Differential Serotonergic Modulation of Synaptic Inputs to the Olfactory Cortex
Abstract
:1. Introduction
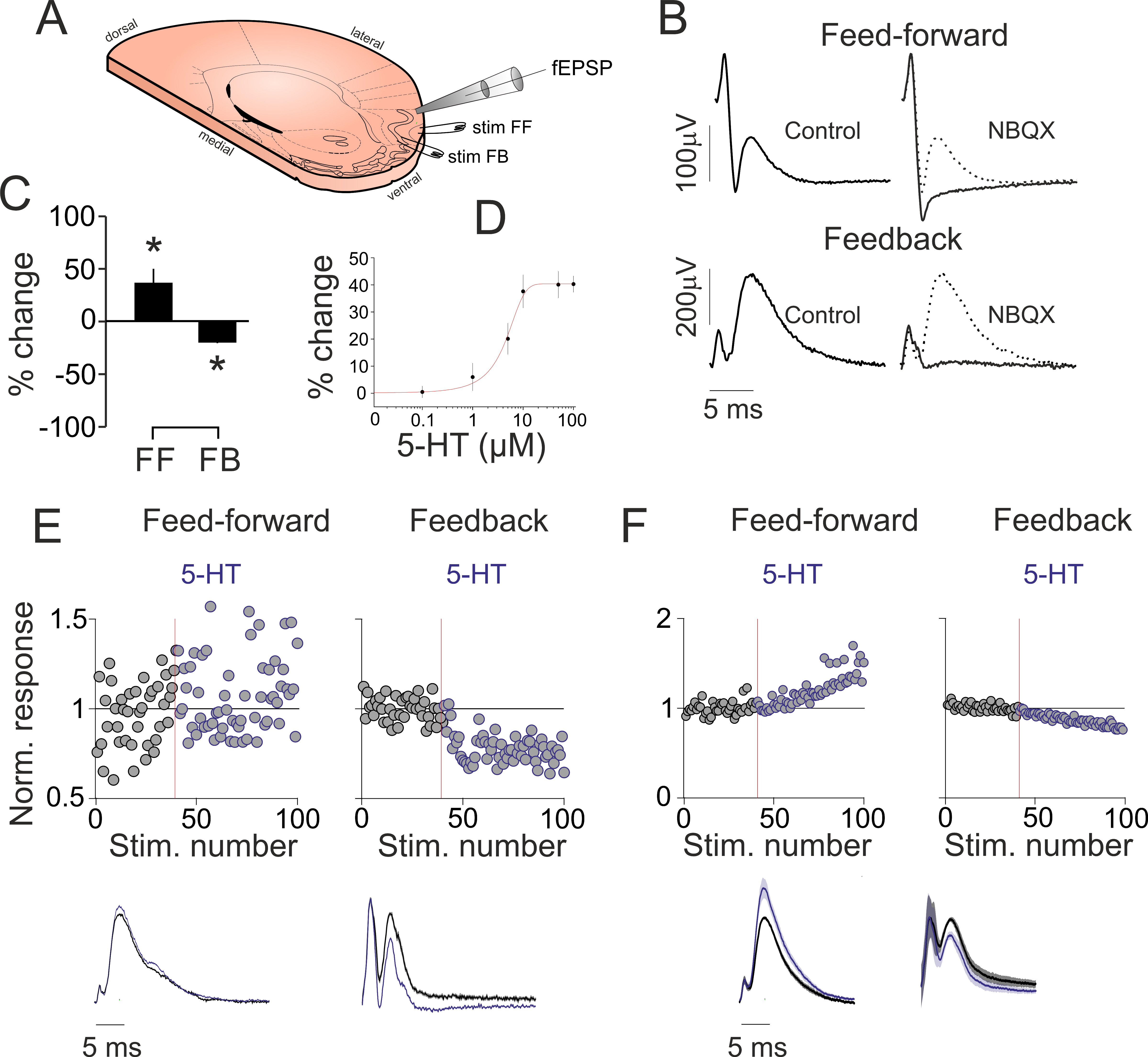
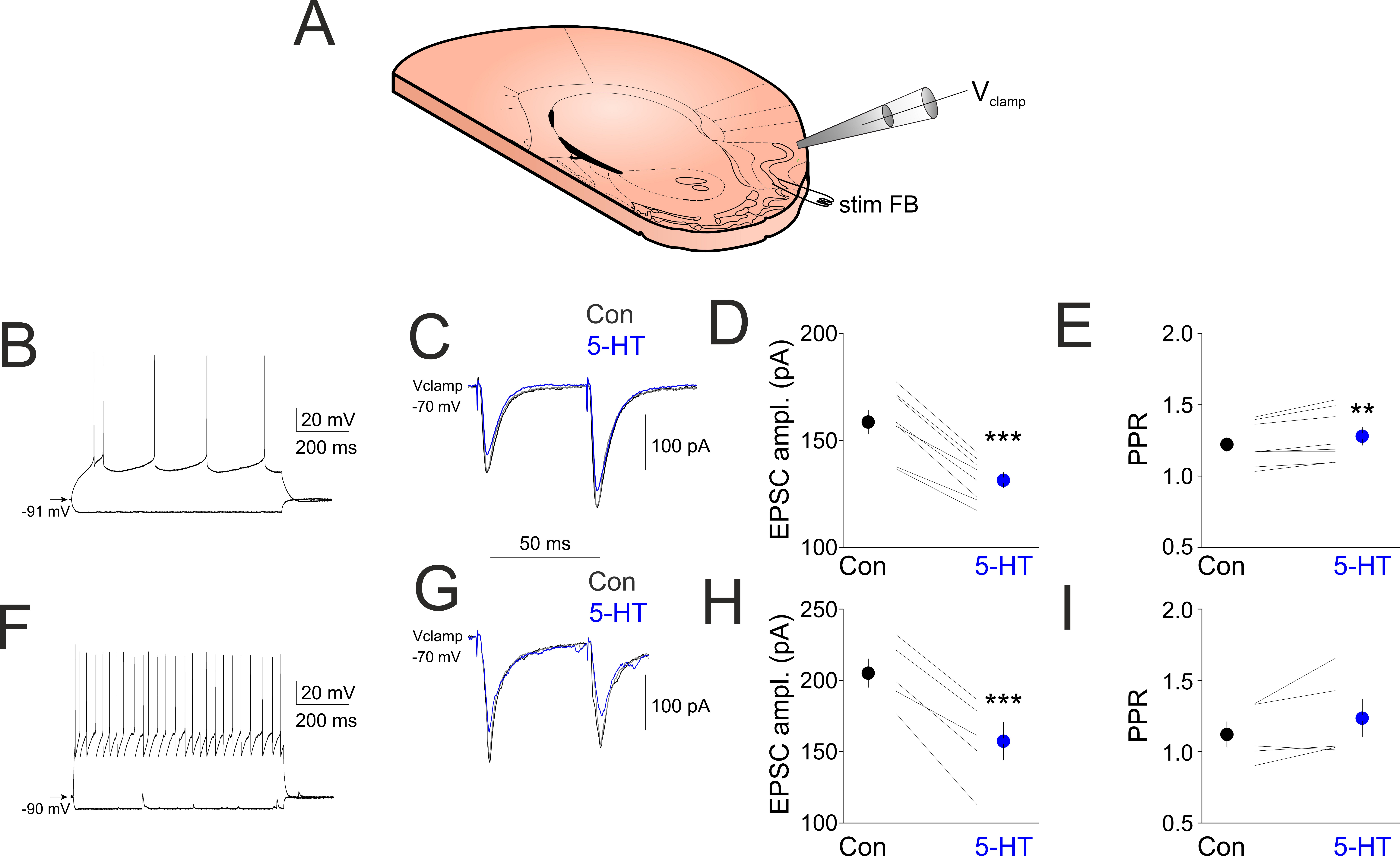
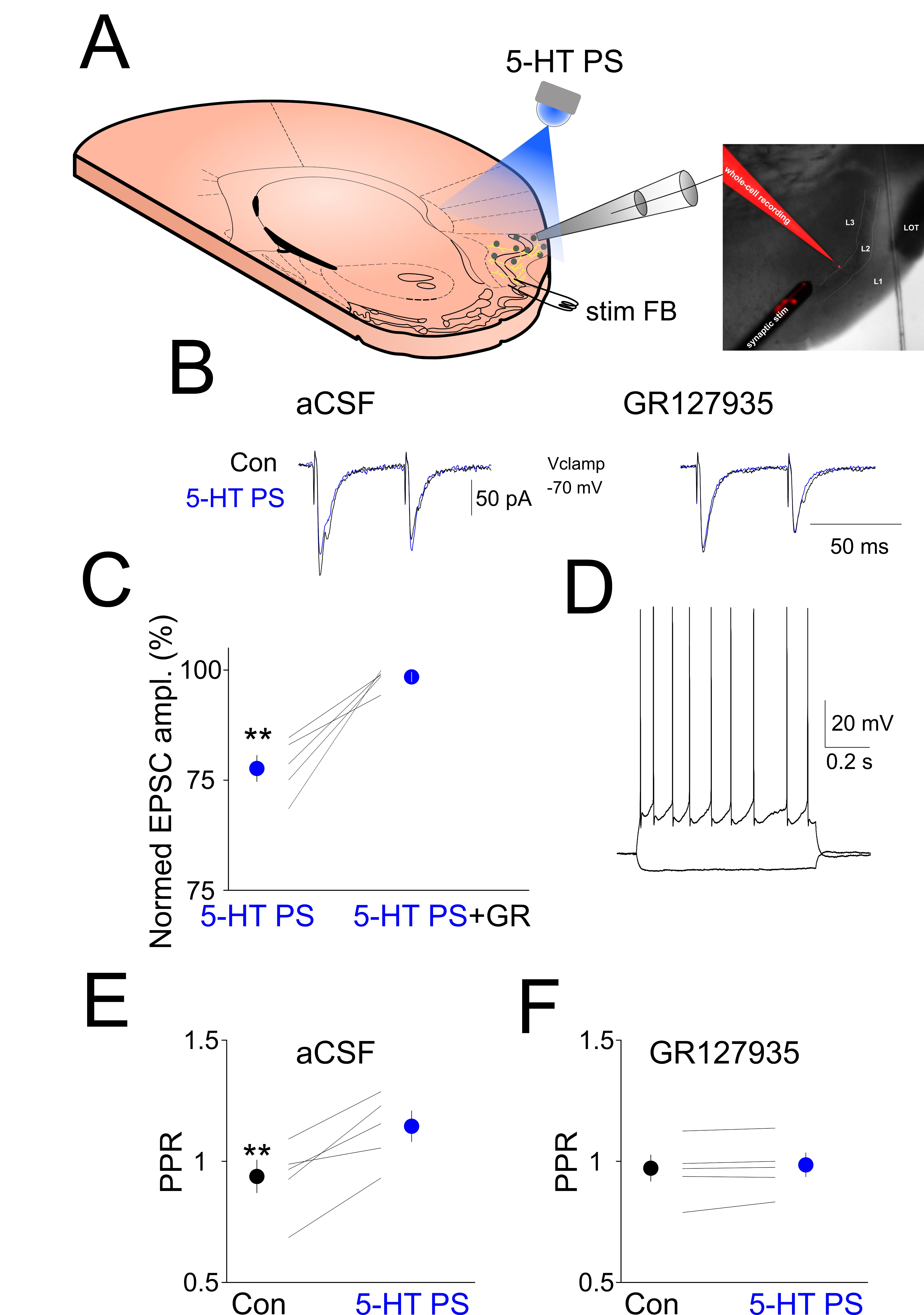
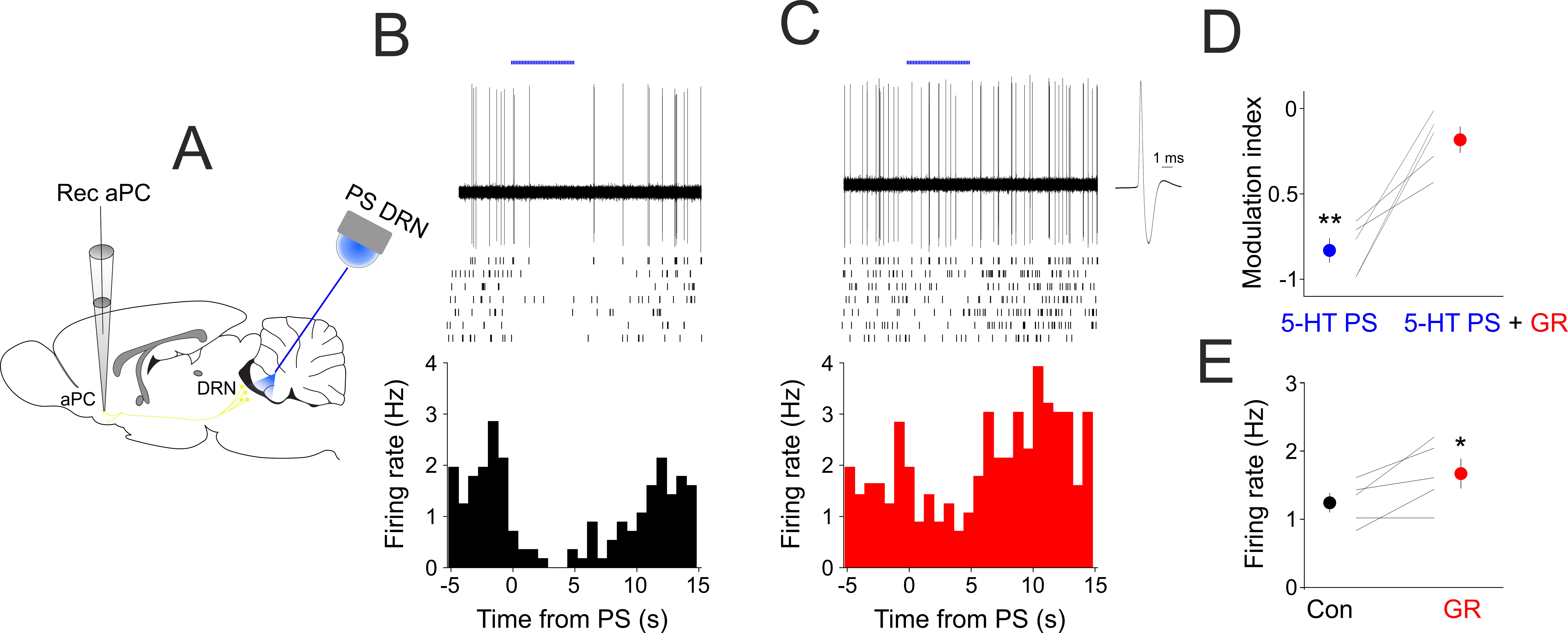
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pollak Dorocic, I.; Fürth, D.; Xuan, Y.; Johansson, Y.; Pozzi, L.; Silberberg, G.; Carlén, M.; Meletis, K. A Whole-Brain Atlas of Inputs to Serotonergic Neurons of the Dorsal and Median Raphe Nuclei. Neuron 2014, 83, 663–678. [Google Scholar] [CrossRef] [Green Version]
- Lottem, E.; Lorincz, M.L.; Mainen, Z.F. Optogenetic Activation of Dorsal Raphe Serotonin Neurons Rapidly Inhibits Spontaneous But Not Odor-Evoked Activity in Olfactory Cortex. J. Neurosci. 2016, 36, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Dugué, G.P.; Lörincz, M.L.; Lottem, E.; Audero, E.; Matias, S.; Correia, P.A.; Léna, C.; Mainen, Z.F. Optogenetic Recruitment of Dorsal Raphe Serotonergic Neurons Acutely Decreases Mechanosensory Responsivity in Behaving Mice. PLoS ONE 2014, 9, e105941. [Google Scholar] [CrossRef]
- Azimi, Z.; Barzan, R.; Spoida, K.; Surdin, T.; Wollenweber, P.; Mark, M.D.; Herlitze, S.; Jancke, D. Separable Gain Control of Ongoing and Evoked Activity in the Visual Cortex by Serotonergic Input. eLife 2020, 9, e53552. [Google Scholar] [CrossRef]
- Davis, M.; Strachan, D.I.; Kass, E. Excitatory and Inhibitory Effects of Serotonin on Sensorimotor Reactivity Measured with Acoustic Startle. Science 1980, 209, 521–523. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Fornal, C.A. Activity of Brain Serotonergic Neurons in the Behaving Animal. Pharmacol. Rev. 1991, 43, 563–578. [Google Scholar]
- Oikonomou, G.; Altermatt, M.; Zhang, R.; Coughlin, G.M.; Montz, C.; Gradinaru, V.; Prober, D.A. The Serotonergic Raphe Promote Sleep in Zebrafish and Mice. Neuron 2019, 103, 686–701.e8. [Google Scholar] [CrossRef] [Green Version]
- Gazea, M.; Furdan, S.; Sere, P.; Oesch, L.; Molnár, B.; Di Giovanni, G.; Fenno, L.E.; Ramakrishnan, C.; Mattis, J.; Deisseroth, K.; et al. Reciprocal Lateral Hypothalamic and Raphe GABAergic Projections Promote Wakefulness. J. Neurosci. 2021, 41, 4840–4849. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Li, Y.; Hu, F.; Lu, Y.; Ma, M.; Feng, Q.; Zhang, J.; Wang, D.; Zeng, J.; et al. Dorsal Raphe Neurons Signal Reward through 5-HT and Glutamate. Neuron 2014, 81, 1360–1374. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.Y.; Amoroso, M.W.; Uchida, N. Serotonergic Neurons Signal Reward and Punishment on Multiple Timescales. eLife 2015, 4, e06346. [Google Scholar] [CrossRef]
- Matias, S.; Lottem, E.; Dugué, G.P.; Mainen, Z.F. Activity Patterns of Serotonin Neurons Underlying Cognitive Flexibility. eLife 2017, 6, e20552. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewcz, C.A.; Mazzone, C.M.; D’Agostino, G.; Halladay, L.R.; Hardaway, J.A.; DiBerto, J.F.; Navarro, M.; Burnham, N.; Cristiano, C.; Dorrier, C.E.; et al. Serotonin Engages an Anxiety and Fear-Promoting Circuit in the Extended Amygdala. Nature 2016, 537, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Morishita, W.; Beier, K.T.; Heifets, B.D.; Malenka, R.C. 5-HT Modulation of a Medial Septal Circuit Tunes Social Memory Stability. Nature 2021, 599, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; Christoffel, D.J.; Heifets, B.D.; Ben-Dor, G.A.; Selimbeyoglu, A.; Hung, L.W.; Deisseroth, K.; Malenka, R.C. 5-HT Release in Nucleus Accumbens Rescues Social Deficits in Mouse Autism Model. Nature 2018, 560, 589–594. [Google Scholar] [CrossRef]
- Pehrson, A.L.; Roberts, D.; Khawaja, A.; McNair, R. The Role of Serotonin Neurotransmission in Rapid Antidepressant Actions. Psychopharmacology 2022, 239, 1823–1838. [Google Scholar] [CrossRef]
- Lörincz, M.; Oláh, M.; Baracskay, P.; Szilágyi, N.; Juhász, G. Propagation of Spike and Wave Activity to the Medial Prefrontal Cortex and Dorsal Raphe Nucleus of WAG/Rij Rats. Physiol. Behav. 2007, 90, 318–324. [Google Scholar] [CrossRef]
- Zhan, Q.; Buchanan, G.F.; Motelow, J.E.; Andrews, J.; Vitkovskiy, P.; Chen, W.C.; Serout, F.; Gummadavelli, A.; Kundishora, A.; Furman, M.; et al. Impaired Serotonergic Brainstem Function during and after Seizures. J. Neurosci. 2016, 36, 2711–2722. [Google Scholar] [CrossRef] [Green Version]
- Datiche, F.; Luppi, P.-H.; Cattarelli, M. Serotonergic and Non-Serotonergic Projections from the Raphe Nuclei to the Piriform Cortex in the Rat: A Cholera Toxin B Subunit (CTb) and 5-HT Immunohistochemical Study. Brain Res. 1995, 671, 27–37. [Google Scholar] [CrossRef]
- Araneda, R.; Andrade, R. 5-Hydroxytryptamine2 and 5-Hydroxytryptamine1A Receptors Mediate Opposing Responses on Membrane Excitability in Rat Association Cortex. Neuroscience 1991, 40, 399–412. [Google Scholar] [CrossRef]
- Gellman, R.L.; Aghajanian, G.K. Serotonin2 Receptor-Mediated Excitation of Interneurons in Piriform Cortex: Antagonism by Atypical Antipsychotic Drugs. Neuroscience 1994, 58, 515–525. [Google Scholar] [CrossRef]
- Marek, G.J.; Aghajanian, G.K. Excitation of Interneurons in Piriform Cortex by 5-Hydroxytryptamine: Blockade by MDL 100,907, a Highly Selective 5-HT2A Receptor Antagonist. Eur. J. Pharmacol. 1994, 259, 137–141. [Google Scholar] [CrossRef]
- Lee, S.; Hjerling-Leffler, J.; Zagha, E.; Fishell, G.; Rudy, B. The Largest Group of Superficial Neocortical GABAergic Interneurons Expresses Ionotropic Serotonin Receptors. J. Neurosci. 2010, 30, 16796–16808. [Google Scholar] [CrossRef] [Green Version]
- Férézou, I.; Cauli, B.; Hill, E.L.; Rossier, J.; Hamel, E.; Lambolez, B. 5-HT3 Receptors Mediate Serotonergic Fast Synaptic Excitation of Neocortical Vasoactive Intestinal Peptide/Cholecystokinin Interneurons. J. Neurosci. 2002, 22, 7389–7397. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, P.W.; Aghajanian, G.K. Serotonin (5-HT) Induces IPSPs in Pyramidal Layer Cells of Rat Piriform Cortex: Evidence for the Involvement of a 5-HT2 -Activated Interneuron. Brain Res. 1990, 506, 62–69. [Google Scholar] [CrossRef]
- Piszár, I.; Lőrincz, M.L. Differential Serotonergic Modulation of Principal Neurons and Interneurons in the Anterior Piriform Cortex. Front. Neuroanat. 2022, 16, 821695. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Liu, P.; Jing, S.; Du, H.; Zhang, L.; Jia, F.; Li, A. Serotonergic Afferents from the Dorsal Raphe Decrease the Excitability of Pyramidal Neurons in the Anterior Piriform Cortex. Proc. Natl. Acad. Sci. USA 2020, 117, 3239–3247. [Google Scholar] [CrossRef]
- Price, J.L. An Autoradiographic Study of Complementary Laminar Patterns of Termination of Afferent Fibers to the Olfactory Cortex. J. Comp. Neurol. 1973, 150, 87–108. [Google Scholar] [CrossRef]
- Haberly, L.B.; Price, J.L. Association and Commissural Fiber Systems of the Olfactory Cortex of the Rat. I. Systems Originating in the Piriform Cortex and Adjacent Areas. J. Comp. Neurol. 1978, 178, 711–740. [Google Scholar] [CrossRef]
- Luskin, M.B.; Price, J. The Laminar Distribution of Intracortical Fibers Originating in the Olfactory Cortex of the Rat. J. Comp. Neurol. 1983, 216, 292–302. [Google Scholar] [CrossRef]
- Hasselmo, M.E.; Bower, J.M. Cholinergic Suppression Specific to Intrinsic Not Afferent Fiber Synapses in Rat Piriform (Olfactory) Cortex. J. Neurophysiol. 1992, 67, 1222–1229. [Google Scholar] [CrossRef]
- Hasselmo, M.E.; Linster, C.; Patil, M.; Ma, D.; Cekic, M. Noradrenergic Suppression of Synaptic Transmission May Influence Cortical Signal-to-Noise Ratio. J. Neurophysiol. 1997, 77, 3326–3339. [Google Scholar] [CrossRef] [PubMed]
- Hadley, J.K.; Halliwell, J.V. Serotonin Modulates Glutamatergic Transmission in the Rat Olfactory Tubercle. Eur. J. Neurosci. 2010, 31, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, D.; Gloveli, T.; Empson, R.M.; Heinemann, U. Comparison of the Effects of Serotonin in the Hippocampus and the Entorhinal Cortex. Mol. Neurobiol. 1998, 17, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Christoffel, D.J.; Walsh, J.J.; Hoerbelt, P.; Heifets, B.D.; Llorach, P.; Lopez, R.C.; Ramakrishnan, C.; Deisseroth, K.; Malenka, R.C. Selective Filtering of Excitatory Inputs to Nucleus Accumbens by Dopamine and Serotonin. Proc. Natl. Acad. Sci. USA 2021, 118, e2106648118. [Google Scholar] [CrossRef] [PubMed]
- Pickard, G.E.; Smith, B.N.; Belenky, M.; Rea, M.A.; Dudek, F.E.; Sollars, P.J. 5-HT 1B Receptor–Mediated Presynaptic Inhibition of Retinal Input to the Suprachiasmatic Nucleus. J. Neurosci. 1999, 19, 4034–4045. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.S.; Han, T.H.; Jo, J.Y.; Kang, G.; Lee, S.Y.; Ryu, P.D.; Im, J.H.; Jeon, B.H.; Park, J.B. Serotonin Inhibits GABA Synaptic Transmission in Presympathetic Paraventricular Nucleus Neurons. Neurosci. Lett. 2008, 439, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.-S.; Cho, J.-H.; An, C.-H.; Jung, J.-K.; Hur, Y.-K.; Choi, J.-K.; Jang, I.-S. 5-HT1B Receptors Inhibit Glutamate Release from Primary Afferent Terminals in Rat Medullary Dorsal Horn Neurons: 5-HT1B Receptors in Trigeminal Primary Afferents. Br. J. Pharmacol. 2012, 167, 356–367. [Google Scholar] [CrossRef] [Green Version]
- Hwang, E.-K.; Chung, J. 5HT1B Receptor-Mediated Pre-Synaptic Depression of Excitatory Inputs to the Rat Lateral Habenula. Neuropharmacology 2014, 81, 153–165. [Google Scholar] [CrossRef]
- Guo, J.-D.; O’Flaherty, B.M.; Rainnie, D.G. Serotonin Gating of Cortical and Thalamic Glutamate Inputs onto Principal Neurons of the Basolateral Amygdala. Neuropharmacology 2017, 126, 224–232. [Google Scholar] [CrossRef]
- Nagata, A.; Nakayama, K.; Nakamura, S.; Mochizuki, A.; Gemba, C.; Aoki, R.; Dantsuji, M.; Maki, K.; Inoue, T. Serotonin1B Receptor-Mediated Presynaptic Inhibition of Proprioceptive Sensory Inputs to Jaw-Closing Motoneurons. Brain Res. Bull. 2019, 149, 260–267. [Google Scholar] [CrossRef]
- Nishijo, T.; Suzuki, E.; Momiyama, T. Serotonin 5-HT 1A and 5-HT 1B Receptor-mediated Inhibition of Glutamatergic Transmission onto Rat Basal Forebrain Cholinergic Neurones. J. Physiol. 2022, 600, 3149–3167. [Google Scholar] [CrossRef] [PubMed]
- Sere, P.; Zsigri, N.; Raffai, T.; Furdan, S.; Győri, F.; Crunelli, V.; Lőrincz, M.L. Activity of the Lateral Hypothalamus during Genetically Determined Absence Seizures. Int. J. Mol. Sci. 2021, 22, 9466. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Masson, J.; Gingrich, J.A.; Rayport, S.; Hen, R. Targeted Gene Expression in Dopamine and Serotonin Neurons of the Mouse Brain. J. Neurosci. Methods 2005, 143, 27–32. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piszár, I.; Lőrincz, M.L. Differential Serotonergic Modulation of Synaptic Inputs to the Olfactory Cortex. Int. J. Mol. Sci. 2023, 24, 1950. https://doi.org/10.3390/ijms24031950
Piszár I, Lőrincz ML. Differential Serotonergic Modulation of Synaptic Inputs to the Olfactory Cortex. International Journal of Molecular Sciences. 2023; 24(3):1950. https://doi.org/10.3390/ijms24031950
Chicago/Turabian StylePiszár, Ildikó, and Magor L. Lőrincz. 2023. "Differential Serotonergic Modulation of Synaptic Inputs to the Olfactory Cortex" International Journal of Molecular Sciences 24, no. 3: 1950. https://doi.org/10.3390/ijms24031950





