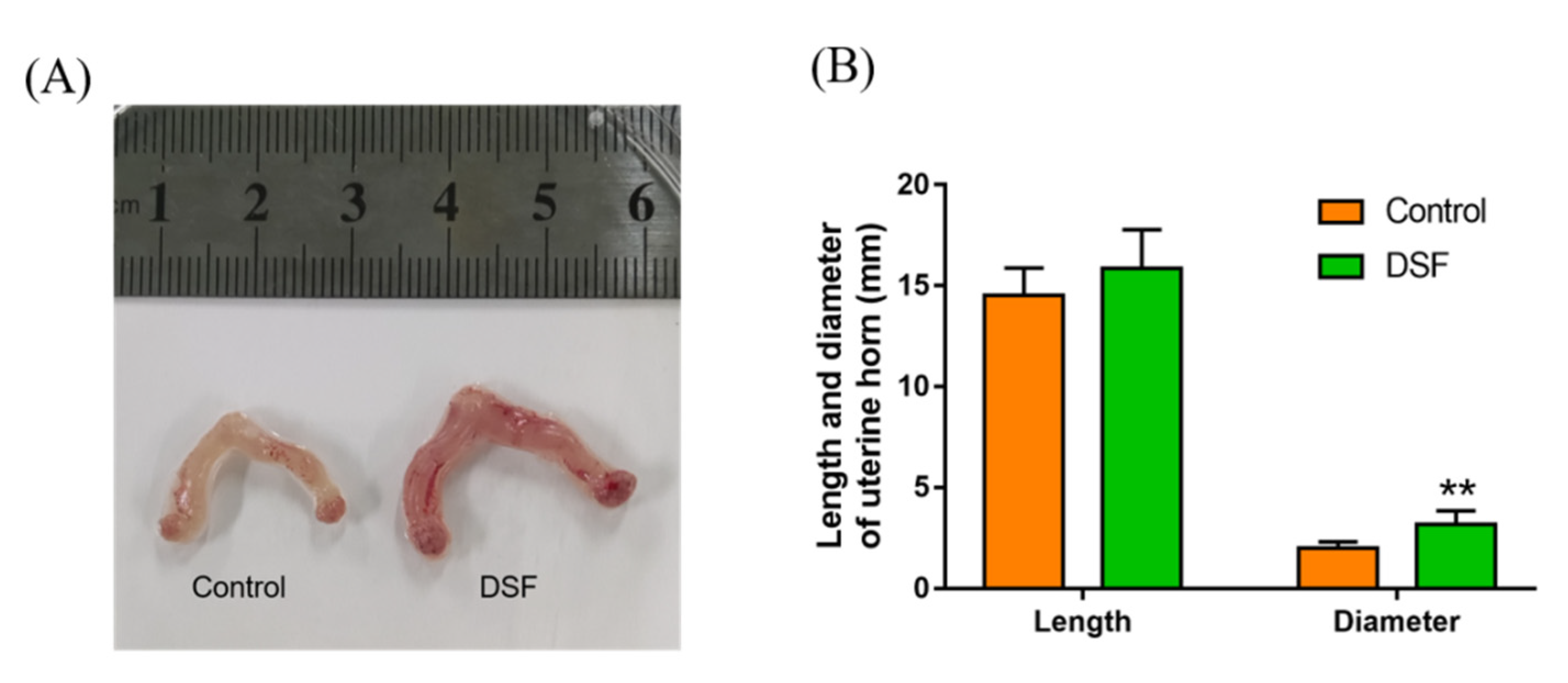
2.10. The Influence of DSF on the Uterus in Pregnant Mice
2.11. Influence of DSF on Mouse Offspring
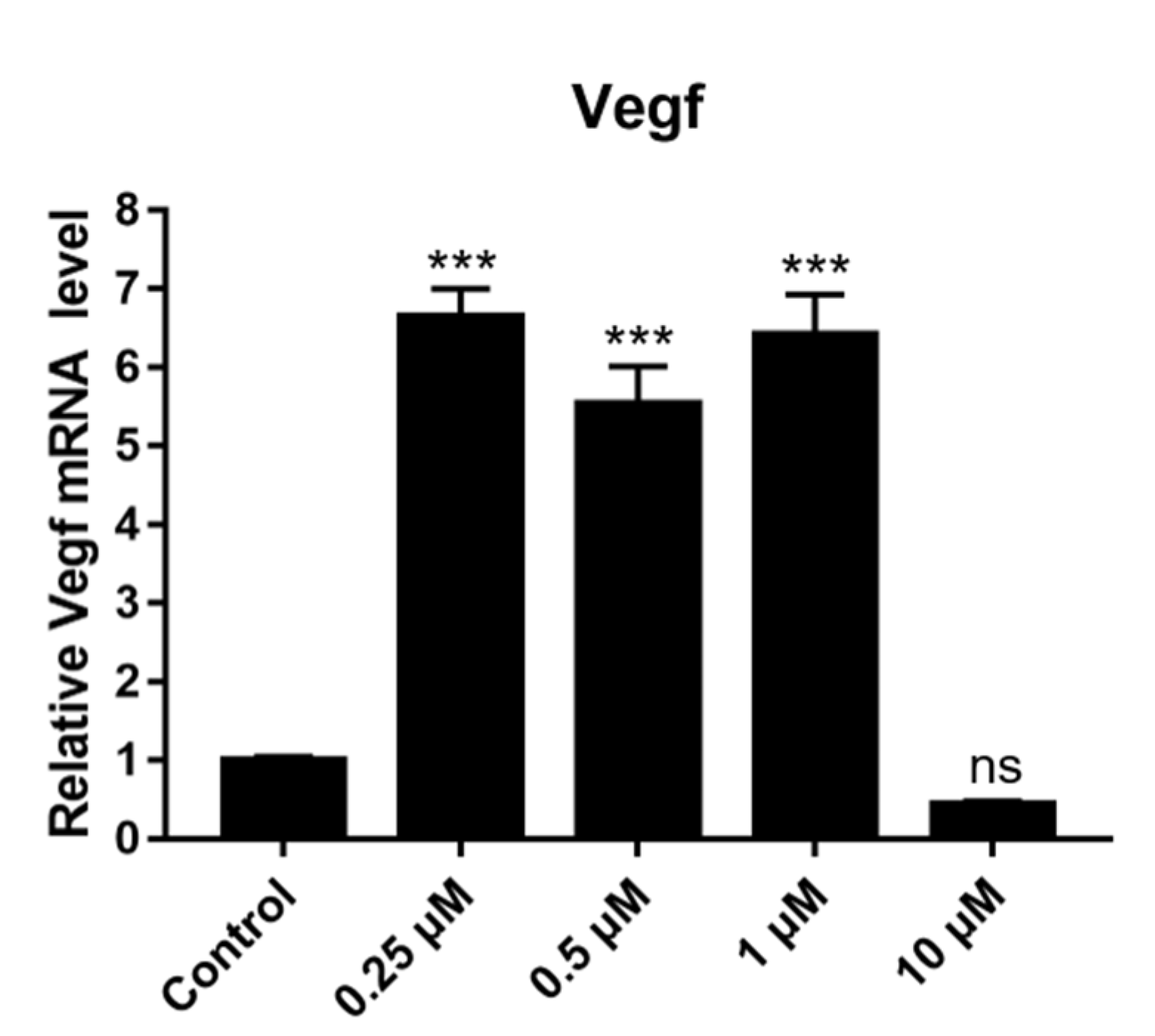
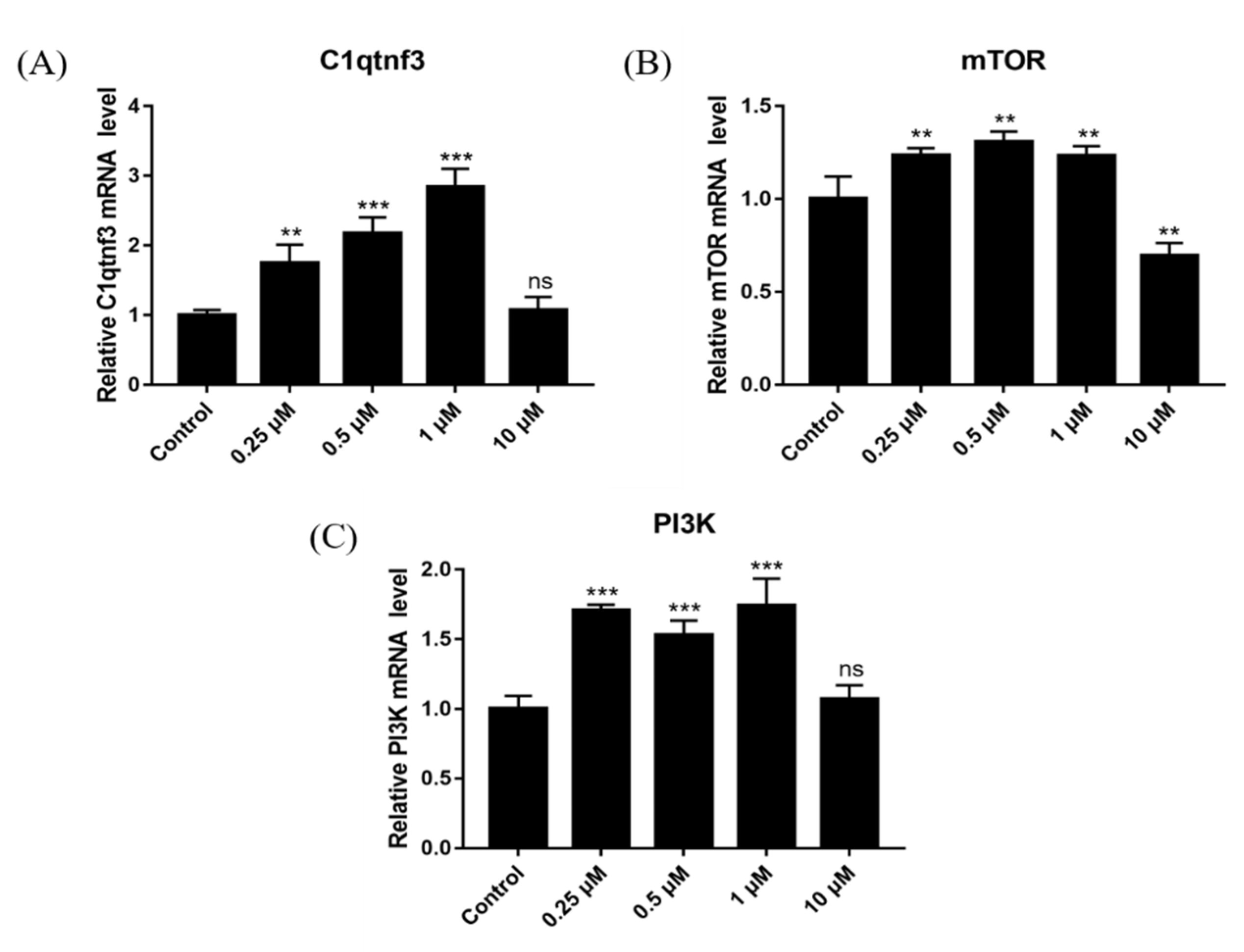
2.12. Effect of DSF on Expression of Angiogenesis-Related Genes
2.13. Effect of DSF on Expression of Follicular Development-Related Genes
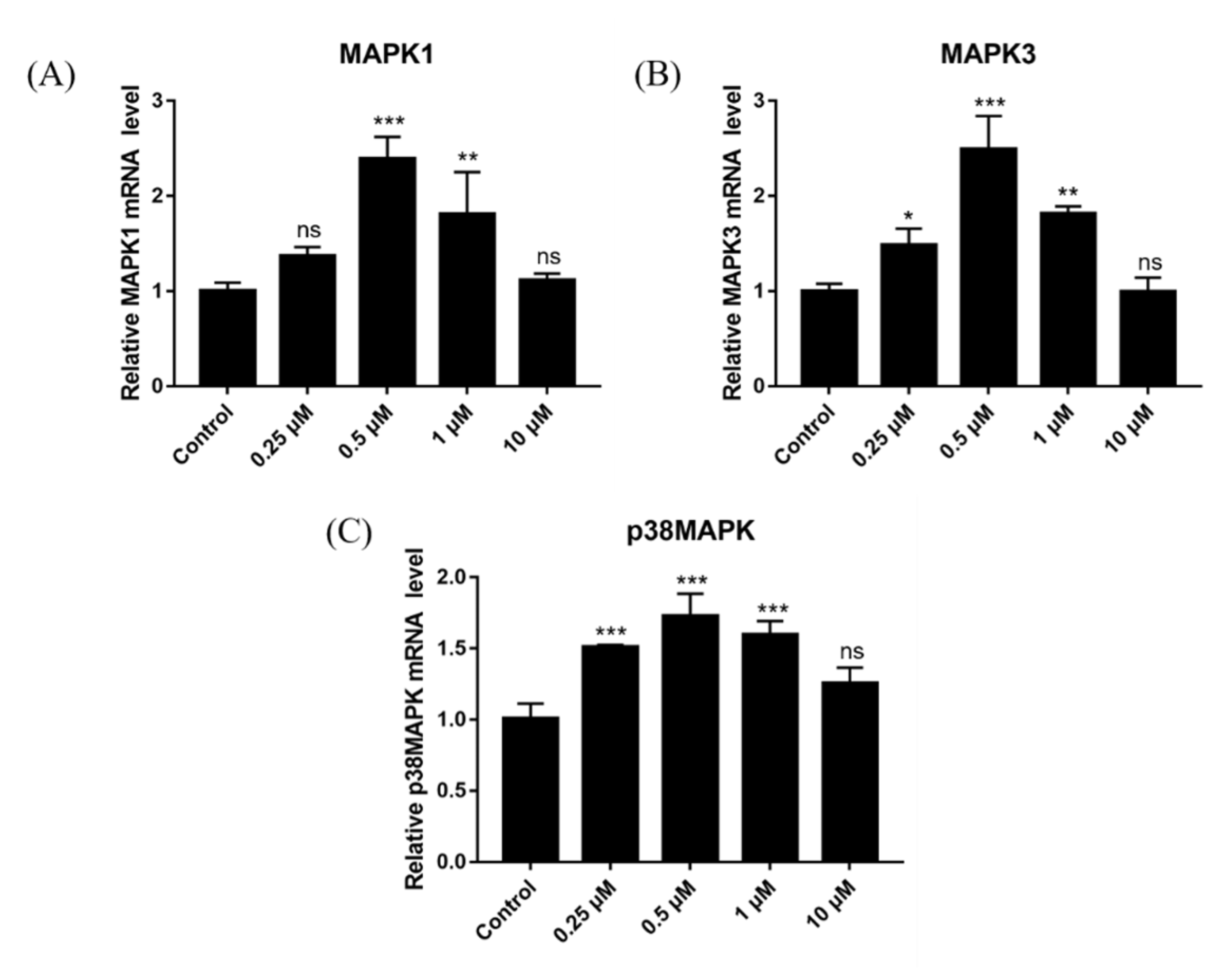
2.14. Effect of DSF on Expression of Ovulation-Related Genes
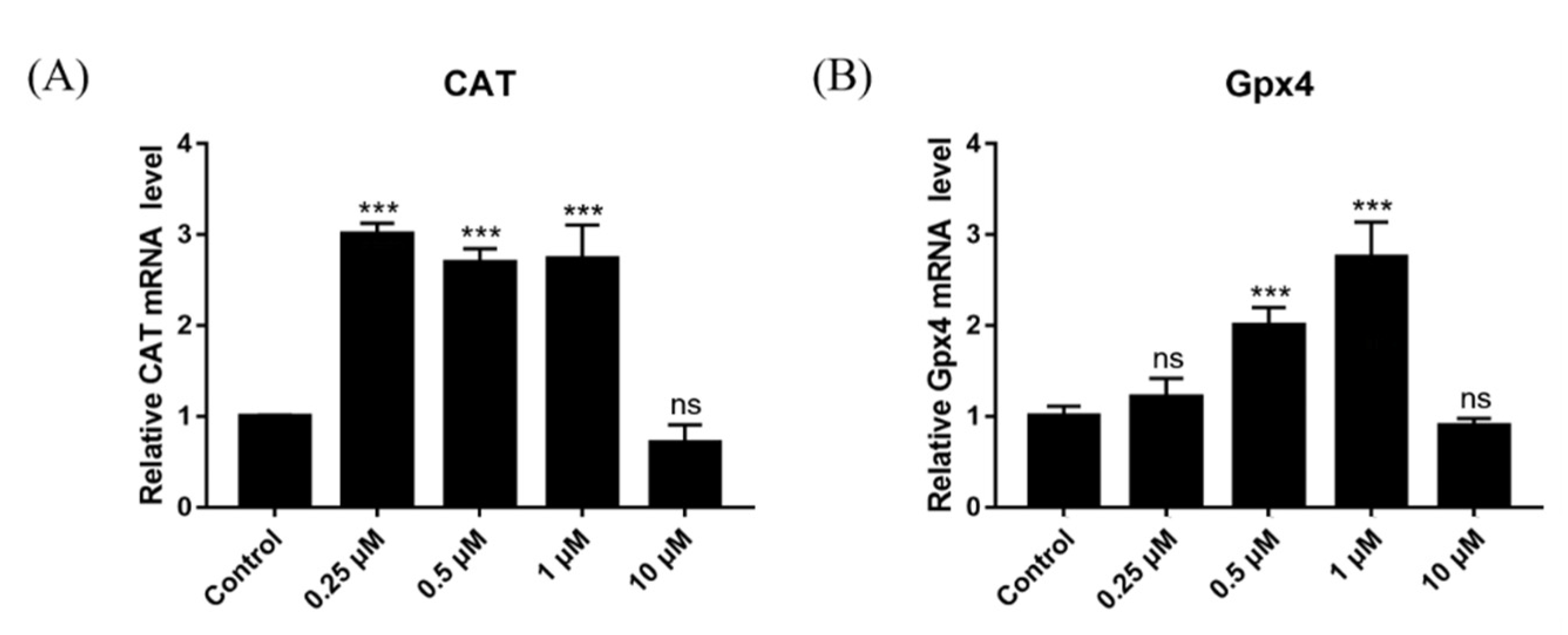
2.15. Effect of DSF on Expression of Antioxidant-Related Genes
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Reagents
4.3. DSF Preparation
4.4. Observation of Vulva and Vaginal Smear
4.5. Blood Collection and Hormone Detection
4.6. Collection of Oocytes
4.7. In Vitro Fertilization (IVF)
4.8. Reactive Oxygen Species (ROS) Detection
4.9. L-Glutathione (GSH) Detection
4.10. Collection of Blastocysts
4.11. Determination of Pregnancy Rate and Litter Size
4.12. Isolation and Culture of Ovarian Granulosa Cells
4.13. RNA Extraction and Quantitative Reverse Transcription PCR (RT-qPCR)
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sobek, A.; Tkadlec, E.; Klaskova, E.; Prochazka, M. Cytoplasmic Transfer Improves Human Egg Fertilization and Embryo Quality: An Evaluation of Sibling Oocytes in Women with Low Oocyte Quality. Reprod. Sci. 2021, 28, 1362–1369. [Google Scholar] [CrossRef]
- Carles, M.; Lefranc, E.; Bosquet, D.; Capelle, S.; Scheffler, F.; Copin, H.; Cabry, R.; Benkhalifa, M. In vitro maturation of oocytes from stimulated IVF-ICSI cycles using autologous cumulus cell co-culture: A preliminary study. Morphologie 2022, 22, 87–89. [Google Scholar] [CrossRef]
- Van der Reest, J.; Nardini Cecchino, G.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their relevance during oocyte ageing. Ageing Res. Rev. 2021, 70, 101378. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Chen, S.Y.; Arur, S. ERK phosphorylates chromosomal axis component HORMA domain protein HTP-1 to regulate oocyte numbers. Sci. Adv. 2020, 6, eabc5580. [Google Scholar] [CrossRef]
- Adrover, J.M.; Carrau, L.; Daßler-Plenker, J.; Bram, Y.; Chandar, V.; Houghton, S.; Redmond, D.; Merrill, J.R.; Shevik, M.; tenOever, B.R.; et al. Disulfiram inhibits neutrophil extracellular trap formation and protects rodents from acute lung injury and SARS-CoV-2 infection. JCI Insight 2022, 7, e157342. [Google Scholar] [CrossRef] [PubMed]
- Bonnekoh, H.; Vera, C.; Abad-Perez, A.; Radetzki, S.; Neuenschwander, M.; Specker, E.; Mahnke, N.A.; Frischbutter, S.; Latz, E.; Nazaré, M.; et al. Topical inflammasome inhibition with disulfiram prevents irritant contact dermatitis. Clin. Transl. Allergy 2021, 11, e12045. [Google Scholar] [CrossRef] [PubMed]
- Corsello, S.M.; Nagari, R.T.; Spangler, R.D.; Rossen, J.; Kocak, M.; Bryan, J.G.; Humeidi, R.; Peck, D.; Wu, X.; Tang, A.A.; et al. Discovering the anti-cancer potential of non-oncology drugs by systematic viability profiling. Nat. Cancer 2020, 1, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, K.A.; Flick-Smith, H.; Barnes, K.B.; Pereira-Leal, J.B.; Surkont, J.; Hampson, R.; Atkins, H.S.; Harding, S.V. Disulfiram, an alcohol dependence therapy, can inhibit the in vitro growth of Francisella tularensis. Int. J. Antimicrob. Agents 2019, 54, 85–88. [Google Scholar] [CrossRef]
- Deng, W.; Yang, Z.; Yue, H.; Ou, Y.; Hu, W.; Sun, P. Disulfiram suppresses NLRP3 inflammasome activation to treat peritoneal and gouty inflammation. Free Radic. Biol. Med. 2020, 152, 8–17. [Google Scholar] [CrossRef]
- Bernier, M.; Mitchell, S.J.; Wahl, D.; Diaz, A.; Singh, A.; Seo, W.; Wang, M.; Ali, A.; Kaiser, T.; Price, N.L.; et al. Disulfiram Treatment Normalizes Body Weight in Obese Mice. Cell Metab. 2020, 32, 203–214.e4. [Google Scholar] [CrossRef]
- McMahon, A.; Chen, W.; Li, F. Old wine in new bottles: Advanced drug delivery systems for disulfiram-based cancer therapy. J. Control Release 2020, 319, 352–359. [Google Scholar] [CrossRef]
- Arcos, A.; de Paola, M.; Gianetti, D.; Acuña, D.; Velásquez, Z.D.; Miró, M.P.; Toro, G.; Hinrichsen, B.; Muñoz, R.I.; Lin, Y.; et al. α-SNAP is expressed in mouse ovarian granulosa cells and plays a key role in folliculogenesis and female fertility. Sci. Rep. 2017, 7, 11765. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Brocker, C.; Koppaka, V.; Chen, Y.; Jackson, B.C.; Matsumoto, A.; Thompson, D.C.; Vasiliou, V. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature 2017, 552, 194–199. [Google Scholar]
- Ren, Y.; Lin, Y.; Chen, J.; Jin, Y. Disulfiram Chelated with Copper Promotes Apoptosis in Osteosarcoma via ROS/Mitochondria Pathway. Biol. Pharm. Bull. 2021, 44, 1557–1564. [Google Scholar] [CrossRef]
- Wehbe, M.; Anantha, M.; Shi, M.; Leung, A.W.; Dragowska, W.H.; Sanche, L.; Bally, M.B. Development and optimization of an injectable formulation of copper diethyldithiocarbamate, an active anticancer agent. Int. J. Nanomed. 2017, 12, 4129–4146. [Google Scholar] [CrossRef] [Green Version]
- Frazier, K.R.; Moore, J.A.; Long, T.E. Antibacterial activity of disulfiram and its metabolites. J. Appl. Microbiol. 2019, 126, 79–86. [Google Scholar] [CrossRef]
- Nechushtan, H.; Hamamreh, Y.; Nidal, S.; Gotfried, M.; Baron, A.; Shalev, Y.I.; Nisman, B.; Peretz, T.; Peylan-Ramu, N. A phase IIb trial assessing the addition of disulfiram to chemotherapy for the treatment of metastatic non-small cell lung cancer. Oncologist 2015, 20, 366–367. [Google Scholar] [CrossRef] [Green Version]
- Anchordoquy, J.M.; Anchordoquy, J.P.; Testa, J.A.; Sirini, M.Á.; Furnus, C.C. Cysteamine. Cell Biol. Int. 2015, 39, 1090–1098. [Google Scholar] [CrossRef]
- Wada, Y.; Ozaki, H.; Abe, N.; Mori, A.; Sakamoto, K.; Nagamitsu, T.; Nakahara, T.; Ishii, K. Role of vascular endothelial growth factor in maintenance of pregnancy in mice. Endocrinology 2013, 154, 900–910. [Google Scholar] [CrossRef]
- Bista, R.; Lee, D.W.; Pepper, O.B.; Azorsa, D.O.; Arceci, R.J.; Aleem, E. Disulfiram overcomes bortezomib and cytarabine resistance in Down-syndrome-associated acute myeloid leukemia cells. J. Exp. Clin. Cancer Res. 2017, 36, 22. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Brocker, C.; Koppaka, V. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stress. Free Radic. Biol. Med. 2013, 56, 89–101. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.; Zhang, X.; Chen, Y.; Chen, Q.; Shen, M.; Liu, H.; Deng, S. Effect of Exogenous Melatonin on the Development of Mice Ovarian Follicles and Follicular Angiogenesis. Int. J. Mol. Sci. 2021, 22, 11262. [Google Scholar] [CrossRef] [PubMed]
- Radaschin, D.S.; Bujoreanu, F.C.; Tatu, A.L. Disulfiram-Induced Baboon Syndrome. Am. J. Ther. 2020, 29, e272–e273. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Michelakos, T.; Wang, B.; Shang, Z.; DeLeo, A.B.; Duan, Z.; Hornicek, F.J.; Schwab, J.H.; Wang, X. Targeting cancer stem cells by disulfiram and copper sensitizes radioresistant chondrosarcoma to radiation. Cancer Lett. 2021, 505, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Omran, Z.; Sheikh, R.; Baothman, O.A.; Zamzami, M.A.; Alarjah, M. Repurposing Disulfiram as an Anti-Obesity Drug: Treating and Preventing Obesity in High-Fat-Fed Rats. Diabetes Metab. Syndr. Obes. 2020, 13, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chaudhary, R.; Cohen, A.L.; Fink, K.; Goldlust, S.; Boockvar, J.; Chinnaiyan, P.; Wan, L.; Marcus, S.; Campian, J.L. Disulfiram attenuates lipopolysaccharide-induced acute kidney injury by suppressing oxidative stress and NLRP3 inflammasome activation in mice. J. Pharm. Pharmacol. 2022, 74, 259–267. [Google Scholar] [CrossRef]
- Wang, X.; Ye, H.; Yang, S.; Sha, X.; Wang, X.; Zhang, T.; Chen, R.; Xiao, W.; Yang, H. Disulfiram Exerts Antifibrotic and Anti-Inflammatory Therapeutic Effects on Perimysial Orbital Fibroblasts in Graves’ Orbitopathy. Int. J. Mol. Sci. 2022, 23, 5261. [Google Scholar] [CrossRef]
- Ou, A.T.; Zhang, J.X.; Fang, Y.F.; Wang, R.; Tang, X.P.; Zhao, P.F.; Zhao, Y.G.; Zhang, M.; Huang, Y.Z. Disulfiram-loaded lactoferrin nanoparticles for treating inflammatory diseases. Acta Pharmacol. Sin. 2021, 42, 1913–1920. [Google Scholar] [CrossRef]
- Sander, P.; Feng, M.; Schweitzer, M.K.; Wilting, F.; Gutenthaler, S.M.; Arduino, D.M.; Fischbach, S.; Dreizehnter, L.; Moretti, A.; Gudermann, T.; et al. Approved drugs ezetimibe and disulfiram enhance mitochondrial Ca2+ uptake and suppress cardiac arrhythmogenesis. Br. J. Pharmacol. 2021, 178, 4518–4532. [Google Scholar] [CrossRef]
- Akison, L.K.; Robertson, S.A.; Gonzalez, M.B.; Richards, J.S.; Smith, C.W.; Russell, D.L.; Robker, R.L. Regulation of the ovarian inflammatory response at ovulation by nuclear progesterone receptor. Am. J. Reprod. Immunol. 2018, 79, e12835. [Google Scholar] [CrossRef]
- Favié, L.M.A.; Peeters-Scholte, C.M.P.C.D.; Bakker, A.; Tjabbes, H.; Egberts, T.C.G.; van Bel, F.; Rademaker, C.M.A.; Vis, P.; Groenendaal, F. Translation from animal to clinical studies, choosing the optimal moment. Pediatr. Res. 2020, 88, 836–837. [Google Scholar] [CrossRef]
- Choi, Y.; Rosewell, K.L.; Brännström, M.; Akin, J.W.; Curry, T.E.; Jo, M. FOS, a Critical Downstream Mediator of PGR and EGF Signaling Necessary for Ovulatory Prostaglandins in the Human Ovary. J. Clin. Endocrinol. Metab. 2018, 103, 4241–4252. [Google Scholar] [CrossRef] [Green Version]
- Pinto, E. Neurobiological mechanisms of alcohol dependence. Rev. Med. Liege 2020, 74, 274–280. [Google Scholar]
- Hu, J.J.; Liu, X.; Xia, S.; Zhang, Z.; Zhang, Y.; Zhao, J.; Ruan, J.; Luo, X.; Lou, X.; Bai, Y.; et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 2020, 21, 736–745. [Google Scholar] [CrossRef]
- Wei, S.; Xiao, Z.; Huang, J.; Peng, Z.; Zhang, B.; Li, W. Disulfiram inhibits oxidative stress and NLRP3 inflammasome activation to prevent LPS-induced cardiac injury. Int. Immunopharmacol. 2022, 105, 108545. [Google Scholar] [CrossRef]
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 2016, 23, 36. [Google Scholar] [CrossRef] [Green Version]
- Khatun, H.; Wada, Y.; Konno, T.; Tatemoto, H.; Yamanaka, K.I. Endoplasmic reticulum stress attenuation promotes bovine oocyte maturation in vitro. Reproduction 2020, 159, 361–370. [Google Scholar] [CrossRef]
- Chelenga, M.; Sakaguchi, K.; Kawano, K.; Furukawa, E.; Yanagawa, Y.; Katagiri, S.; Nagano, M. Low oxygen environment and astaxanthin supplementation promote the developmental competence of bovine oocytes derived from early antral follicles during 8 days of in vitro growth in a gas-permeable culture device. Theriogenology 2022, 177, 116–126. [Google Scholar] [CrossRef]
- Liu, Y.J.; Ji, D.M.; Liu, Z.B.; Wang, T.J.; Xie, F.F.; Zhang, Z.G.; Wei, Z.L.; Zhou, P.; Cao, Y.X. Melatonin maintains mitochondrial membrane potential and decreases excessive intracellular Ca2+ levels in immature human oocytes. Life Sci. 2019, 235, 116810. [Google Scholar] [CrossRef]
- Ding, H.; Zhu, T.; Yin, X.; Liu, J.; Zhang, L.; Bernier, M.; Zhao, R. Pyrrolidine dithiocarbamate protects pancreatic β-cells from oxidative damage through regulation of FoxO1 activity in type 2 diabetes rats. Acta Biochim. Biophys. Sin. 2014, 46, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Lodde, V.; Luciano, A.M.; Musmeci, G.; Miclea, I.; Tessaro, I.; Aru, M.; Albertini, D.F.; Franciosi, F. A Nuclear and Cytoplasmic Characterization of Bovine Oocytes Reveals That Cysteamine Partially Rescues the Embryo Development in a Model of Low Ovarian Reserve. Animals 2021, 11, 1936. [Google Scholar] [CrossRef] [PubMed]
- Betarelli, R.P.; Rocco, M.; Yeste, M.; Fernández-Novell, J.M.; Placci, A.; Azevedo Pereira, B.; Castillo-Martín, M.; Estrada, E.; Peña, A.; Zangeronimo, M.G.; et al. The achievement of boar sperm in vitro capacitation is related to an increase of disrupted disulphide bonds and intracellular reactive oxygen species levels. Andrology 2018, 6, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yao, Y.; Li, K.; Jiao, L.; Zhu, J.; Ni, C.; Li, M.; Dou, Q.P.; Yang, H. An Updated Review of Disulfiram: Molecular Targets and Strategies for Cancer Treatment. Curr. Pharm. Des. 2019, 25, 3248–3256. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Yang, Z.; Yue, H.; Ou, Y.; Hu, W.; Sun, P. Ovulation: Parallels with Inflammatory Processes. Endocr. Rev. 2019, 40, 369–416. [Google Scholar]
- Kim, K.H.; Kim, Y.H.; Son, J.E.; Lee, J.H.; Kim, S.; Choe, M.S.; Moon, J.H.; Zhong, J.; Fu, K.; Lenglin, F.; et al. Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage. Cell Res. 2017, 27, 1309–1326. [Google Scholar] [CrossRef]
- Jo, Y.J.; Yoon, S.B.; Park, B.J.; Lee, S.I.; Kim, K.J.; Kim, S.Y.; Kim, M.; Lee, J.K.; Lee, S.Y.; Lee, D.H.; et al. Particulate Matter Exposure During Oocyte Maturation: Cell Cycle Arrest, ROS Generation, and Early Apoptosis in Mice. Front. Cell Dev. Biol. 2020, 8, 602097. [Google Scholar] [CrossRef]
- Liegner, K.B. Disulfiram (Tetraethylthiuram Disulfide) in the Treatment of Lyme Disease and Babesiosis: Report of Experience in Three Cases. Antibiotics 2019, 8, 72. [Google Scholar] [CrossRef] [Green Version]
- Paule, S.G.; Heng, S.; Samarajeewa, N.; Li, Y.; Mansilla, M.; Webb, A.I.; Nebl, T.; Young, S.L.; Lessey, B.A.; Hull, M.L.; et al. Podocalyxin is a key negative regulator of human endometrial epithelial receptivity for embryo implantation. Hum. Reprod. 2021, 36, 1353–1366. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Shi, X.; Zhang, C.; Song, G.; Huang, D. The Factors and Pathways Regulating the Activation of Mammalian Primordial Follicles in vivo. Front. Cell Dev. Biol. 2020, 8, 575706. [Google Scholar] [CrossRef]
- Zha, J.; Chen, F.; Dong, H.; Shi, P.; Yao, Y.; Zhang, Y.; Li, R.; Wang, S.; Li, P.; Wang, W.; et al. Disulfiram targeting lymphoid malignant cell lines via ROS-JNK activation as well as Nrf2 and NF-kB pathway inhibition. J. Transl. Med. 2014, 12, 163. [Google Scholar] [CrossRef] [Green Version]
- Rajin, T.R.; Patra, M.K.; Sheikh, P.A.; Singh, A.K.; Mishra, G.K.; Karikalan, M.; Singh, S.K.; Kumar, H.; Gaur, G.K.; Krishnaswamy, N. Expression of kisspeptin and its receptor in different functional classes of ovarian follicle in the buffalo (Bubalus bubalis). Theriogenology 2022, 179, 87–96. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Z.; Brown, S.; Kannappan, V.; Tawari, P.E.; Jiang, W.; Irache, J.M.; Tang, J.Z.; Armesilla, A.L.; Darling, J.L.; et al. Liposome encapsulated Disulfiram inhibits NFκB pathway and targets breast cancer stem cells in vitro and in vivo. Oncotarget 2014, 5, 7471–7485. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Wilson, K.; Hannon, P.R.; Rosewell, K.L.; Brännström, M.; Akin, J.W.; Curry, T.E., Jr.; Jo, M. Coordinated regulation among progesterone, prostaglandins, and EGF-like factors in human ovulatory follicles. J. Clin. Endocrinol. Metab. 2017, 102, 1971–1982. [Google Scholar] [CrossRef]
- Mao, Z.; Yang, L.; Lu, X.; Tan, A.; Wang, Y.; Ding, F.; Xiao, L.; Qi, X.; Yu, Y. C1QTNF3 in the murine ovary and its function in folliculogenesis. Reproduction 2018, 155, 333–346. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Shi, H.; Jin, Y.; Li, X.; Pan, J.; Lai, Y.; Lin, Y.; Jin, Y.; Roy, G.; Zhao, A.; et al. Adiponectin Deficiency Leads to Female Subfertility and Ovarian Dysfunctions in Mice. Endocrinology 2016, 157, 4875–4887. [Google Scholar] [CrossRef]
- Majera, D.; Skrott, Z.; Bouchal, J.; Bartkova, J.; Simkova, D.; Gachechiladze, M.; Steigerova, J.; Kurfurstova, D.; Gursky, J.; Korinkova, G.; et al. Targeting genotoxic and proteotoxic stress-response pathways in human prostate cancer by clinically available PARP inhibitors, vorinostat and disulfiram. Prostate 2019, 79, 352–362. [Google Scholar] [CrossRef]
- Luo, Q.Y.; Huo, P.; Wang, L.L.; Wu, X.H. The influencing mechanism of mTOR signal pathway mediated by mitofusin-2 in development of follicle. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2212–2217. [Google Scholar]
- Guo, J.; Zhang, T.; Guo, Y.; Sun, T.; Li, H.; Zhang, X.; Yin, H.; Cao, G.; Yin, Y.; Wang, H.; et al. Oocyte stage-specific effects of MTOR determine granulosa cell fate and oocyte quality in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E5326–E5333. [Google Scholar] [CrossRef] [Green Version]
- Matsika, A.; Srinivasan, B.; Day, C. Cancer stem cell markers in prostate cancer: An immunohistochemical study of ALDH1, SOX2 and EZH2. Pathology 2015, 47, 622–628. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Liu, X.; Zhang, C.; Shang, W.; Xue, J.; Chen, R.; Xing, Y.; Song, D.; Xu, R. Ginsenoside Rg1 promotes cerebral angiogenesis via the PI3K/Akt/mTOR signaling pathway in ischemic mice. Eur. J. Pharmacol. 2019, 856, 172418. [Google Scholar] [CrossRef]
- Khristi, V.; Chakravarthi, V.P.; Singh, P.; Ghosh, S.; Pramanik, A.; Ratri, A.; Borosha, S.; Roby, K.F.; Wolfe, M.W.; Rumi, M.A.K. ESR2 regulates granulosa cell genes essential for follicle maturation and ovulation. Mol. Cell. Endocrinol. 2018, 474, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Semplici, B.; Luongo, F.P.; Passaponti, S.; Landi, C.; Governini, L.; Morgante, G.; De Leo, V.; Piomboni, P.; Luddi, A. Bitter Taste Receptors Expression in Human Granulosa and Cumulus Cells: New Perspectives in Female Fertility. Cells 2021, 10, 3127. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Ma, Y.; Tong, X.; Yang, W.; Dai, Y.; Pan, Y.; Ren, P.; Liu, L.; Fan, H.Y.; Zhang, Y.; et al. Metformin inhibits testosterone-induced endoplasmic reticulum stress in ovarian granulosa cells via inactivation of p38 MAPK. Hum. Reprod. 2020, 35, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, Y.; Kanemori, Y.; Tanaka, F.; Baba, T. Possible role of p38 MAPK-MNK1-EMI2 cascade in metaphase-II arrest of mouse oocytes. Biol. Reprod. 2014, 91, 45. [Google Scholar] [CrossRef]
- Procházka, R.; Bartková, A.; Němcová, L.; Murín, M.; Gad, A.; Marcollová, K.; Kinterová, V.; Lucas-Hahn, A.; Laurinčík, J. The Role of MAPK3/1 and AKT in the Acquisition of High Meiotic and Developmental Competence of Porcine Oocytes Cultured In Vitro in FLI Medium. Int. J. Mol. Sci. 2021, 22, 11148. [Google Scholar] [CrossRef]
- Bender, H.R.; Campbell, G.E.; Aytoda, P.; Mathiesen, A.H.; Duffy, D.M. Thrombospondin 1 (THBS1) Promotes Follicular Angiogenesis, Luteinization, and Ovulation in Primates. Front. Endocrinol. 2019, 10, 727. [Google Scholar] [CrossRef] [Green Version]
- Campbell, G.E.; Bender, H.R.; Parker, G.A.; Curry, T.E.; Duffy, D.M. Neurotensin: A novel mediator of ovulation? FASEB J. 2021, 35, e21481. [Google Scholar] [CrossRef]

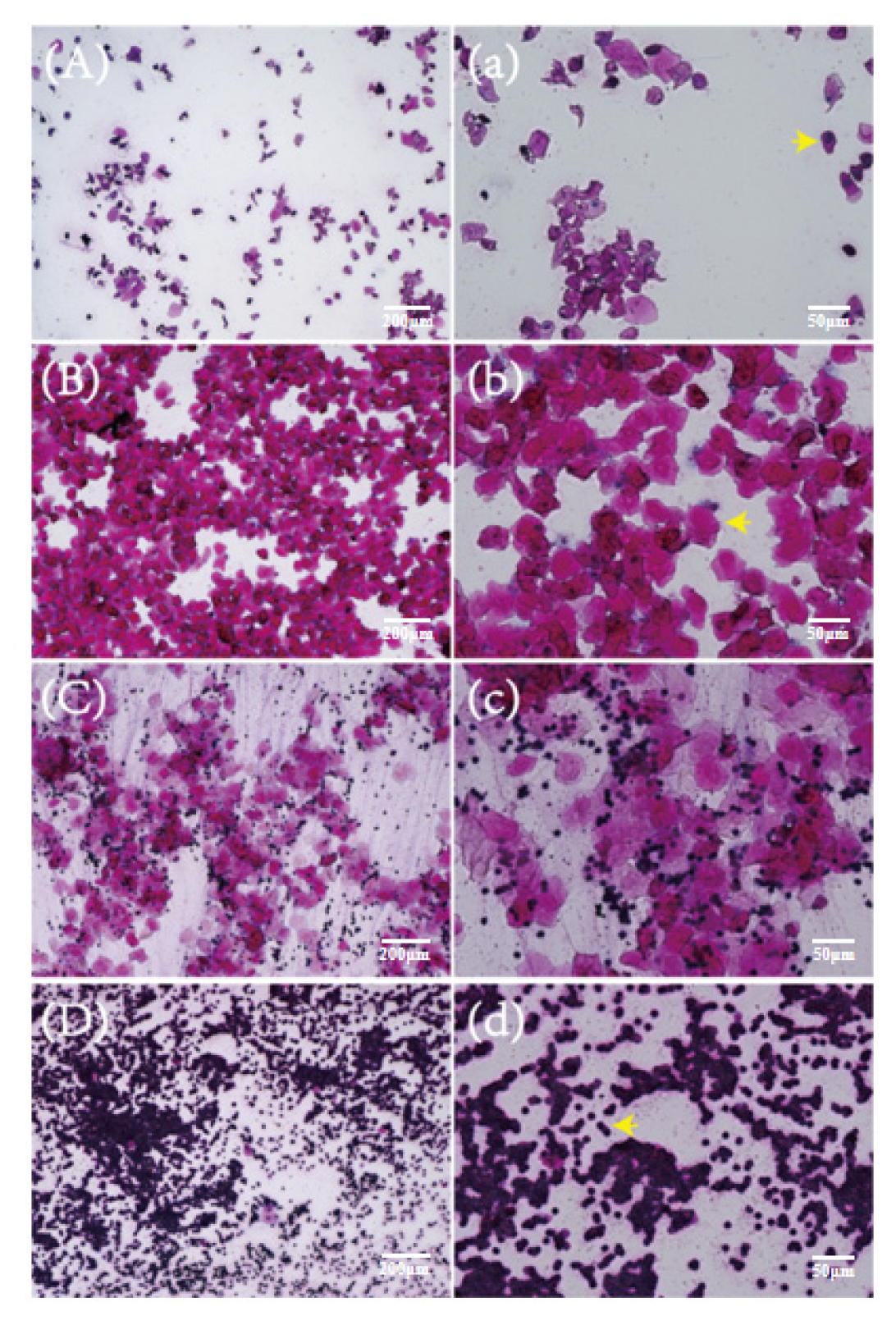

| Duration of Different Estrus Periods (Days) | Control | DSF 50 mg/kg | DSF 100 mg/kg |
|---|---|---|---|
| Proestrus | 0.75 ± 0.11 | 1.00 ± 0.16 | 1.00 ± 0.13 |
| Estrus | 1.17 ± 0.17 | 1.00 ± 0.13 | 0.92 ± 0.08 |
| Metoestrus | 0.83 ± 0.11 | 0.67 ± 0.11 | 0.92 ± 0.15 |
| Dioestrus | 1.67 ± 0.22 a | 2.92 ± 0.28 b | 3.42 ± 0.15 b |
| Estrus cycle | 4.40 ± 0.24 a | 5.60 ± 0.23 b | 6.25 ± 0.21 b |
| Groups | Pregnancy Rate | Litter Size |
|---|---|---|
| Control | 45% (9/20) | 10.33 ± 1.73 |
| DSF | 67% (12/18) | 13.55 ± 1.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, M.; Luo, Y.; Wang, C.; Lei, A. Effect of Disulfiram on the Reproductive Capacity of Female Mice. Int. J. Mol. Sci. 2023, 24, 2371. https://doi.org/10.3390/ijms24032371
Teng M, Luo Y, Wang C, Lei A. Effect of Disulfiram on the Reproductive Capacity of Female Mice. International Journal of Molecular Sciences. 2023; 24(3):2371. https://doi.org/10.3390/ijms24032371
Chicago/Turabian StyleTeng, Mingming, Yuan Luo, Chan Wang, and Anmin Lei. 2023. "Effect of Disulfiram on the Reproductive Capacity of Female Mice" International Journal of Molecular Sciences 24, no. 3: 2371. https://doi.org/10.3390/ijms24032371





