A Cell-Based Assessment of the Muscle Anabolic Potential of Blue Whiting (Micromesistius poutassou) Protein Hydrolysates
Abstract
:1. Introduction
2. Results
2.1. Degree of Hydrolysis (DH) and Molecular Mass Distribution
2.2. Total Amino Acid Analysis
2.3. Cell Viability Assay (WST-1)
2.4. Myotube Growth and Thickness
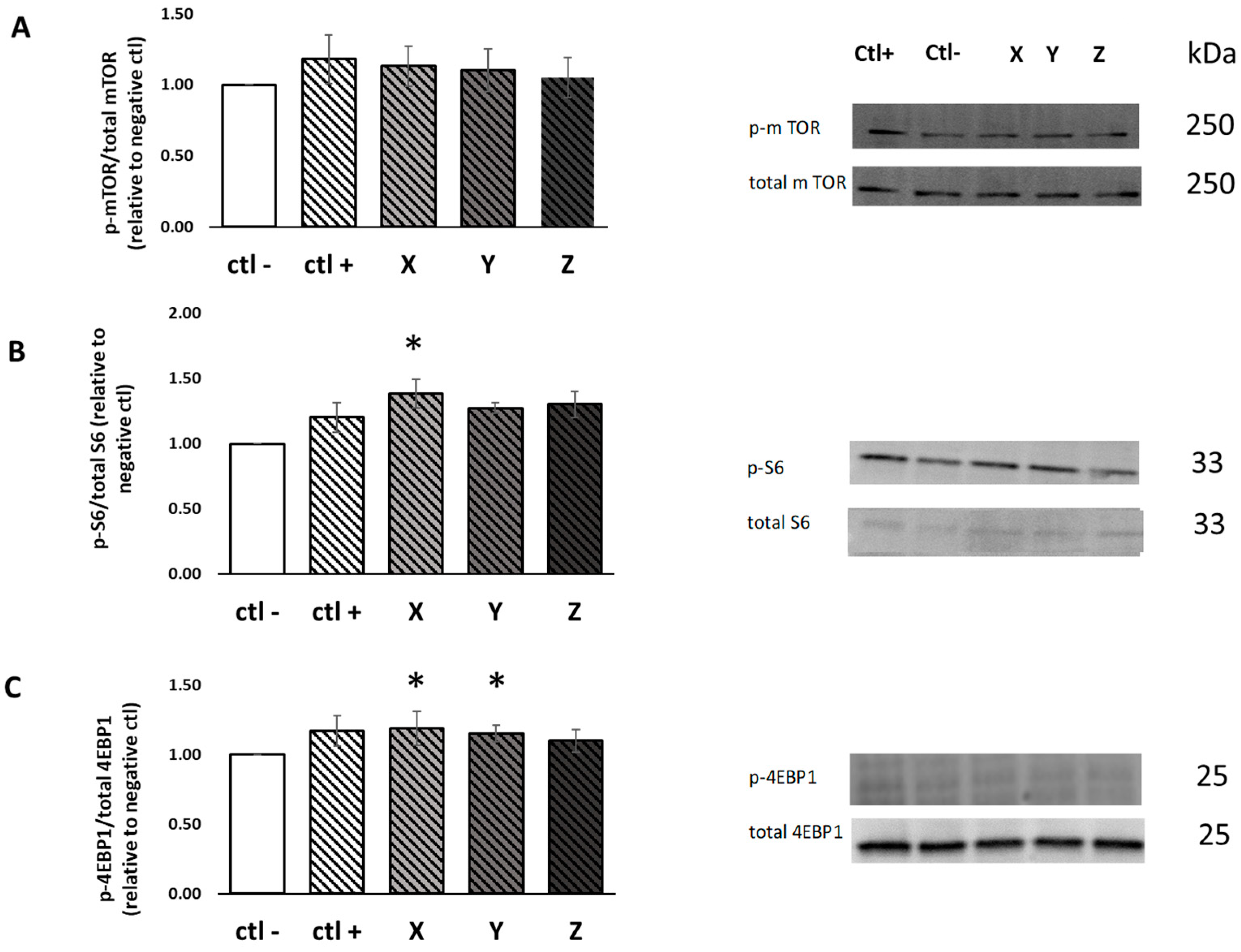
2.5. Muscle Protein Synthesis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Generation of BWPHs by Direct Enzymatic Hydrolysis
4.3. Simulated Gastrointestinal Digestion (SGID)
4.4. Amino Acid Quantification, Degree of Hydrolysis (DH) and Molecular Mass Distribution Profiles
4.5. Cell Culture
4.6. WST-1 Cell Viability Assay
4.7. Electrical Impedance Measurement
4.8. Myotube Diameter Measurement
4.9. Preparation of Cellular Protein Lysates
4.10. Western Blot Method and Analysis
4.11. Muscle Protein Synthesis (MPS)
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolfe, R.R. The role of dietary protein in optimizing muscle mass, function and health outcomes in older individuals. Br. J. Nutr. 2012, 108, S88–S93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, S.T.; Cooley, I.D. The effect of physiological stimuli on sarcopenia; impact of Notch and Wnt signaling on impaired aged skeletal muscle repair. Int. J. Biol. Sci. 2012, 8, 731–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güller, I.; Russell, A.P. MicroRNAs in skeletal muscle: Their role and regulation in development, disease and function. J. Physiol. 2010, 588, 4075–4087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- English, K.L.; Paddon-Jones, D. Protecting muscle mass and function in older adults during bed rest. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Traylor, D.A.; Gorissen, S.H.; Phillips, S.M. Perspective: Protein requirements and optimal intakes in aging: Are we ready to recommend more than the recommended daily allowance? Adv. Nutr. 2018, 9, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Burd, N.A.; Gorissen, S.H.; Van Loon, L.J. Anabolic resistance of muscle protein synthesis with aging. Exerc. Sport Sci. Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2004, 19, 422–424. [Google Scholar] [CrossRef] [Green Version]
- Braga-Pontes, C.; Ribeiro, V.S.; Pereira, C.D. The Role of Macronutrients in Diets for Weight Control. Underst. Obes. Its Causes Impact Life 2020, 1, 236–270. [Google Scholar]
- Walker, D.K.; Dickinson, J.M.; Timmerman, K.L.; Drummond, M.J.; Reidy, P.T.; Fry, C.S.; Gundermann, D.M.; Rasmussen, B. Exercise, amino acids and aging in the control of human muscle protein synthesis. Med. Sci. Sports Exerc. 2011, 43, 2249–2258. [Google Scholar] [CrossRef] [Green Version]
- Ter Borg, S.; Luiking, Y.; van Helvoort, A.; Boirie, Y.; Schols, J.; de Groot, C. Low levels of branched chain amino acids, eicosapentaenoic acid and micronutrients are associated with low muscle mass, strength and function in community-dwelling older adults. J. Nutr. Health Aging 2019, 23, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.E.; Phillips, S.M. Maximizing muscle protein anabolism: The role of protein quality. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. Regulation of muscle protein by amino acids. J. Nutr. 2002, 132, 3219S–3224S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, L.M.; A Winter, J.; Cameron-Smith, D.; Enslen, M.; Farnfield, M.; Decombaz, J. Effect of intake of different dietary protein sources on plasma amino acid profiles at rest and after exercise. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 452–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvan, E.; Arentson-Lantz, E.; Lamon, S.; Paddon-Jones, D. Protecting skeletal muscle with protein and amino acid during periods of disuse. Nutrients 2016, 8, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proud, C.G. Regulation of mammalian translation factors by nutrients. Eur. J. Biochem. 2002, 269, 5338–5349. [Google Scholar] [CrossRef]
- Kimball, S.R.; Jefferson, L.S. Control of protein synthesis by amino acid availability. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 63–67. [Google Scholar] [CrossRef]
- Rennie, M. Exercise-and nutrient-controlled mechanisms involved in maintenance of the musculoskeletal mass. Biochem. Soc. Trans. 2007, 35, 1302–1305. [Google Scholar] [CrossRef]
- Morgan, P.T.; Breen, L. The role of protein hydrolysates for exercise-induced skeletal muscle recovery and adaptation: A current perspective. Nutr. Metab. 2021, 18, 44. [Google Scholar] [CrossRef]
- Ishak, N.; Sarbon, N. A review of protein hydrolysates and bioactive peptides deriving from wastes generated by fish processing. Food Bioprocess Technol. 2018, 11, 2–16. [Google Scholar] [CrossRef]
- Dale, H.F.; Madsen, L.; Lied, G.A. Fish–derived proteins and their potential to improve human health. Nutr. Rev. 2019, 77, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Kandyliari, A.; Mallouchos, A.; Papandroulakis, N.; Golla, J.P.; Lam, T.T.; Sakellari, A.; Karavoltsos, S.; Vasiliou, V.; Kapsokefalou, M. Nutrient composition and fatty acid and protein profiles of selected fish by-products. Foods 2020, 9, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.P.; FitzGerald, R.J. Blue whiting (Micromesistius poutassou) muscle protein hydrolysate with in vitro and in vivo antidiabetic properties. J. Funct. Foods 2018, 40, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Lees, M.; Nolan, D.; Amigo-Benavent, M.; Raleigh, C.; Khatib, N.; Harnedy-Rothwell, P.; FitzGerald, R.; Egan, B.; Carson, B. A Fish-Derived Protein Hydrolysate Induces Postprandial Aminoacidaemia and Skeletal Muscle Anabolism in an In Vitro Cell Model Using Ex Vivo Human Serum. Nutrients 2021, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Rigon, C.; Perna, S.; Gasparri, C.; Iannello, G.; Akber, R.; Alalwan, T.A.; Freije, A.M. Novel insights on intake of fish and prevention of sarcopenia: All reasons for an adequate consumption. Nutrients 2020, 12, 307. [Google Scholar] [CrossRef] [Green Version]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from fish by-product protein hydrolysates and its functional properties: An overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef]
- Lees, M.J.; Carson, B.P. The potential role of fish-derived protein hydrolysates on metabolic health, skeletal muscle mass and function in ageing. Nutrients 2020, 12, 2434. [Google Scholar] [CrossRef]
- Cordeiro, E.M.; de Oliveira, G.V.; Volino-Souza, M.; Velozo, O.d.C.; Alvares, T.S. Effects of fish protein hydrolysate ingestion on postexercise aminoacidemia compared with whey protein hydrolysate in young individuals. J. Food Sci. 2020, 85, 21–27. [Google Scholar] [CrossRef]
- Yoshie-Stark, Y.; Tsukamoto, M.; Futagawa, K.; Kubota, M.; Ogushi, M. Bioactivities of surimi from southern blue whiting prepared by different ways. Food Chem. 2008, 113, 47–52. [Google Scholar] [CrossRef]
- Harnedy-Rothwell, P.A.; Khatib, N.; Sharkey, S.; Lafferty, R.A.; Gite, S.; Whooley, J.; O’Harte, F.P.M.; FitzGerald, R.J. Physicochemical, Nutritional and In Vitro Antidiabetic Characterisation of Blue Whiting (Micromesistius poutassou) Protein Hydrolysates. Mar. Drugs 2021, 19, 383. [Google Scholar] [CrossRef]
- Cudennec, B.; Fouchereau-Peron, M.; Ferry, F.; Duclos, E.; Ravallec, R. In Vitro and in vivo evidence for a satiating effect of fish protein hydrolysate obtained from blue whiting (Micromesistius poutassou) muscle. J. Funct. Foods 2012, 4, 271–277. [Google Scholar] [CrossRef]
- Nobile, V.; Duclos, E.; Michelotti, A.; Bizzaro, G.; Negro, M.; Soisson, F. Supplementation with a fish protein hydrolysate (Micromesistius poutassou): Effects on body weight, body composition, and CCK/GLP-1 secretion. Food Nutr. Res. 2016, 60, 29857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carson, B.P.; Patel, B.; Amigo-Benavent, M.; Pauk, M.; Kumar Gujulla, S.; Murphy, S.M.; Kiely, P.A.; Jakeman, P.M. Regulation of muscle protein synthesis in an in vitro cell model using ex vivo human serum. Exp. Physiol. 2018, 103, 783–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, B.; Pauk, M.; Amigo-Benavent, M.; Nongonierma, A.B.; Fitzgerald, R.J.; Jakeman, P.M.; Carson, B.P. A cell-based evaluation of a non-essential amino acid formulation as a non-bioactive control for activation and stimulation of muscle protein synthesis using ex vivo human serum. PLoS ONE 2019, 14, e0220757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Fang, C.-H.; Li, B.G.; Wang, J.J.; Fischer, J.E.; Hasselgren, P.O. Insulin-like growth factor 1 stimulates protein synthesis and inhibits protein breakdown in muscle from burned rats. J. Parenter. Enter. Nutr. 1997, 21, 245–251. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [Green Version]
- Anthony, J.C.; Yoshizawa, F.; Anthony, T.G.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 2000, 130, 2413–2419. [Google Scholar] [CrossRef] [Green Version]
- Bolster, D.R.; Vary, T.C.; Kimball, S.R.; Jefferson, L.S. Leucine regulates translation initiation in rat skeletal muscle via enhanced eIF4G phosphorylation. J. Nutr. 2004, 134, 1704–1710. [Google Scholar] [CrossRef] [Green Version]
- Hornberger, T.A.; Sukhija, K.B.; Wang, X.-R.; Chien, S. mTOR is the rapamycin-sensitive kinase that confers mechanically-induced phosphorylation of the hydrophobic motif site Thr (389) in p70S6k. FEBS Lett. 2007, 581, 4562–4566. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.A.; Mabrey, D.M.; Frey, J.W.; Miu, M.H.; Schmidt, E.K.; Pierre, P.; Hornberger, T.A. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J. 2011, 25, 1028–1039. [Google Scholar] [CrossRef] [Green Version]
- Roeseler, D.A.; McGraw, N.J.; Butteiger, D.N.; Shah, N.; Hall-Porter, J.; Mukherjea, R.; Krul, E.S. Muscle protein signaling in C2C12 cells is stimulated to similar degrees by diverse commercial food protein sources and experimental soy protein hydrolysates. J. Agric. Food Chem. 2017, 65, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-H.; Choung, S.-Y. Oyster hydrolysates attenuate muscle atrophy via regulating protein turnover and mitochondria biogenesis in C2C12 cell and immobilized mice. Nutrients 2021, 13, 4385. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Fujitani, M.; Mizushige, T.; Kawabata, F.; Hayamizu, K.; Uozumi, K.; Hara, Y.; Sawai, M.; Uehigashi, R.; Okada, S.; et al. Dietary Alaska Pollack Protein Induces Acute and Sustainable Skeletal Muscle Hypertrophy in Rats. Nutrients 2022, 14, 547. [Google Scholar] [CrossRef]
- Morisasa, M.; Yoshida, E.; Fujitani, M.; Kimura, K.; Uchida, K.; Kishida, T.; Mori, T.; Goto-Inoue, N. Fish Protein Promotes Skeletal Muscle Hypertrophy via the Akt/mTOR Signaling Pathways. J. Nutr. Sci. Vitaminol. 2022, 68, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Bernard, H.; Murray, B.; MacDonald, J.; Pentzien, A.-K.; Wright, G.; Wal, J.-M.; Struthers, A.; Meisel, H.; FitzGerald, R. In Vitro generation and stability of the lactokinin β-lactoglobulin fragment (142–148). J. Dairy Sci. 2004, 87, 3845–3857. [Google Scholar] [CrossRef] [Green Version]
- Deacon, C.F. Peptide degradation and the role of DPP-4 inhibitors in the treatment of type 2 diabetes. Peptides 2018, 100, 150–157. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Spellman, D.; Kenny, P.; O’Cuinn, G.; FitzGerald, R.J. Aggregation properties of whey protein hydrolysates generated with Bacillus licheniformis proteinase activities. J. Agric. Food Chem. 2005, 53, 1258–1265. [Google Scholar] [CrossRef]
- Murphy, S.M.; Kiely, M.; Jakeman, P.M.; Kiely, P.A.; Carson, B.P. Optimization of an in vitro bioassay to monitor growth and formation of myotubes in real time. Biosci. Rep. 2016, 36, e00330. [Google Scholar] [CrossRef] [PubMed]
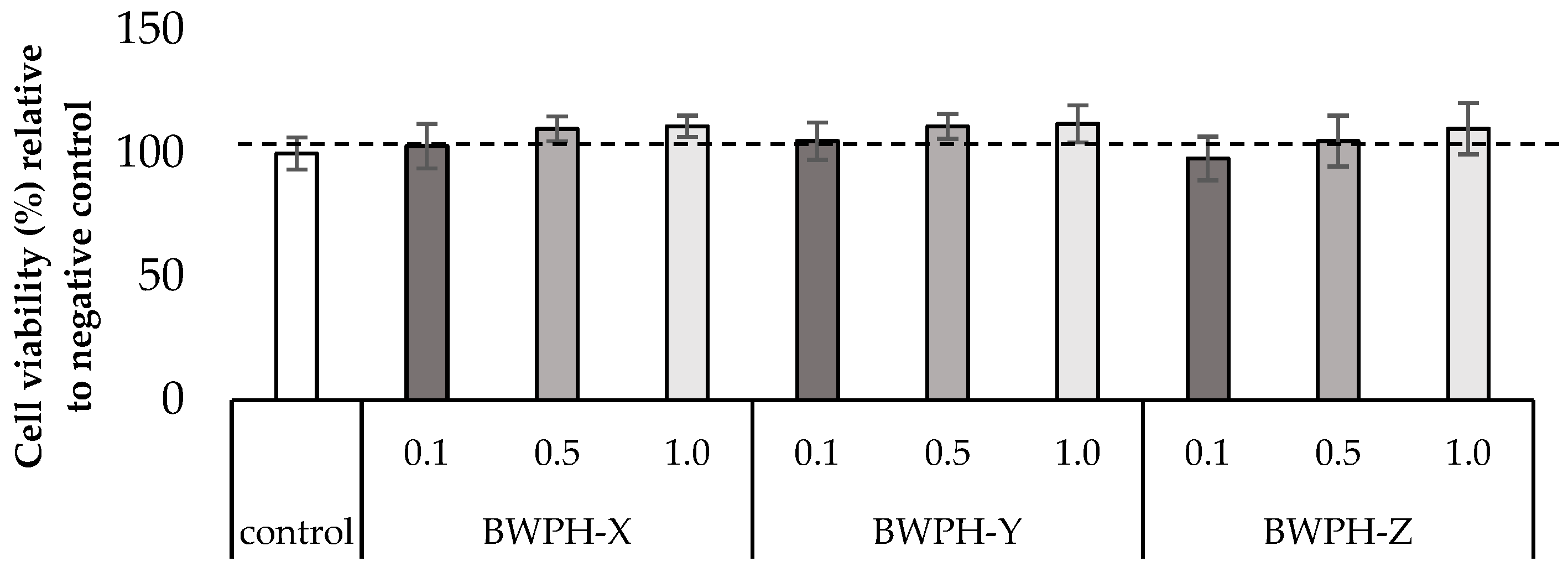


| Test Sample | DH (%) | Molecular Mass Distribution (%) | |||
|---|---|---|---|---|---|
| >10 kDa | 5–10 kDa | 1–5 kDa | <1 kDa | ||
| BWPH-X BWPH-X-SGID | 25.89 ± 0.21 * | 2.13 | 4.74 | 15.61 | 77.5 |
| 37.29 ± 0.24 | 0 | 0.25 | 2.63 | 97.12 | |
| BWPH-Y BWPH-Y-SGID | 26.81 ± 0.82 * | 1.95 | 4.97 | 38.69 | 54.39 |
| 35.48 ± 0.27 | 0.01 | 0.30 | 13.03 | 86.67 | |
| BWPH-Z BWPH-Z-SGID | 27.16 ± 0.68 * | 1.51 | 3.72 | 32.16 | 62.6 |
| 33.41 ± 0.91 | 0.01 | 0.21 | 11.04 | 88.75 | |
| AA | AA Content of BWPHs (g·kg−1) | |||
|---|---|---|---|---|
| BWPH-X | BWPH-Y | BWPH-Z | ||
| Non essential (NEAA) | Cys | 5.3 | 5.4 | 12.4 |
| Arg | 58.5 | 58.7 | 50.1 | |
| Asx | 95.2 | 90.9 | 82.9 | |
| Pro | 37.5 | 36.7 | 28.8 | |
| Ser | 42.5 | 40.9 | 37.1 | |
| Glx | 146.0 | 139.0 | 133.7 | |
| Gly | 62.6 | 60.2 | 58.7 | |
| Ala | 59.9 | 57.8 | 52.6 | |
| Tyr | 26.2 | 25.5 | 14.6 | |
| Essential amino acids (EAA) | Val | 31.4 | 30.1 | 33.8 |
| Ile | 24.6 | 23.8 | 27.3 | |
| Leu | 56.2 | 55.3 | 53.7 | |
| Trp | 5.7 | 5.3 | 5.9 | |
| Phe | 26.5 | 26.0 | 26.3 | |
| His | 15.9 | 15.6 | 25.3 | |
| Lys | 80 | 75.7 | 71.5 | |
| Met | 19.1 | 19.2 | 26.0 | |
| Thr | 34.9 | 34.0 | 31.5 | |
| EAA | 294.3 | 285.0 | 301.3 | |
| NEAA | 533.7 | 515.1 | 470.9 | |
| EAA:NEAA | 0.55 | 0.55 | 0.6 | |
| BCAA | 112.2 | 109.2 | 114.9 | |
| TAA | 828.0 | 800.1 | 772.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shekoohi, N.; Amigo-Benavent, M.; Wesley Peixoto da Fonseca, G.; Harnedy-Rothwell, P.A.; FitzGerald, R.J.; Carson, B.P. A Cell-Based Assessment of the Muscle Anabolic Potential of Blue Whiting (Micromesistius poutassou) Protein Hydrolysates. Int. J. Mol. Sci. 2023, 24, 2001. https://doi.org/10.3390/ijms24032001
Shekoohi N, Amigo-Benavent M, Wesley Peixoto da Fonseca G, Harnedy-Rothwell PA, FitzGerald RJ, Carson BP. A Cell-Based Assessment of the Muscle Anabolic Potential of Blue Whiting (Micromesistius poutassou) Protein Hydrolysates. International Journal of Molecular Sciences. 2023; 24(3):2001. https://doi.org/10.3390/ijms24032001
Chicago/Turabian StyleShekoohi, Niloofar, Miryam Amigo-Benavent, Guilherme Wesley Peixoto da Fonseca, Pádraigín A. Harnedy-Rothwell, Richard J. FitzGerald, and Brian P. Carson. 2023. "A Cell-Based Assessment of the Muscle Anabolic Potential of Blue Whiting (Micromesistius poutassou) Protein Hydrolysates" International Journal of Molecular Sciences 24, no. 3: 2001. https://doi.org/10.3390/ijms24032001






