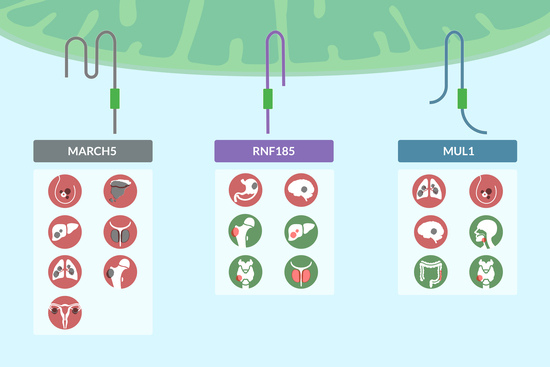
Role of the Mitochondrial E3 Ubiquitin Ligases as Possible Therapeutic Targets in Cancer Therapy
Abstract
:1. Introduction
2. MARCH5
3. RNF185
4. MUL1
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pickart, C.M.; Eddins, M.J. Ubiquitin: Structures, Functions, Mechanisms. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2004, 1695, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Tracz, M.; Bialek, W. Beyond K48 and K63: Non-Canonical Protein Ubiquitination. Cell. Mol. Biol. Lett. 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Schulman, B.A.; Harper, J.W. Ubiquitin-like Protein Activation by E1 Enzymes: The Apex for Downstream Signalling Pathways. Nat. Rev. Mol. Cell Biol. 2009, 10, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.D.; Ritterhoff, T.; Klevit, R.E.; Brzovic, P.S. E2 Enzymes: More than Just Middle Men. Cell Res. 2016, 26, 423–440. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, J.; Chen, D.; Wang, Y. E3 Ubiquitin Ligases: Styles, Structures and Functions. Mol. Biomed. 2021, 2, 23. [Google Scholar] [CrossRef]
- Senft, D.; Qi, J.; Ronai, Z.A. Ubiquitin Ligases in Oncogenic Transformation and Cancer Therapy. Nat. Rev. Cancer 2017, 18, 69–88. [Google Scholar] [CrossRef]
- Lescouzères, L.; Bomont, P. E3 Ubiquitin Ligases in Neurological Diseases: Focus on Gigaxonin and Autophagy. Front. Physiol. 2020, 11, 1022. [Google Scholar] [CrossRef]
- Saravanan, K.M.; Kannan, M.; Meera, P.; Bharathkumar, N.; Anand, T. E3 Ligases: A Potential Multi-Drug Target for Different Types of Cancers and Neurological Disorders. Future Med. Chem. 2022, 14, 187–201. [Google Scholar] [CrossRef]
- Yonashiro, R.; Ishido, S.; Kyo, S.; Fukuda, T.; Goto, E.; Matsuki, Y.; Ohmura-Hoshino, M.; Sada, K.; Hotta, H.; Yamamura, H.; et al. A Novel Mitochondrial Ubiquitin Ligase Plays a Critical Role in Mitochondrial Dynamics. EMBO J. 2006, 25, 3618–3626. [Google Scholar] [CrossRef]
- Di Gregorio, J.; Petricca, S.; Iorio, R.; Toniato, E.; Flati, V. Mitochondrial and Metabolic Alterations in Cancer Cells. Eur. J. Cell Biol. 2022, 101, 151225. [Google Scholar] [CrossRef]
- Xu, S.; Cherok, E.; Das, S.; Li, S.; Roelofs, B.A.; Ge, S.X.; Polster, B.M.; Boyman, L.; Lederer, W.J.; Wang, C.; et al. Mitochondrial E3 Ubiquitin Ligase MARCH5 Controls Mitochondrial Fission and Cell Sensitivity to Stress-Induced Apoptosis through Regulation of MiD49 Protein. Mol. Biol. Cell 2016, 27, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, L.; Cheng, Q.; Li, Y.; Wu, H.; Zhang, W.; Wang, Y.; Sehgal, S.A.; Siraj, S.; Wang, X.; et al. Mitochondrial E3 Ligase MARCH5 Regulates FUNDC1 to Fine-tune Hypoxic Mitophagy. EMBO Rep. 2017, 18, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Oanh, N.T.K.; Heo, J.; Kim, S.-G.; Lee, H.-S.; Lee, H.; Lee, J.-H.; Kang, H.C.; Lim, W.; Yoo, Y.-S.; et al. Dual Targeting of RIG-I and MAVS by MARCH5 Mitochondria Ubiquitin Ligase in Innate Immunity. Cell. Signal. 2020, 67, 109520. [Google Scholar] [CrossRef]
- Takeda, K.; Nagashima, S.; Shiiba, I.; Uda, A.; Tokuyama, T.; Ito, N.; Fukuda, T.; Matsushita, N.; Ishido, S.; Iwawaki, T.; et al. MITOL Prevents ER Stress-Induced Apoptosis by IRE1α Ubiquitylation at ER-Mitochondria Contact Sites. EMBO J. 2019, 38, e100999. [Google Scholar] [CrossRef]
- Yoo, Y.-S.; Park, Y.-Y.; Kim, J.-H.; Cho, H.; Kim, S.-H.; Lee, H.-S.; Kim, T.-H.; Sun Kim, Y.; Lee, Y.; Kim, C.-J.; et al. The Mitochondrial Ubiquitin Ligase MARCH5 Resolves MAVS Aggregates during Antiviral Signalling. Nat. Commun. 2015, 6, 7910. [Google Scholar] [CrossRef]
- Kim, S.-H.; Park, Y.-Y.; Yoo, Y.-S.; Cho, H. FEBS Press. Self-clearance Mechanism of Mitochondrial E3 ligase MARCH5 Contributes to Mitochondria Quality Control. FEBS J. 2015, 283, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Shiiba, I.; Takeda, K.; Nagashima, S.; Yanagi, S. Overview of Mitochondrial E3 Ubiquitin Ligase MITOL/MARCH5 from Molecular Mechanisms to Diseases. Int. J. Mol. Sci. 2020, 21, 3781. [Google Scholar] [CrossRef]
- Tang, H.; Peng, S.; Dong, Y.; Yang, X.; Yang, P.; Yang, L.; Yang, B.; Bao, G. MARCH5 Overexpression Contributes to Tumor Growth and Metastasis and Associates with Poor Survival in Breast Cancer. Cancer Manag. Res. 2018, 11, 201–215. [Google Scholar] [CrossRef]
- Chu, P.-Y.; Tzeng, Y.-D.T.; Chiu, Y.-H.; Lin, H.-Y.; Kuo, C.-H.; Hou, M.-F.; Li, C.-J. Multi-Omics Reveals the Immunological Role and Prognostic Potential of Mitochondrial Ubiquitin Ligase MARCH5 in Human Breast Cancer. Biomedicines 2021, 9, 1329. [Google Scholar] [CrossRef]
- Hu, J.; Meng, Y.; Zhang, Z.; Yan, Q.; Jiang, X.; Lv, Z.; Hu, L. MARCH5 RNA Promotes Autophagy, Migration, and Invasion of Ovarian Cancer Cells. Autophagy 2017, 13, 333–344. [Google Scholar] [CrossRef]
- Liu, R.; Zeng, L.-W.; Li, H.-F.; Shi, J.-G.; Zhong, B.; Shu, H.-B.; Li, S. PD-1 Signaling Negatively Regulates the Common Cytokine Receptor γ Chain via MARCH5-Mediated Ubiquitination and Degradation to Suppress Anti-Tumor Immunity. Cell Res. 2023. [Google Scholar] [CrossRef]
- Kong, Y.; Jiang, J.; Huang, Y.; Li, L.; Liu, X.; Jin, Z.; Wei, F.; Liu, X.; Zhang, S.; Duan, X.; et al. Endoplasmic Reticulum Stress in Melanoma Pathogenesis and Resistance. Biomed. Pharmacother. 2022, 155, 113741. [Google Scholar] [CrossRef]
- Cerezo, M.; Lehraiki, A.; Millet, A.; Rouaud, F.; Plaisant, M.; Jaune, E.; Botton, T.; Ronco, C.; Abbe, P.; Amdouni, H.; et al. Compounds Triggering ER Stress Exert Anti-Melanoma Effects and Overcome BRAF Inhibitor Resistance. Cancer Cell 2016, 29, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Wieder, S.Y.; Serasinghe, M.N.; Sung, J.C.; Choi, D.C.; Birge, M.B.; Yao, J.L.; Bernstein, E.; Celebi, J.T.; Chipuk, J.E. Activation of the Mitochondrial Fragmentation Protein DRP1 Correlates with BRAF(V600E) Melanoma. J. Investig. Dermatol. 2015, 135, 2544–2547. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yi, X.; Guo, S.; Wang, S.; Ma, J.; Zhao, T.; Shi, Q.; Tian, Y.; Wang, H.; Jia, L.; et al. The XBP1–MARCH5–MFN2 Axis Confers Endoplasmic Reticulum Stress Resistance by Coordinating Mitochondrial Fission and Mitophagy in Melanoma. J. Investig. Dermatol. 2021, 141, 2932–2943.e12. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cheng, X.; Lu, J.; Wei, J.; Fu, G.; Zhu, F.; Jia, C.; Zhou, L.; Xie, H.; Zheng, S. Mitofusin-2 Is a Novel Direct Target of P53. Biochem. Biophys. Res. Commun. 2010, 400, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, J.; Zhu, F.; Wei, J.; Jia, C.; Zhang, Y.; Zhou, L.; Xie, H.; Zheng, S. Pro-Apoptotic and Anti-Proliferative Effects of Mitofusin-2 via Bax Signaling in Hepatocellular Carcinoma Cells. Med. Oncol. 2010, 29, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chen, G.; Li, X.; Wu, X.; Chang, Z.; Xu, J.; Zhu, Y.; Yin, P.; Liang, X.; Dong, L. MFN2 Suppresses Cancer Progression through Inhibition of mTORC2/Akt Signaling. Sci. Rep. 2017, 7, 41718. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Chen, K.; Lima, P.D.A.; Mewburn, J.; Wu, D.; Al-Qazazi, R.; Jones, O.; Tian, L.; Potus, F.; Bonnet, S.; et al. PINK1-induced Phosphorylation of Mitofusin 2 at Serine 442 Causes Its Proteasomal Degradation and Promotes Cell Proliferation in Lung Cancer and Pulmonary Arterial Hypertension. FASEB J. 2021, 35, e21771. [Google Scholar] [CrossRef]
- Subramanian, A.; Andronache, A.; Li, Y.-C.; Wade, M. Inhibition of MARCH5 Ubiquitin Ligase Abrogates MCL1-Dependent Resistance to BH3 Mimetics via NOXA. Oncotarget 2016, 7, 15986–16002. [Google Scholar] [CrossRef]
- Haschka, M.D.; Karbon, G.; Soratroi, C.; O’Neill, K.L.; Luo, X.; Villunger, A. MARCH5-Dependent Degradation of MCL1/NOXA Complexes Defines Susceptibility to Antimitotic Drug Treatment. Cell Death Differ. 2020, 27, 2297–2312. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Roberts, A.W.; Spencer, A.; Rosenberg, A.S.; Siegel, D.; Walter, R.B.; Caenepeel, S.; Hughes, P.; McIver, Z.; Mezzi, K.; et al. Targeting MCL-1 in Hematologic Malignancies: Rationale and Progress. Blood Rev. 2020, 44, 100672. [Google Scholar] [CrossRef] [PubMed]
- Al-Odat, O.; von Suskil, M.; Chitren, R.; Elbezanti, W.; Srivastava, S.; Budak-Alpdogan, T.; Jonnalagadda, S.; Aggarwal, B.; Pandey, M. Mcl-1 Inhibition: Managing Malignancy in Multiple Myeloma. Front. Pharmacol. 2021, 12, 699629. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wan, J.; Zhang, W.; Hao, S. MCL-1 or BCL-xL-Dependent Resistance to the BCL-2 Antagonist (ABT-199) Can Be Overcome by Specific Inhibitor as Single Agents and in Combination with ABT-199 in Acute Myeloid Leukemia Cells. Leuk. Lymphoma 2019, 60, 2170–2180. [Google Scholar] [CrossRef] [PubMed]
- Munkhbaatar, E.; Dietzen, M.; Agrawal, D.; Anton, M.; Jesinghaus, M.; Boxberg, M.; Pfarr, N.; Bidola, P.; Uhrig, S.; Höckendorf, U.; et al. MCL-1 Gains Occur with High Frequency in Lung Adenocarcinoma and Can Be Targeted Therapeutically. Nat. Commun. 2020, 11, 4527. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, S.A.; Breitenbuecher, F.; Soni, A.; Paul-Konietzko, K.; Ziegler, S.; Sak, A.; Iliakis, G.; Schuler, M. Deregulated BCL-2 Family Proteins Impact on Repair of DNA Double-Strand Breaks and Are Targets to Overcome Radioresistance in Lung Cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Tan, S.; Zou, F.; Yu, J.; Zhang, L. FBW7 Mutations Mediate Resistance of Colorectal Cancer to Targeted Therapies by Blocking Mcl-1 Degradation. Oncogene 2017, 36, 787–796. [Google Scholar] [CrossRef]
- El Khouri, E.; Le Pavec, G.; Toledano, M.B.; Delaunay-Moisan, A. RNF185 Is a Novel E3 Ligase of Endoplasmic Reticulum-Associated Degradation (ERAD) That Targets Cystic Fibrosis Transmembrane Conductance Regulator (CFTR). J. Biol. Chem. 2013, 288, 31177–31191. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, L.; Hong, Z.; Lv, Z.; Mao, Z.; Tang, Y.; Kong, X.; Li, S.; Cui, Y.; Liu, H.; et al. The E3 Ubiquitin Ligase RNF185 Facilitates the cGAS-Mediated Innate Immune Response. PLoS Pathog. 2017, 13, e1006264. [Google Scholar] [CrossRef]
- Tang, F.; Wang, B.; Li, N.; Wu, Y.; Jia, J.; Suo, T.; Chen, Q.; Liu, Y.-J.; Tang, J. RNF185, a Novel Mitochondrial Ubiquitin E3 Ligase, Regulates Autophagy through Interaction with BNIP1. PLoS ONE 2011, 6, e24367. [Google Scholar] [CrossRef]
- Huang, C.; Li, K.; Huang, R.; Zhu, J.; Yang, J. RNF185-AS1 Promotes Hepatocellular Carcinoma Progression through Targeting miR-221-5p/Integrin Β5 Axis. Life Sci. 2021, 267, 118928. [Google Scholar] [CrossRef]
- Song, Q.; An, Q.; Niu, B.; Lu, X.; Zhang, N.; Cao, X. Role of miR-221/222 in Tumor Development and the Underlying Mechanism. J. Oncol. 2019, 2019, 7252013. [Google Scholar] [CrossRef]
- Di Martino, M.T.; Arbitrio, M.; Caracciolo, D.; Cordua, A.; Cuomo, O.; Grillone, K.; Riillo, C.; Caridà, G.; Scionti, F.; Labanca, C.; et al. miR-221/222 as Biomarkers and Targets for Therapeutic Intervention on Cancer and Other Diseases: A Systematic Review. Mol. Ther.—Nucleic Acids 2022, 27, 1191–1224. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, M.; Song, Y.; Yang, N. RNF185 Antisense RNA 1 (RNF185-AS1) Promotes Proliferation, Migration, and Invasion in Papillary Thyroid Carcinoma. Anti-Cancer Drugs 2022, 33, 595–606. [Google Scholar] [CrossRef]
- Van Espen, B.; Oo, H.Z.; Collins, C.; Fazli, L.; Molinolo, A.; Yip, K.; Murad, R.; Gleave, M.; Ronai, Z.A. RNF185 Control of COL3A1 Expression Limits Prostate Cancer Migration and Metastatic Potential. Mol. Cancer Res. 2023; ahead of print. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Guo, Z.; Liu, H. COL3A1 Overexpression Associates with Poor Prognosis and Cisplatin Resistance in Lung Cancer. Balk. Med. J. 2022, 39, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Iwase, I.; Yamasaki, Y.; Takai, T.; Wu, Y.; Kanemoto, S.; Matsuhisa, K.; Asada, R.; Okuma, Y.; Watanabe, T.; et al. Genome-Wide Identification and Gene Expression Profiling of Ubiquitin Ligases for Endoplasmic Reticulum Protein Degradation. Sci. Rep. 2016, 6, 30955. [Google Scholar] [CrossRef] [PubMed]
- van de Weijer, M.L.; Krshnan, L.; Liberatori, S.; Guerrero, E.N.; Robson-Tull, J.; Hahn, L.; Lebbink, R.J.; Wiertz, E.J.H.J.; Fischer, R.; Ebner, D.; et al. Quality Control of ER Membrane Proteins by the RNF185/Membralin Ubiquitin Ligase Complex. Mol. Cell 2020, 80, 374–375. [Google Scholar] [CrossRef] [PubMed]
- Kaluzhskiy, L.; Ershov, P.; Yablokov, E.; Shkel, T.; Grabovec, I.; Mezentsev, Y.; Gnedenko, O.; Usanov, S.; Shabunya, P.; Fatykhava, S.; et al. Human Lanosterol 14-Alpha Demethylase (CYP51A1) Is a Putative Target for Natural Flavonoid Luteolin 7,3′-Disulfate. Molecules 2021, 26, 2237. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Botas, J.; Ferruelo, A.J.; Suárez, Y.; Gómez-Coronado, D.; Lasunción, M.A. Induction of Apoptosis in P53-Null HL-60 Cells by Inhibition of Lanosterol 14-α Demethylase. Biochimie 1998, 80, 887–894. [Google Scholar] [CrossRef]
- Shteinberg, M.; Haq, I.J.; Polineni, D.; Davies, J.C. Cystic Fibrosis. Lancet 2021, 397, 2195–2211. [Google Scholar] [CrossRef] [PubMed]
- Graeber, S.Y.; Mall, M.A. The Future of Cystic Fibrosis Treatment: From Disease Mechanisms to Novel Therapeutic Approaches. Lancet 2023, 402, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Parisi, G.F.; Papale, M.; Pecora, G.; Rotolo, N.; Manti, S.; Russo, G.; Leonardi, S. Cystic Fibrosis and Cancer: Unraveling the Complex Role of CFTR Gene in Cancer Susceptibility. Cancers 2023, 15, 4244. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Blankenheim, Z.; Scott, P.M.; Cormier, R.T. CFTR and Gastrointestinal Cancers: An Update. J. Pers. Med. 2022, 12, 868. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Liu, X.; Wang, L.; Zou, L.; Jiang, X.; Li, A.; Zhou, J. Targeting JWA for Cancer Therapy: Functions, Mechanisms and Drug Discovery. Cancers 2022, 14, 4655. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Wang, Q.; Wang, Z.; Chen, J.; Yan, D.; Zhou, Y.; Li, A.; Zhang, R.; Wang, S.; Zhou, J. RNF185 Modulates JWA Ubiquitination and Promotes Gastric Cancer Metastasis. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2018, 1864, 1552–1561. [Google Scholar] [CrossRef]
- de Martino, M.; Klatte, T.; Haitel, A.; Marberger, M. Serum Cell-free DNA in Renal Cell Carcinoma. Cancer 2012, 118, 82–90. [Google Scholar] [CrossRef]
- Principi, E.; Sordo, E.; Bianchi, G.; Ravera, S.; Morini, M.; Tomati, V.; Pastorino, C.; Zara, F.; Bruno, C.; Eva, A.; et al. Targeting of Ubiquitin E3 Ligase RNF5 as a Novel Therapeutic Strategy in Neuroectodermal Tumors. Cancers 2022, 14, 1802. [Google Scholar] [CrossRef]
- Lin, K.; Shen, S.-H.; Lu, F.; Zheng, P.; Wu, S.; Liao, J.; Jiang, X.; Zeng, G.; Wei, D. CRISPR Screening of E3 Ubiquitin Ligases Reveals Ring Finger Protein 185 as a Novel Tumor Suppressor in Glioblastoma Repressed by Promoter Hypermethylation and miR-587. J. Transl. Med. 2022, 20, 96. [Google Scholar] [CrossRef]
- Li, W.; Bengtson, M.H.; Ulbrich, A.; Matsuda, A.; Reddy, V.A.; Orth, A.; Chanda, S.K.; Batalov, S.; Joazeiro, C.A.P. Genome-Wide and Functional Annotation of Human E3 Ubiquitin Ligases Identifies MULAN, a Mitochondrial E3 That Regulates the Organelle’s Dynamics and Signaling. PLoS ONE 2008, 3, e1487. [Google Scholar] [CrossRef]
- Peng, J.; Ren, K.-D.; Yang, J.; Luo, X.-J. Mitochondrial E3 Ubiquitin Ligase 1: A Key Enzyme in Regulation of Mitochondrial Dynamics and Functions. Mitochondrion 2016, 28, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Prudent, J.; Zunino, R.; Sugiura, A.; Mattie, S.; Shore, G.C.; McBride, H.M. MAPL SUMOylation of Drp1 Stabilizes an ER/Mitochondrial Platform Required for Cell Death. Mol. Cell 2015, 59, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Cheng, X.-T.; Lin, M.-Y.; Huang, N.; Sheng, Z.-H. Mul1 Restrains Parkin-Mediated Mitophagy in Mature Neurons by Maintaining ER-Mitochondrial Contacts. Nat. Commun. 2019, 10, 3645. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qi, W.; Chen, G.; Feng, D.; Liu, J.; Ma, B.; Zhou, C.; Mu, C.; Zhang, W.; Chen, Q.; et al. Mitochondrial Outer-Membrane E3 Ligase MUL1 Ubiquitinates ULK1 and Regulates Selenite-Induced Mitophagy. Autophagy 2015, 11, 1216–1229. [Google Scholar] [CrossRef]
- Cilenti, L.; Mahar, R.; Di Gregorio, J.; Ambivero, C.T.; Merritt, M.E.; Zervos, A.S. Regulation of Metabolism by Mitochondrial MUL1 E3 Ubiquitin Ligase. Front. Cell Dev. Biol. 2022, 10, 904728. [Google Scholar] [CrossRef]
- Ni, G.; Konno, H.; Barber, G.N. Ubiquitination of STING at Lysine 224 Controls IRF3 Activation. Sci. Immunol. 2017, 2, eaah7119. [Google Scholar] [CrossRef]
- Lee, M.-S.; Lee, S.-O.; Lee, M.-K.; Yi, G.-S.; Lee, C.-K.; Ryu, K.-S.; Chi, S.-W. Solution Structure of MUL1-RING Domain and Its Interaction with P53 Transactivation Domain. Biochem. Biophys. Res. Commun. 2019, 516, 533–539. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, J.; Li, H.-L.; Liu, T.; Wang, Y.-Y.; Waterman, P.; Mao, A.-P.; Xu, L.-G.; Zhai, Z.; Liu, D.; et al. GIDE Is a Mitochondrial E3 Ubiquitin Ligase That Induces Apoptosis and Slows Growth. Cell Res. 2008, 18, 900–910. [Google Scholar] [CrossRef]
- Braschi, E.; Zunino, R.; McBride, H.M. MAPL Is a New Mitochondrial SUMO E3 Ligase That Regulates Mitochondrial Fission. EMBO Rep. 2009, 10, 748–754. [Google Scholar] [CrossRef]
- Jenkins, K.; Khoo, J.J.; Sadler, A.; Piganis, R.; Wang, D.; Borg, N.A.; Hjerrild, K.; Gould, J.; Thomas, B.J.; Nagley, P.; et al. Mitochondrially Localised MUL1 Is a Novel Modulator of Antiviral Signaling. Immunol. Cell Biol. 2013, 91, 321–330. [Google Scholar] [CrossRef]
- Cilenti, L.; Ambivero, C.T.; Ward, N.; Alnemri, E.S.; Germain, D.; Zervos, A.S. Inactivation of Omi/HtrA2 Protease Leads to the Deregulation of Mitochondrial Mulan E3 Ubiquitin Ligase and Increased Mitophagy. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Puri, R.; Yang, H.; Lizzio, M.A.; Wu, C.; Sheng, Z.-H.; Guo, M. MUL1 Acts in Parallel to the PINK1/Parkin Pathway in Regulating Mitofusin and Compensates for Loss of PINK1/Parkin. eLife 2014, 3, e01958. [Google Scholar] [CrossRef] [PubMed]
- Cilenti, L.; Di Gregorio, J.; Ambivero, C.T.; Andl, T.; Liao, R.; Zervos, A.S. Mitochondrial MUL1 E3 Ubiquitin Ligase Regulates Hypoxia Inducible Factor (HIF-1α) and Metabolic Reprogramming by Modulating the UBXN7 Cofactor Protein. Sci. Rep. 2020, 10, 1609. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Cheng, X.-T.; Lin, M.-Y.; Huang, N.; Sheng, Z.-H. Defending Stressed Mitochondria: Uncovering the Role of MUL1 in Suppressing Neuronal Mitophagy. Autophagy 2020, 16, 176–178. [Google Scholar] [CrossRef]
- Gao, A.; Wang, M.; Tang, X.; Shi, G.; Hou, K.; Fang, J.; Zhou, L.; Zhou, H.; Jiang, W.; Li, Y.; et al. NDP52 SUMOylation Contributes to Low-dose X-rays-induced Cardiac Hypertrophy through PINK1/Parkin-mediated Mitophagy via MUL1/SUMO2 Signalling. J. Cell. Physiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.J.; Candau, R.; Bernardi, H. AMP-Activated Protein Kinase Stabilizes FOXO3 in Primary Myotubes. Biochem. Biophys. Res. Commun. 2018, 499, 493–498. [Google Scholar] [CrossRef] [PubMed]
- van Wijk, S.J.L.; de Vries, S.J.; Kemmeren, P.; Huang, A.; Boelens, R.; Bonvin, A.M.J.J.; Timmers, H.T.M. A Comprehensive Framework of E2–RING E3 Interactions of the Human Ubiquitin–Proteasome System. Mol. Syst. Biol. 2009, 5, 295. [Google Scholar] [CrossRef]
- Calle, X.; Garrido-Moreno, V.; Lopez-Gallardo, E.; Norambuena-Soto, I.; Martínez, D.; Peñaloza-Otárola, A.; Troncossi, A.; Guerrero-Moncayo, A.; Ortega, A.; Maracaja-Coutinho, V.; et al. Mitochondrial E3 Ubiquitin Ligase 1 (MUL1) as a Novel Therapeutic Target for Diseases Associated with Mitochondrial Dysfunction. IUBMB Life 2022, 74, 850–865. [Google Scholar] [CrossRef]
- Jung, J.H.; Bae, S.; Lee, J.Y.; Woo, S.R.; Cha, H.J.; Yoon, Y.; Suh, K.-S.; Lee, S.-J.; Park, I.-C.; Jin, Y.-W.; et al. E3 Ubiquitin Ligase Hades Negatively Regulates the Exonuclear Function of P53. Cell Death Differ. 2011, 18, 1865–1875. [Google Scholar] [CrossRef]
- Subhasree, N.; Jiangjiang, Q.; Kalkunte, S.; Minghai, W.; Ruiwen, Z. The MDM2-P53 Pathway Revisited. J. Biomed. Res. 2013, 27, 254. [Google Scholar] [CrossRef]
- Min, B.; Ryu, J.; Chi, S.-W.; Yi, G.-S. Ubiquitination-Dependent Degradation of P73 by the Mitochondrial E3 Ubiquitin Ligase Hades. Biochem. Biophys. Res. Commun. 2015, 467, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Kim, H.J.; Park, M.K.; Huh, J.W.; Park, H.Y.; Ha, S.Y.; Shin, J.-H.; Lee, Y.-S. Mitochondrial E3 Ubiquitin Protein Ligase 1 Mediates Cigarette Smoke–Induced Endothelial Cell Death and Dysfunction. Am. J. Respir. Cell Mol. Biol. 2016, 54, 284–296. [Google Scholar] [CrossRef]
- Fan, Y.; Peng, X.; Li, B.; Zhao, G. Development of Autophagy Signature-Based Prognostic Nomogram for Refined Glioma Survival Prognostication. BioMed Res. Int. 2020, 2020, 1872962. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lin, N. A Risk Signature of Three Autophagy-Related Genes for Predicting Lower Grade Glioma Survival Is Associated with Tumor Immune Microenvironment. Genomics 2021, 113, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhou, J.; Du, W.; Ning, W.; Zhang, Y.; Zeng, Y.; Liu, Z.; Huang, J.-A. AKT2 Drives Cancer Progression and Is Negatively Modulated by miR-124 in Human Lung Adenocarcinoma. Respir. Res. 2020, 21, 227. [Google Scholar] [CrossRef]
- Revathidevi, S.; Munirajan, A.K. Akt in Cancer: Mediator and More. Semin. Cancer Biol. 2019, 59, 80–91. [Google Scholar] [CrossRef]
- Lee, J.; An, S.; Jung, J.; Kim, K.; Kim, J.; An, I.-S.; Bae, S. MUL1 E3 Ligase Regulates the Antitumor Effects of Metformin in Chemoresistant Ovarian Cancer Cells via AKT Degradation. Int. J. Oncol. 2019, 54, 1833–1842. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, H.J.; Kim, H.-J.; Kim, D.H.; Han, J.H.; Byeon, H.K.; Lee, K.; Kim, C.-H. HSPA5 Negatively Regulates Lysosomal Activity through Ubiquitination of MUL1 in Head and Neck Cancer. Autophagy 2018, 14, 385–403. [Google Scholar] [CrossRef]
- Di Gregorio, J.; Cilenti, L.; Ambivero, C.T.; Andl, T.; Liao, R.; Zervos, A.S. UBXN7 Cofactor of CRL3KEAP1 and CRL2VHL Ubiquitin Ligase Complexes Mediates Reciprocal Regulation of NRF2 and HIF-1α Proteins. Biochim. Biophys. Acta. Mol. Cell Res. 2021, 1868, 118963. [Google Scholar] [CrossRef]
- Jun, J.C.; Rathore, A.; Younas, H.; Gilkes, D.; Polotsky, V.Y. Hypoxia-Inducible Factors and Cancer. Curr. Sleep Med. Rep. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Sharma, A.; Sinha, S.; Shrivastava, N. Therapeutic Targeting Hypoxia-Inducible Factor (HIF-1) in Cancer: Cutting Gordian Knot of Cancer Cell Metabolism. Front. Genet. 2022, 13, 849040. [Google Scholar] [CrossRef] [PubMed]
- Ito, S. Proteasome Inhibitors for the Treatment of Multiple Myeloma. Cancers 2020, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Ahmed, Z.S.; Huang, X.; Yang, Q.; Ekinci, E.; Neslund-Dudas, C.M.; Mitra, B.; Elnady, F.A.; Ahn, Y.-H.; Yang, H.; et al. Discovering Proteasomal Deubiquitinating Enzyme Inhibitors for Cancer Therapy: Lessons from Rational Design, Nature and Old Drug Reposition. Future Med. Chem. 2018, 10, 2087–2108. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Gregorio, J.; Appignani, M.; Flati, V. Role of the Mitochondrial E3 Ubiquitin Ligases as Possible Therapeutic Targets in Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 17176. https://doi.org/10.3390/ijms242417176
Di Gregorio J, Appignani M, Flati V. Role of the Mitochondrial E3 Ubiquitin Ligases as Possible Therapeutic Targets in Cancer Therapy. International Journal of Molecular Sciences. 2023; 24(24):17176. https://doi.org/10.3390/ijms242417176
Chicago/Turabian StyleDi Gregorio, Jacopo, Martina Appignani, and Vincenzo Flati. 2023. "Role of the Mitochondrial E3 Ubiquitin Ligases as Possible Therapeutic Targets in Cancer Therapy" International Journal of Molecular Sciences 24, no. 24: 17176. https://doi.org/10.3390/ijms242417176