Exogenous Nitric Oxide Alleviates Water Deficit and Increases the Seed Production of an Endemic Amazonian Canga Grass
Abstract
:1. Introduction
2. Results
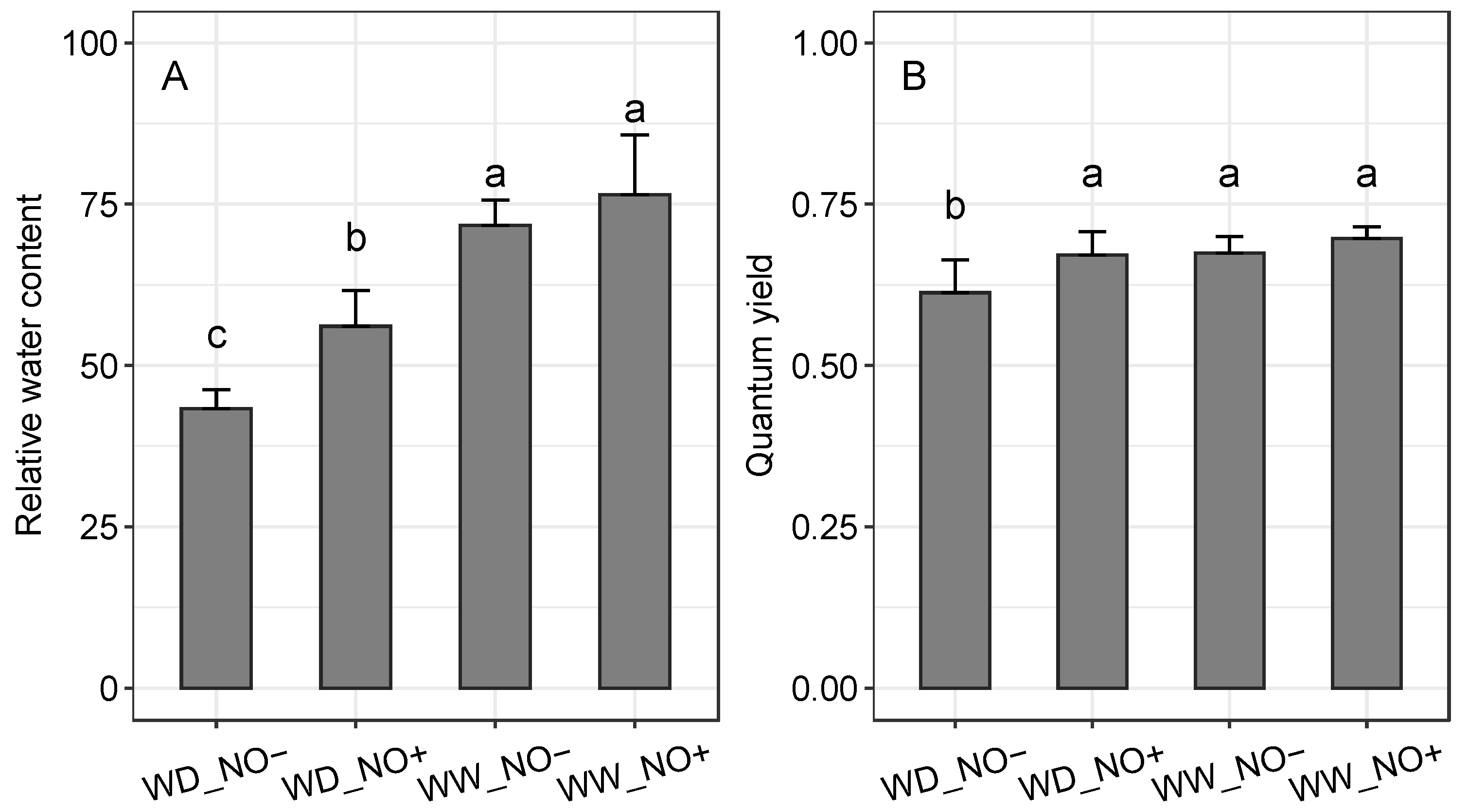
2.1. Relative Water Content and Photosynthetic Efficiency
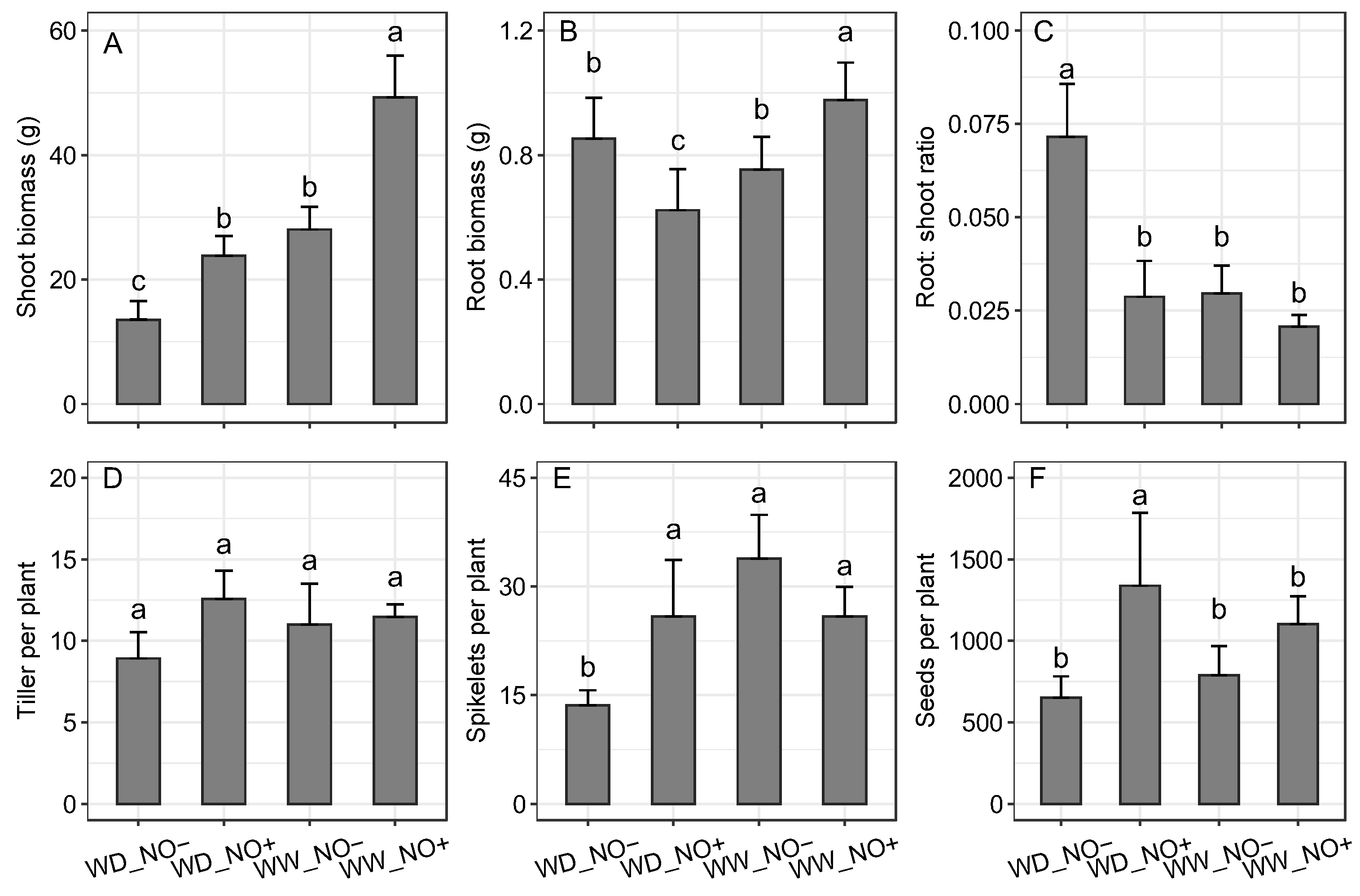
2.2. Biomass
2.3. Anatomical Traits
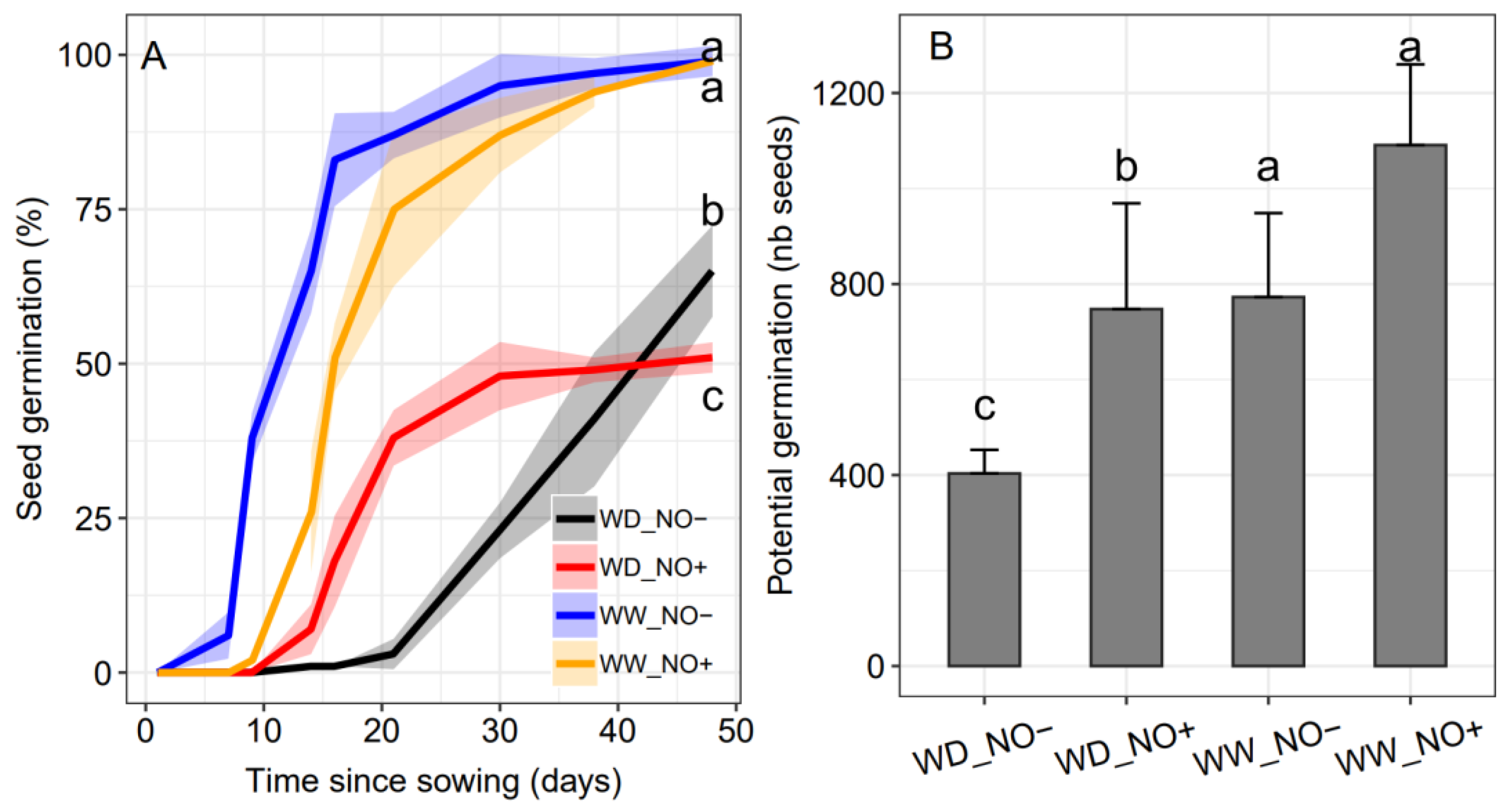
2.4. Seed Germination
2.5. Proteomics Profile
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Design
4.3. Relative Water Content
4.4. Photosynthetic Efficiency
4.5. Tillering Rate and Biomass Production
4.6. Anatomical Analyses
4.7. Germination Assay
4.8. Proteomic Profile
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zappi, D.C.; Moro, M.F.; Meagher, T.R.; Nic Lughadha, E. Plant Biodiversity Drivers in Brazilian Campos Rupestres: Insights from Phylogenetic Structure. Front. Plant Sci. 2017, 8, 2141. [Google Scholar] [CrossRef]
- Jacobi, C.M.; Do Carmo, F.F.; De Campos, I.C. Soaring Extinction Threats to Endemic Plants in Brazilian Metal-Rich Regions. AMBIO 2011, 40, 540–543. [Google Scholar] [CrossRef]
- Viana, P.L.; Mota, N.F.D.O.; Gil, A.D.S.B.; Salino, A.; Zappi, D.C.; Harley, R.M.; Ilkiu-Borges, A.L.; Secco, R.D.S.; Almeida, T.E.; Watanabe, M.T.C.; et al. Flora das Cangas da Serra dos Carajás, Pará, Brasil: História, Área de Estudos e Metodologia. Rodriguésia 2016, 67, 1107–1124. [Google Scholar] [CrossRef]
- Mota, N.F.D.O.; Watanabe, M.T.C.; Zappi, D.C.; Hiura, A.L.; Pallos, J.; Viveros, R.S.; Giulietti, A.M.; Viana, P.L. Cangas da Amazônia: A Vegetação Única de Carajás Evidenciada Pela Lista de Fanerógamas. Rodriguésia 2018, 69, 1435–1488. [Google Scholar] [CrossRef]
- Skirycz, A.; Castilho, A.; Chaparro, C.; Carvalho, N.; Tzotzos, G.; Siqueira, J.O. Canga Biodiversity, a Matter of Mining. Front. Plant Sci. 2014, 5, 653. [Google Scholar] [CrossRef]
- Silveira, N.M.; Frungillo, L.; Marcos, F.C.C.; Pelegrino, M.T.; Miranda, M.T.; Seabra, A.B.; Salgado, I.; Machado, E.C.; Ribeiro, R.V. Exogenous Nitric Oxide Improves Sugarcane Growth and Photosynthesis under Water Deficit. Planta 2016, 244, 181–190. [Google Scholar] [CrossRef]
- Giulietti, A.M.; Giannini, T.C.; Mota, N.F.O.; Watanabe, M.T.C.; Viana, P.L.; Pastore, M.; Silva, U.C.S.; Siqueira, M.F.; Pirani, J.R.; Lima, H.C.; et al. Edaphic Endemism in the Amazon: Vascular Plants of the Canga of Carajás, Brazil. Bot. Rev. 2019, 85, 357–383. [Google Scholar] [CrossRef]
- Ranjan, R. Assessing the Impact of Mining on Deforestation in India. Resour. Policy 2019, 60, 23–35. [Google Scholar] [CrossRef]
- Gastauer, M.; Massante, J.C.; Ramos, S.J.; Da Silva, R.D.S.S.; Boanares, D.; Guedes, R.S.; Caldeira, C.F.; Medeiros-Sarmento, P.S.; De Castro, A.F.; Prado, I.G.D.O.; et al. Revegetation on Tropical Steep Slopes after Mining and Infrastructure Projects: Challenges and Solutions. Sustainability 2022, 14, 17003. [Google Scholar] [CrossRef]
- Boechat, S.D.C.; Longhi-Wagner, H.M. O Gênero Sporobolus (Poaceae: Chloridoideae) No Brasil. Acta Bot. Bras. 1995, 9, 21–86. [Google Scholar] [CrossRef]
- Siqueira-Silva, A.I.; Rios, C.O.; Pereira, E.G. Iron Toxicity Resistance Strategies in Tropical Grasses: The Role of Apoplastic Radicular Barriers. J. Environ. Sci. 2019, 78, 257–266. [Google Scholar] [CrossRef]
- Gastauer, M.; Silva, J.R.; Caldeira Junior, C.F.; Ramos, S.J.; Souza Filho, P.W.M.; Furtini Neto, A.E.; Siqueira, J.O. Mine Land Rehabilitation: Modern Ecological Approaches for More Sustainable Mining. J. Clean. Prod. 2018, 172, 1409–1422. [Google Scholar] [CrossRef]
- Caldeira, C.F.; Lima, M.O.; Ramos, S.J.; Gastauer, M. Native Amazonian Canga Grasses Show Distinct Nitrogen Growth Responses in Iron Mining Substrates. Plants 2021, 10, 849. [Google Scholar] [CrossRef]
- Leger, E.A.; Baughman, O.W. What Seeds to Plant in the Great Basin? Comparing Traits Prioritized in Native Plant Cultivars and Releases with Those That Promote Survival in the Field. Nat. Areas J. 2015, 35, 54–68. [Google Scholar] [CrossRef]
- Faurès, J.M.; Hoogeveen, J.; Bruinsma, J. The FAO Irrigated Area Forecast for 2030; Food and Agricultural Organization: Rome, Italy, 2002; pp. 1–14. [Google Scholar]
- Kim, J.-Y.; Mahé, A.; Brangeon, J.; Prioul, J.-L. A Maize Vacuolar Invertase, IVR 2, Is Induced by Water Stress. Organ/Tissue Specificity and Diurnal Modulation of Expression. Plant Physiol. 2000, 124, 71–84. [Google Scholar] [CrossRef]
- Delledonne, M. NO News Is Good News for Plants. Curr. Opin. Plant Biol. 2005, 8, 390–396. [Google Scholar] [CrossRef]
- Wendehenne, D.; Pugin, A.; Klessig, D.F.; Durner, J. Nitric Oxide: Comparative Synthesis and Signaling in Animal and Plant Cells. Trends Plant Sci. 2001, 6, 177–183. [Google Scholar] [CrossRef]
- Neill, S.J.; Desikan, R.; Clarke, A.; Hancock, J.T. Nitric Oxide Is a Novel Component of Abscisic Acid Signaling in Stomatal Guard Cells. Plant Physiol. 2002, 128, 13–16. [Google Scholar] [CrossRef]
- Graziano, M.; Beligni, M.V.; Lamattina, L. Nitric Oxide Improves Internal Iron Availability in Plants. Plant Physiol. 2002, 130, 1852–1859. [Google Scholar] [CrossRef]
- Sanz, L.; Albertos, P.; Mateos, I.; Sánchez-Vicente, I.; Lechón, T.; Fernández-Marcos, M.; Lorenzo, O. Nitric Oxide (NO) and Phytohormones Crosstalk during Early Plant Development. J. Exp. Bot. 2015, 66, 2857–2868. [Google Scholar] [CrossRef]
- Salgado, I.; Carmen Martínez, M.; Oliveira, H.C.; Frungillo, L. Nitric Oxide Signaling and Homeostasis in Plants: A Focus on Nitrate Reductase and S-Nitrosoglutathione Reductase in Stress-Related Responses. Braz. J. Bot. 2013, 36, 89–98. [Google Scholar] [CrossRef]
- Simontacchi, M.; Galatro, A.; Ramos-Artuso, F.; Santa-María, G.E. Plant Survival in a Changing Environment: The Role of Nitric Oxide in Plant Responses to Abiotic Stress. Front. Plant Sci. 2015, 6, 977. [Google Scholar] [CrossRef]
- Rezayian, M.; Ebrahimzadeh, H.; Niknam, V. Nitric Oxide Stimulates Antioxidant System and Osmotic Adjustment in Soybean Under Drought Stress. J. Soil. Sci. Plant Nutr. 2020, 20, 1122–1132. [Google Scholar] [CrossRef]
- Rahimian Boogar, A.; Salehi, H.; Jowkar, A. Exogenous Nitric Oxide Alleviates Oxidative Damage in Turfgrasses under Drought Stress. S. Afr. J. Bot. 2014, 92, 78–82. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.-G.; Yun, B.-W. Nitric Oxide Regulates Plant Responses to Drought, Salinity, and Heavy Metal Stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Buet, A.; Galatro, A.; Ramos-Artuso, F.; Simontacchi, M. Nitric Oxide and Plant Mineral Nutrition: Current Knowledge. J. Exp. Bot. 2019, 70, 4461–4476. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Aziz, U.; Alsahli, A.A.; Alyemeni, M.N.; Ahmad, P. Influence of Exogenous Salicylic Acid and Nitric Oxide on Growth, Photosynthesis, and Ascorbate-Glutathione Cycle in Salt Stressed Vigna Angularis. Biomolecules 2019, 10, 42. [Google Scholar] [CrossRef]
- Rather, B.A.; Masood, A.; Sehar, Z.; Majid, A.; Anjum, N.A.; Khan, N.A. Mechanisms and Role of Nitric Oxide in Phytotoxicity-Mitigation of Copper. Front. Plant Sci. 2020, 11, 675. [Google Scholar] [CrossRef]
- Habib, N.; Ali, Q.; Ali, S.; Javed, M.T.; Zulqurnain Haider, M.; Perveen, R.; Shahid, M.R.; Rizwan, M.; Abdel-Daim, M.M.; Elkelish, A.; et al. Use of Nitric Oxide and Hydrogen Peroxide for Better Yield of Wheat (Triticum aestivum L.) under Water Deficit Conditions: Growth, Osmoregulation, and Antioxidative Defense Mechanism. Plants 2020, 9, 285. [Google Scholar] [CrossRef]
- Salahuddin, M.; Nawaz, F.; Shahbaz, M.; Naeem, M.; Zulfiqar, B.; Shabbir, R.N.; Hussain, R.A. Effect of Exogenous Nitric Oxide (NO) Supply on Germination and Seedling Growth of Mungbean (Cv. Nm-54) under Salinity Stress. LR 2017, 40, 846–852. [Google Scholar] [CrossRef]
- Munawar, A.; Akram, N.A.; Ahmad, A.; Ashraf, M. Nitric Oxide Regulates Oxidative Defense System, Key Metabolites and Growth of Broccoli (Brassica oleracea L.) Plants under Water Limited Conditions. Sci. Hortic. 2019, 254, 7–13. [Google Scholar] [CrossRef]
- Montilla-Bascón, G.; Rubiales, D.; Hebelstrup, K.H.; Mandon, J.; Harren, F.J.M.; Cristescu, S.M.; Mur, L.A.J.; Prats, E. Reduced Nitric Oxide Levels during Drought Stress Promote Drought Tolerance in Barley and Is Associated with Elevated Polyamine Biosynthesis. Sci. Rep. 2017, 7, 13311. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Liu, Y.; Zhang, Q.; Wei, Q.; Zhang, W. Nitric Oxide Enhances Salt Tolerance in Maize Seedlings through Increasing Activities of Proton-Pump and Na+/H+ Antiport in the Tonoplast. Planta 2006, 224, 545–555. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Wahid, A.; Rehman, H. Exogenously Applied Nitric Oxide Enhances the Drought Tolerance in Fine Grain Aromatic Rice (Oryza sativa L.). J. Agron. Crop Sci. 2009, 195, 254–261. [Google Scholar] [CrossRef]
- Majeed, S.; Nawaz, F.; Naeem, M.; Ashraf, M.Y. Effect of Exogenous Nitric Oxide on Sulfur and Nitrate Assimilation Pathway Enzymes in Maize (Zea mays L.) under Drought Stress. Acta Physiol. Plant 2018, 40, 206. [Google Scholar] [CrossRef]
- Hatamzadeh, A.; Molaahmad Nalousi, A.; Ghasemnezhad, M.; Biglouei, M.H. The Potential of Nitric Oxide for Reducing Oxidative Damage Induced by Drought Stress in Two Turfgrass Species, Creeping Bentgrass and Tall Fescue. Grass Forage Sci. 2015, 70, 538–548. [Google Scholar] [CrossRef]
- Kabiri, R.; Hatami, A.; Oloumi, H.; Naghizadeh, M.; Nasibi, F.; Tahmasebi, Z. Foliar Application of Melatonin Induces Tolerance to Drought Stress in Moldavian Balm Plants (Dracocephalum moldavica) through Regulating the Antioxidant System. Folia Hortic. 2018, 30, 155–167. [Google Scholar] [CrossRef]
- Meier, I.C.; Leuschner, C. Belowground Drought Response of European Beech: Fine Root Biomass and Carbon Partitioning in 14 Mature Stands across a Precipitation Gradient: Belowground Drought Response of Beech. Glob. Change Biol. 2008, 14, 2081–2095. [Google Scholar] [CrossRef]
- Niu, G.; Fang, Y.; Chang, L.; Jin, J.; Yuan, H.; Zeng, X. Enhancing the Noah-MP Ecosystem Response to Droughts with an Explicit Representation of Plant Water Storage Supplied by Dynamic Root Water Uptake. J. Adv. Model. Earth Syst. 2020, 12, e2020MS002062. [Google Scholar] [CrossRef]
- Lombardo, M.C.; Graziano, M.; Polacco, J.C.; Lamattina, L. Nitric Oxide Functions as a Positive Regulator of Root Hair Development. Plant Signal. Behav. 2006, 1, 28–33. [Google Scholar] [CrossRef]
- Yemets, A.I.; Krasylenko, Y.A.; Sheremet, Y.A.; Blume, Y.B. Microtubule Reorganization as a Response to Implementation of NO Signals in Plant Cells. Cytol. Genet. 2009, 43, 73–79. [Google Scholar] [CrossRef]
- Vadez, V. Root Hydraulics: The Forgotten Side of Roots in Drought Adaptation. Field Crops Res. 2014, 165, 15–24. [Google Scholar] [CrossRef]
- Sharp, R.E. Interaction with Ethylene: Changing Views on the Role of Abscisic Acid in Root and Shoot Growth Responses to Water Stress: ABA, Ethylene and Root and Shoot Growth. Plant Cell Environ. 2002, 25, 211–222. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Yin, H.; Liu, X.; Sun, H.; Mi, Q. Exogenous Nitric Oxide Improves Antioxidative Capacity and Reduces Auxin Degradation in Roots of Medicago Truncatula Seedlings under Cadmium Stress. Plant Soil. 2010, 326, 321–330. [Google Scholar] [CrossRef]
- Imran, M.; Shazad, R.; Bilal, S.; Imran, Q.M.; Khan, M.; Kang, S.-M.; Khan, A.L.; Yun, B.-W.; Lee, I.-J. Exogenous Melatonin Mediates the Regulation of Endogenous Nitric Oxide in Glycine max L. to Reduce Effects of Drought Stress. Environ. Exp. Bot. 2021, 188, 104511. [Google Scholar] [CrossRef]
- Dunlap, J.M.; Stettler, R.F. Variation in Leaf Epidermal and Stomatal Traits of Populus trichocarpa from Two Transects across the Washington Cascades. Can. J. Bot. 2001, 79, 528–536. [Google Scholar] [CrossRef]
- Martínez, J.P.; Silva, H.; Ledent, J.F.; Pinto, M. Effect of Drought Stress on the Osmotic Adjustment, Cell Wall Elasticity and Cell Volume of Six Cultivars of Common Beans (Phaseolus vulgaris L.). Eur. J. Agron. 2007, 26, 30–38. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of Leaf Stomatal Density to Water Status and Its Relationship with Photosynthesis in a Grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef]
- Charfeddine, S.; Saïdi, M.N.; Charfeddine, M.; Gargouri-Bouzid, R. Genome-Wide Identification and Expression Profiling of the Late Embryogenesis Abundant Genes in Potato with Emphasis on Dehydrins. Mol. Biol. Rep. 2015, 42, 1163–1174. [Google Scholar] [CrossRef]
- Close, T.J. Dehydrins: Emergence of a biochemical role of a family of plant dehydration proteins. Physiologia Plantarum 1996, 97, 795–803. [Google Scholar] [CrossRef]
- Allagulova, C.R.; Gimalov, F.R.; Shakirova, F.M.; Vakhitov, V.A. The Plant Dehydrins: Structure and Putative Functions. Biochemistry 2003, 68, 945–951. [Google Scholar] [CrossRef]
- Yongchun, W. The Expression of Dehydrin in Wheat Leaves under Drought Stress and Its Relationship with Water. J. Northwest Agric. Forest. Univ. 2010, 38, 69–75. [Google Scholar]
- Hundertmark, M.; Hincha, D.K. LEA (Late Embryogenesis Abundant) Proteins and Their Encoding Genes in Arabidopsis thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef]
- Bies-Ethève, N.; Gaubier-Comella, P.; Debures, A.; Lasserre, E.; Jobet, E.; Raynal, M.; Cooke, R.; Delseny, M. Inventory, Evolution and Expression Profiling Diversity of the LEA (Late Embryogenesis Abundant) Protein Gene Family in Arabidopsis thaliana. Plant Mol. Biol. 2008, 67, 107–124. [Google Scholar] [CrossRef]
- Borisjuk, L.; Macherel, D.; Benamar, A.; Wobus, U.; Rolletschek, H. Low Oxygen Sensing and Balancing in Plant Seeds: A Role for Nitric Oxide. New Phytol. 2007, 176, 813–823. [Google Scholar] [CrossRef]
- Hepworth, S.R.; Zhang, Y.; McKim, S.; Li, X.; Haughn, G.W. BLADE-ON-PETIOLE–Dependent Signaling Controls Leaf and Floral Patterning in Arabidopsis. Plant Cell 2005, 17, 1434–1448. [Google Scholar] [CrossRef]
- Zhang, B.; Holmlund, M.; Lorrain, S.; Norberg, M.; Bakó, L.; Fankhauser, C.; Nilsson, O. BLADE-ON-PETIOLE Proteins Act in an E3 Ubiquitin Ligase Complex to Regulate PHYTOCHROME INTERACTING FACTOR 4 Abundance. eLife 2017, 6, e26759. [Google Scholar] [CrossRef]
- Chahtane, H.; Zhang, B.; Norberg, M.; LeMasson, M.; Thévenon, E.; Bakó, L.; Benlloch, R.; Holmlund, M.; Parcy, F.; Nilsson, O.; et al. LEAFY activity is post-transcriptionally regulated by BLADE ON PETIOLE2 and CULLIN3 in Arabidopsis. New Phytol. 2018, 220, 579–592. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Guo, J.; Yang, Y.; Li, B.; Zhang, L. Nitric Oxide Functions as a Signal in Salt Resistance in the Calluses from Two Ecotypes of Reed. Plant Physiol. 2004, 134, 849–857. [Google Scholar] [CrossRef]
- Dietz, K.J.; Tavakoli, N.; Kluge, C.; Mimura, T.; Sharma, S.S.; Harris, G.C.; Chardonnens, A.N.; Golldack, D. Significance of the V-Type ATPase for the Adaptation to Stressful Growth Conditions and Its Regulation on the Molecular and Biochemical Level. J. Exp. Bot. 2001, 52, 1969–1980. [Google Scholar] [CrossRef]
- Tuteja, N.; Mahajan, S. Calcium Signaling Network in Plants: An Overview. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef]
- Nevill, P.G.; Cross, A.T.; Dixon, K.W. Ethical Seed Sourcing Is a Key Issue in Meeting Global Restoration Targets. Curr. Biol. 2018, 28, R1378–R1379. [Google Scholar] [CrossRef]
- Bethke, P.C.; Gubler, F.; Jacobsen, J.V.; Jones, R.L. Dormancy of Arabidopsis Seeds and Barley Grains Can Be Broken by Nitric Oxide. Planta 2004, 219, 847–855. [Google Scholar] [CrossRef]
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the Mark: Early Seed Germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef]
- Nonogaki, H. Seed Germination and Dormancy: The Classic Story, New Puzzles, and Evolution. J. Integr. Plant Biol. 2019, 61, 541–563. [Google Scholar] [CrossRef]
- Nonogaki, H. A Repressor Complex Silencing ABA Signaling in Seeds? J. Exp. Bot. 2020, 71, 2847–2853. [Google Scholar] [CrossRef]
- Sokolovski, S.; Hills, A.; Gay, R.; Garcia-Mata, C.; Lamattina, L.; Blatt, M.R. Protein Phosphorylation Is a Prerequisite for Intracellular Ca2+ Release and Ion Channel Control by Nitric Oxide and Abscisic Acid in Guard Cells: Nitric Oxide-Mediated Ca2+ Signalling and Protein Phosphorylation. Plant J. 2005, 43, 520–529. [Google Scholar] [CrossRef]
- Matilla, A.J.; Matilla-Vázquez, M.A. Involvement of Ethylene in Seed Physiology. Plant Sci. 2008, 175, 87–97. [Google Scholar] [CrossRef]
- Iglesias-Fernández, R.; Matilla, A. After-Ripening Alters the Gene Expression Pattern of Oxidases Involved in the Ethylene and Gibberellin Pathways during Early Imbibition of Sisymbrium officinale L. Seeds. J. Exp. Bot. 2009, 60, 1645–1661. [Google Scholar] [CrossRef]
- Linkies, A.; Leubner-Metzger, G. Beyond Gibberellins and Abscisic Acid: How Ethylene and Jasmonates Control Seed Germination. Plant Cell Rep. 2012, 31, 253–270. [Google Scholar] [CrossRef]
- Martínez, C.; Manzano, S.; Megías, Z.; Garrido, D.; Picó, B.; Jamilena, M. Involvement of Ethylene Biosynthesis and Signalling in Fruit Set and Early Fruit Development in Zucchini Squash (Cucurbita pepo L.). BMC Plant Biol. 2013, 13, 139. [Google Scholar] [CrossRef]
- Jamaux, I.; Steinmetz, A.; Belhassen, E. Looking for Molecular and Physiological Markers of Osmotic Adjustment in Sunflower. New Phytol. 1997, 137, 117–127. [Google Scholar] [CrossRef]
- Johansen, D. Plant Microtechnique, 1st ed.; McGraw-Hill: New York, NY, USA, 1940; Volume 1. [Google Scholar]
- O’Brien, T.P.; Feder, N.; McCully, M.E. Polychromatic Staining of Plant Cell Walls by Toluidine Blue-O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Segatto, F.B.; Bisognin, D.A.; Benedetti, M.; Costa, L.C.D.; Rampelotto, M.V.; Nicoloso, F.T. Técnica Para o Estudo da Anatomia da Epiderme Foliar de Batata. Cienc. Rural. 2004, 34, 1597–1601. [Google Scholar] [CrossRef]
- Wang, W.; Vignani, R.; Scali, M.; Cresti, M. A Universal and Rapid Protocol for Protein Extraction from Recalcitrant Plant Tissues for Proteomic Analysis. Electrophoresis 2006, 27, 2782–2786. [Google Scholar] [CrossRef]
- Herrera, H.; Valadares, R.; Oliveira, G.; Fuentes, A.; Almonacid, L.; Do Nascimento, S.V.; Bashan, Y.; Arriagada, C. Adaptation and Tolerance Mechanisms Developed by Mycorrhizal Bipinnula Fimbriata Plantlets (Orchidaceae) in a Heavy Metal-Polluted Ecosystem. Mycorrhiza 2018, 28, 651–663. [Google Scholar] [CrossRef]
- Meyer, D.; Zeileis, A.; Hornik, K. Vcd: Visualizing Categorical Data, R Package Version 1.4-11. 2022. Available online: https://cran.r-project.org/package=vcd (accessed on 13 November 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
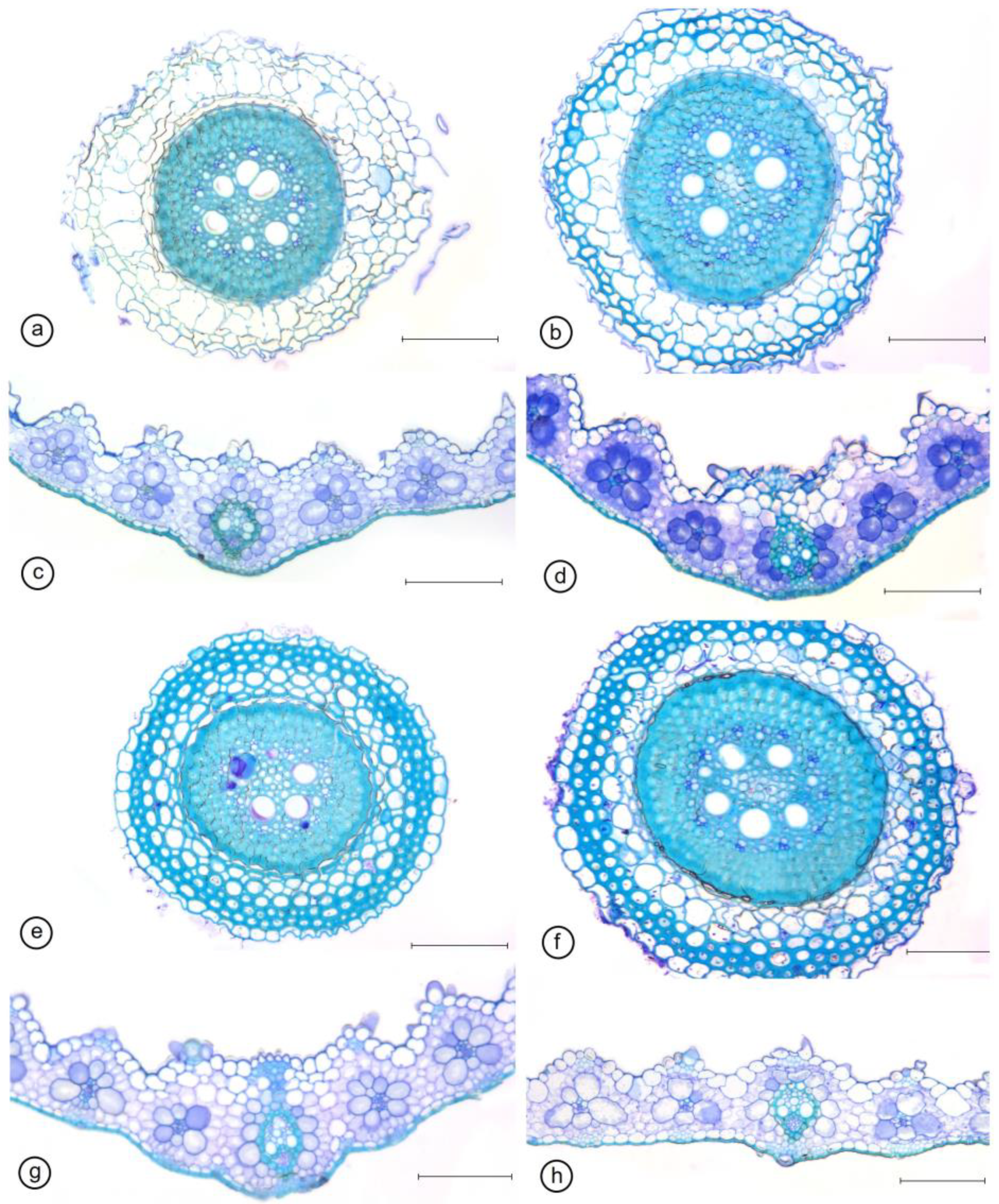

| Substrate | ETAd (µm) | ETAb (µm) | BCT (µm) | MT (µm) | BST (µm) | VBT (µm) | MD (µm) |
|---|---|---|---|---|---|---|---|
| WD_NO− | 11.47 ± 1.2 b | 5.38 ± 0.7 b | 21.22 ± 2.9 b | 126.09 ± 14.9 a | 81.34 ± 9.5 a | 45.63 ± 5.4 b | 13.18 ± 0.81 b |
| WD_NO+ | 11.33 ± 0.8 b | 5.7 ± 0.7 b | 19.45 ± 2.9 b | 110.74 ± 5.9 b | 84.79 ± 5.9 a | 44.32 ± 2.6 b | 13.98 ± 0.71 b |
| WW_NO− | 15.15 ± 0.9 a | 9.19 ± 1.2 a | 27.53 ± 1.6 a | 121.83 ±7.8 ab | 85.59 ± 6.6 a | 50.3 ±3.1 ab | 15.74 ± 1.71 a |
| WW_NO+ | 15.45 ± 0.9 a | 6.69 ± 0.7 b | 25.44 ± 1.4 a | 127 ± 13.5 a | 85.6 ± 6.0 a | 52.73 ± 4.3 a | 14.65 ± 1.14 a |
| Substrate | SD-Ad (Stomata per mm2) | PD-Ad (µm) | ED-Ad (µm) | SF-Ad | SD-Ab (Stomata per mm2) | PD-Ab (µm) | ED-Ab (µm) | SF-Ab |
|---|---|---|---|---|---|---|---|---|
| WD_NO− | 162.3 ± 11.9 b | 22.6 ± 1.0 b | 12.5 ± 1.3 a | 1.83 ± 0.2 b | 69 ± 9.6 b | 27.3 ± 1.4 b | 13.4 ± 1.1 c | 2.06 ± 0.2 b |
| WD_NO+ | 176 ± 12.5 a | 22.2 ± 1.4 b | 11.3 ± 1.0 a | 1.97 ± 0.2 a | 98 ± 11.8 a | 29.7 ± 2.1 a | 12.2 ± 0.7 d | 2.45 ± 0.2 a |
| WW_NO− | 139 ± 14.6 c | 24.5 ± 1.2 a | 13.9 ± 1.0 a | 1.76 ± 0.1 c | 61 ± 8.4 b | 29.4 ± 2.5 a | 16.1 ± 0.9 a | 1.84 ± 0.2 c |
| WW_NO+ | 131.3 ± 13.9 c | 24.7 ± 2.4 a | 13.5 ± 1.2 a | 1.84 ± 0.2 b | 73 ± 14.3 b | 25.9 ± 1.9 c | 14.5 ± 0.9 b | 1.80 ± 0.2 c |
| Substrate | RET (µm) | RDT (µm) | RCT (µm) | VCD (µm) | RMD (µm) |
|---|---|---|---|---|---|
| WD_NO− | 10.5 ± 0.65 c | 7.12 ± 0.95 b | 70.98 ± 6.96 a | 260.55 ± 30.13 a | 35.02 ± 2.83 a |
| WD_NO+ | 10.5 ± 1.42 c | 5.29 ± 1.82 c | 79.38 ± 12.13 a | 226.4 ± 14.98 b | 31.55 ± 5.24 a |
| WW_NO− | 21.37 ± 0.79 b | 9.52 ± 1 a | 79.27 ± 13.37 a | 225.01 ± 31.37 b | 31.67 ± 3.47 a |
| WW_NO+ | 17.15 ± 1.26 a | 7.9 ± 0.59 b | 75.72 ± 5.97 a | 248.08 ± 38.38 a | 30.23 ± 3.84 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boanares, D.; Da-Silva, C.J.; Costa, K.J.A.; Filgueira, J.P.P.S.; Salles, M.L.O.C.; Neto, L.P.; Gastauer, M.; Valadares, R.; Medeiros, P.S.; Ramos, S.J.; et al. Exogenous Nitric Oxide Alleviates Water Deficit and Increases the Seed Production of an Endemic Amazonian Canga Grass. Int. J. Mol. Sci. 2023, 24, 16676. https://doi.org/10.3390/ijms242316676
Boanares D, Da-Silva CJ, Costa KJA, Filgueira JPPS, Salles MLOC, Neto LP, Gastauer M, Valadares R, Medeiros PS, Ramos SJ, et al. Exogenous Nitric Oxide Alleviates Water Deficit and Increases the Seed Production of an Endemic Amazonian Canga Grass. International Journal of Molecular Sciences. 2023; 24(23):16676. https://doi.org/10.3390/ijms242316676
Chicago/Turabian StyleBoanares, Daniela, Cristiane J. Da-Silva, Keila Jamille Alves Costa, Joana Patrícia Pantoja Serrão Filgueira, Marina Ludmila Oliveira Conor Salles, Luiz Palhares Neto, Markus Gastauer, Rafael Valadares, Priscila Sanjuan Medeiros, Silvio Junio Ramos, and et al. 2023. "Exogenous Nitric Oxide Alleviates Water Deficit and Increases the Seed Production of an Endemic Amazonian Canga Grass" International Journal of Molecular Sciences 24, no. 23: 16676. https://doi.org/10.3390/ijms242316676






