Effect of Octacalcium Phosphate Crystals on the Osteogenic Differentiation of Tendon Stem/Progenitor Cells In Vitro
Abstract
:1. Introduction
2. Results
2.1. Identification of Stemness in TSPCs
2.2. Proliferation and Alkaline Phosphatase (ALP) Activities of TSPCs Treated with Calcium Phosphate Granules
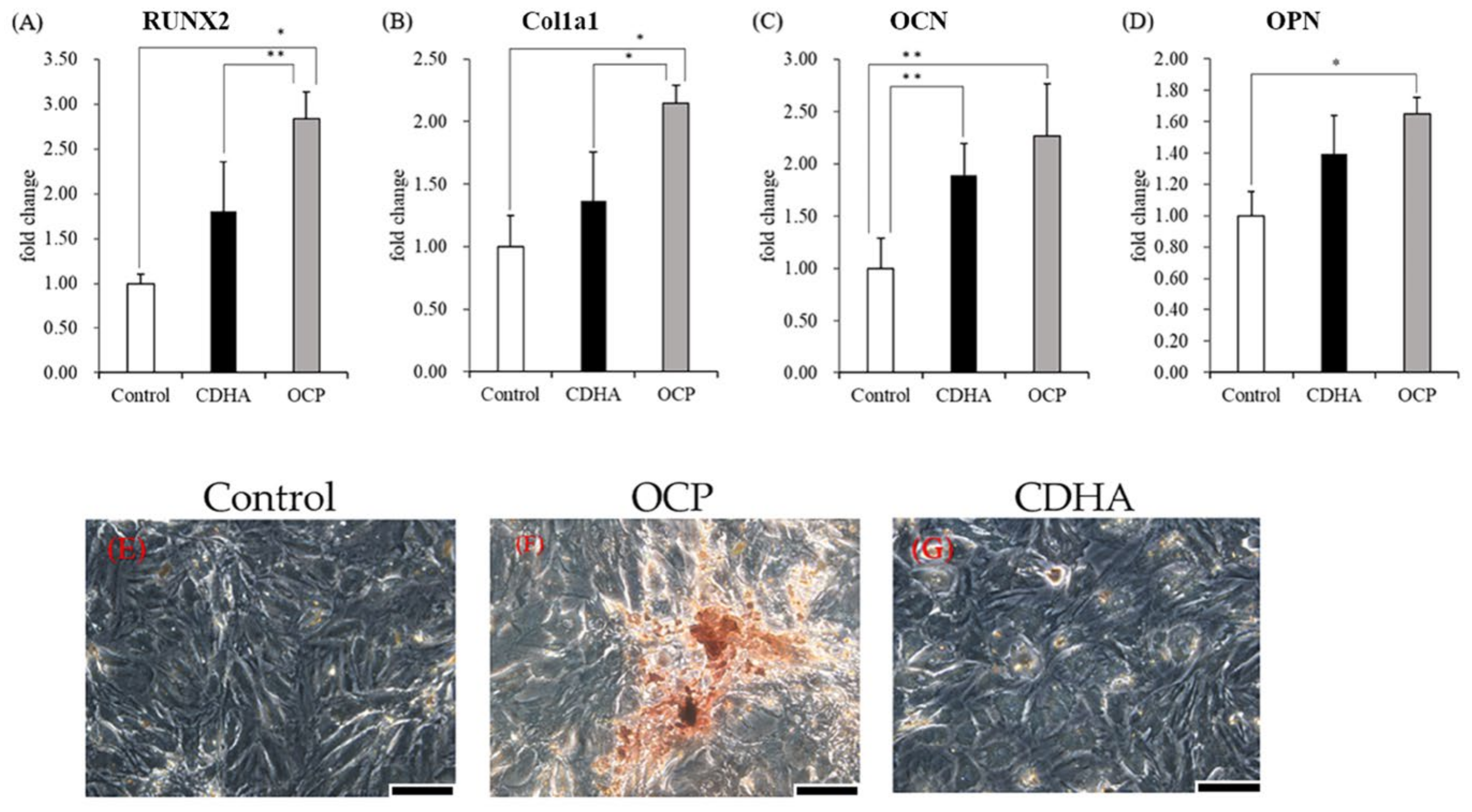
2.3. Osteogenic-Related Gene Expression and Calcified Nodules Formation of TSPCs Treated with Calcium Phosphate Granules
2.4. Proliferation and ALP Activity of TSPCs Treated with Calcium Phosphate–Conditioned Media
2.5. Changes in Ion Compositions and the Degree of Supersaturation (DS) with Respect to the Calcium Phosphates in the Culture Media
2.6. Characterization of the Calcium Phosphate Granules Incubated with the TSPCs
3. Discussion
4. Materials and Methods
4.1. Synthesis of Calcium Phosphate Granules
4.2. TSPCs Isolation, Identification, and Culture
4.3. Analysis of the Proliferation and Osteoblastic Differentiation of TSPCs Cultured with Calcium Phosphate Granules
4.4. Analysis of the Osteoblastic Differentiation of TSPCs Treated with Conditioned Media
4.5. DNA Concentration Measurement and ALP Activity Assay
4.6. Real-Time qPCR
4.7. Alizarin Red Staining
4.8. FTIR Analysis of the Calcium Phosphate Granules Incubated with TSPCs
4.9. Measurement of Ion Composition and the Calculation of the DS with Respect to Calcium Phosphates in the Cell Culture Environment
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, W.E.; Smith, J.P.; Lehr, J.R.; Frazier, A.W. Octacalcium phosphate and hydroxyapatite: Crystallographic and chemical relations between octacalcium phosphate and hydroxyapatite. Nature 1962, 196, 1050–1055. [Google Scholar] [CrossRef]
- Meyer, J.L.; Eanes, E.D. A thermodynamic analysis of the amorphous to crystalline calcium phosphate transformation. Calcif. Tissue Res. 1978, 25, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.E. Crystal growth of bone mineral. Clin. Orthop. Relat. Res. 1966, 44, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.E.; Eidelman, N.; Tomazic, B. Octacalcium phosphate as a precursor in biomineral formation. Adv. Dent. Res. 1987, 1, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Eidelman, N.; Chow, L.C.; Brown, W.E. Calcium phosphate phase transformations in serum. Calcif. Tissue Int. 1987, 41, 18–26. [Google Scholar] [CrossRef]
- Suzuki, O.; Nakamura, M.; Miyasaka, Y.; Kagayama, M.; Sakurai, M. Bone formation on synthetic precursors of hydroxyapatite. Tohoku J. Exp. Med. 1991, 164, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, O.; Shiwaku, Y.; Hamai, R. Octacalcium phosphate bone substitute materials: Comparison between properties of biomaterials and other calcium phosphate materials. Dent. Mater. J. 2020, 39, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, O.; Kamakura, S.; Katagiri, T.; Nakamura, M.; Zhao, B.; Honda, Y.; Kamijo, R. Bone formation enhanced by implanted octacalcium phosphate involving conversion into Ca-deficient hydroxyapatite. Biomaterials 2006, 27, 2671–2681. [Google Scholar] [CrossRef]
- Murakami, Y.; Honda, Y.; Anada, T.; Shimauchi, H.; Suzuki, O. Comparative study on bone regeneration by synthetic octacalcium phosphate with various granule sizes. Acta Biomater. 2010, 6, 1542–1548. [Google Scholar] [CrossRef]
- Honda, Y.; Anada, T.; Kamakura, S.; Morimoto, S.; Kuriyagawa, T.; Suzuki, O. The effect of microstructure of octacalcium phosphate on the bone regenerative property. Tissue Eng. Part A 2009, 15, 1965–1973. [Google Scholar] [CrossRef]
- Suzuki, O.; Kamakura, S.; Katagiri, T. Surface chemistry and biological responses to synthetic octacalcium phosphate. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77, 201–212. [Google Scholar] [CrossRef]
- Shelton, R.M.; Liu, Y.; Cooper, P.R.; Gbureck, U.; German, M.J.; Barralet, J.E. Bone marrow cell gene expression and tissue construct assembly using octacalcium phosphate microscaffolds. Biomaterials 2006, 27, 2874–2881. [Google Scholar] [CrossRef]
- Liu, Y.; Cooper, P.R.; Barralet, J.E.; Shelton, R.M. Influence of calcium phosphate crystal assemblies on the proliferation and osteogenic gene expression of rat bone marrow stromal cells. Biomaterials 2007, 28, 1393–1403. [Google Scholar] [CrossRef]
- Anada, T.; Kumagai, T.; Honda, Y.; Masuda, T.; Kamijo, R.; Kamakura, S.; Yoshihara, N.; Kuriyagawa, T.; Shimauchi, H.; Suzuki, O. Dose-dependent osteogenic effect of octacalcium phosphate on mouse bone marrow stromal cells. Tissue Eng. Part A 2008, 14, 965–978. [Google Scholar] [CrossRef]
- Kawai, T.; Anada, T.; Honda, Y.; Kamakura, S.; Matsui, K.; Matsui, A.; Sasaki, K.; Morimoto, S.; Echigo, S.; Suzuki, O. Synthetic octacalcium phosphate augments bone regeneration correlated with its content in collagen scaffold. Tissue Eng. Part A 2009, 15, 23–32. [Google Scholar] [CrossRef]
- Sato, T.; Anada, T.; Hamai, R.; Shiwaku, Y.; Tsuchiya, K.; Sakai, S.; Baba, K.; Sasaki, K.; Suzuki, O. Culture of hybrid spheroids composed of calcium phosphate materials and mesenchymal stem cells on an oxygen-permeable culture device to predict in vivo bone forming capability. Acta Biomater. 2019, 88, 477–490. [Google Scholar] [CrossRef]
- Sai, Y.; Shiwaku, Y.; Anada, T.; Tsuchiya, K.; Takahashi, T.; Suzuki, O. Capacity of octacalcium phosphate to promote osteoblastic differentiation toward osteocytes in vitro. Acta Biomater. 2018, 69, 362–371. [Google Scholar] [CrossRef]
- Hirayama, B.; Anada, T.; Shiwaku, Y.; Miyatake, N.; Tsuchiya, K.; Nakamura, M.; Takahashi, T.; Suzuki, O. Immune cell response and subsequent bone formation induced by implantation of octacalcium phosphate in a rat tibia defect. RSC Adv. 2016, 6, 57475–57484. [Google Scholar] [CrossRef]
- Takami, M.; Mochizuki, A.; Yamada, A.; Tachi, K.; Zhao, B.; Miyamoto, Y.; Anada, T.; Honda, Y.; Inoue, T.; Nakamura, M.; et al. Osteoclast differentiation induced by synthetic octacalcium phosphate through receptor activator of NF-kappaB ligand expression in osteoblasts. Tissue Eng. Part A 2009, 15, 3991–4000. [Google Scholar] [CrossRef]
- Kurobane, T.; Shiwaku, Y.; Anada, T.; Hamai, R.; Tsuchiya, K.; Baba, K.; Iikubo, M.; Takahashi, T.; Suzuki, O. Angiogenesis involvement by octacalcium phosphate-gelatin composite-driven bone regeneration in rat calvaria critical-sized defect. Acta Biomater. 2019, 88, 514–526. [Google Scholar] [CrossRef]
- Itoigawa, Y.; Suzuki, O.; Sano, H.; Anada, T.; Handa, T.; Hatta, T.; Kuwahara, Y.; Takahashi, A.; Ezoe, Y.; Kaneko, K.; et al. The role of an octacalcium phosphate in the re-formation of infraspinatus tendon insertion. J. Shoulder Elbow Surg. 2015, 24, e175–e184. [Google Scholar] [CrossRef] [PubMed]
- Holladay, C.; Abbah, S.A.; O’Dowd, C.; Pandit, A.; Zeugolis, D.I. Preferential tendon stem cell response to growth factor supplementation. J. Tissue Eng. Regen. Med. 2016, 10, 783–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, D.L.; Juncosa, N.; Dressler, M.R. Functional efficacy of tendon repair processes. Annu. Rev. Biomed. Eng. 2004, 6, 303–329. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yin, H.; Brochhausen, C.; Pfeifer, C.G.; Alt, V.; Docheva, D. Aged tendon stem/progenitor cells are less competent to form 3D tendon organoids due to cell autonomous and matrix production deficits. Front. Bioeng. Biotechnol. 2020, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, L.; Xu, J.; Yang, Z.; Wu, T.; Zhang, J.; Shi, L.; Zhu, D.; Zhang, J.; Li, G. MiR-378a suppresses tenogenic differentiation and tendon repair by targeting at TGF-β2. Stem Cell Res. Ther. 2019, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Genin, G.M.; Thomopoulos, S. The tendon-to-bone attachment: Unification through disarray. Nat. Mater. 2017, 16, 607–608. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhang, W.X.; Wang, L.N.; Ming, Y.Q.; Li, Y.L.; Ni, G.X. Stem cell therapies in tendon-bone healing. World J. Stem Cells 2021, 13, 753–775. [Google Scholar] [CrossRef]
- Lui, P.; Zhang, P.; Chan, K.; Qin, L. Biology and augmentation of tendon-bone insertion repair. J. Orthop. Surg. Res. 2010, 5, 59. [Google Scholar] [CrossRef] [Green Version]
- Thomopoulos, S.; Genin, G.M.; Galatz, L.M. The development and morphogenesis of the tendon-to-bone insertion what development can teach us about healing. J. Musculoskelet. Neuronal Interact. 2010, 10, 35–45. [Google Scholar]
- Mutsuzaki, H.; Kinugasa, T.; Sakane, M. Safety and feasibility of using calcium phosphate hybridization method for quadriceps tendon-bone graft in anterior cruciate ligament reconstruction. J. Orthop. 2019, 16, 422–425. [Google Scholar] [CrossRef]
- Mutsuzaki, H.; Sakane, M.; Ito, A.; Nakajima, H.; Hattori, S.; Miyanaga, Y.; Tanaka, J.; Ochiai, N. The interaction between osteoclast-like cells and osteoblasts mediated by nanophase calcium phosphate-hybridized tendons. Biomaterials 2005, 26, 1027–1034. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Kogure, T.; Sakane, M.; Tanaka, S.; Osaka, A.; Tanaka, J. Microstructure analysis of calcium phosphate formed in tendon. J. Mater. Sci. Mater. Med. 2003, 14, 883–889. [Google Scholar] [CrossRef]
- Chen, C.H.; Liu, H.W.; Tsai, C.L.; Yu, C.M.; Lin, I.H.; Hsiue, G.H. Photoencapsulation of bone morphogenetic protein-2 and periosteal progenitor cells improve tendon graft healing in a bone tunnel. Am. J. Sports Med. 2008, 36, 461–473. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, W.J.; Shih, C.H.; Yang, C.Y.; Liu, S.J.; Lin, P.Y. Enveloping the tendon graft with periosteum to enhance tendon-bone healing in a bone tunnel: A biomechanical and histologic study in rabbits. Arthroscopy 2003, 19, 290–296. [Google Scholar] [CrossRef]
- Stähelin, A.C.; Weiler, A. All-inside anterior cruciate ligament reconstruction using semitendinosus tendon and soft threaded biodegradable interference screw fixation. Arthroscopy 1997, 13, 773–779. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.H. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet. Disord. 2010, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Rui, Y.F.; Lui, P.P.; Li, G.; Fu, S.C.; Lee, Y.W.; Chan, K.M. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng. Part A 2010, 16, 1549–1558. [Google Scholar] [CrossRef] [Green Version]
- Adams, S.B.; Thorpe, M.A.; Parks, B.G.; Aghazarian, G.; Allen, E.; Schon, L.C. Stem cell-bearing suture improves Achilles tendon healing in a rat model. Foot Ankle Int. 2014, 35, 293–299. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, H.; Gao, S.; Yang, M.; Lyu, J.; Tang, K. Effect of tendon stem cells in chitosan/β-glycerophosphate/collagen hydrogel on Achilles tendon healing in a rat model. Med. Sci. Monit. 2017, 23, 4633–4643. [Google Scholar] [CrossRef]
- Lui, P.P.; Chan, K.M. Tendon-derived stem cells (TDSCs): From basic science to potential roles in tendon pathology and tissue engineering applications. Stem Cell Rev. Rep. 2011, 7, 883–897. [Google Scholar] [CrossRef]
- Ahmad, Z.; Wardale, J.; Brooks, R.; Henson, F.; Noorani, A.; Rushton, N. Exploring the application of stem cells in tendon repair and regeneration. Arthroscopy 2012, 28, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.; Wong, O.T.; Lee, Y.W. Application of tendon-derived stem cell sheet for the promotion of graft healing in anterior cruciate ligament reconstruction. Am. J. Sports Med. 2014, 42, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Thomopoulos, S.; Williams, G.R.; Soslowsky, L.J. Tendon to bone healing: Differences in biomechanical, structural, and compositional properties due to a range of activity levels. J. Biomech. Eng. 2003, 125, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.W.; Qin, L.; Tai, J.K.; Lee, S.K.; Leung, K.S.; Chan, K.M. Engineered allogeneic chondrocyte pellet for reconstruction of fibrocartilage zone at bone-tendon junction--a preliminary histological observation. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 70, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Harryman, D.T., 2nd; Mack, L.A.; Wang, K.Y.; Jackins, S.E.; Richardson, M.L.; Matsen, F.A. 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J. Bone Joint Surg. Am. 1991, 73, 982–989. [Google Scholar] [CrossRef]
- Galatz, L.M.; Ball, C.M.; Teefey, S.A.; Middleton, W.D.; Yamaguchi, K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J. Bone Joint Surg. Am. 2004, 86, 219–224. [Google Scholar] [CrossRef]
- Wu, Y.F.; Chen, C.; Tang, J.B.; Mao, W.F. Growth and stem cell characteristics of tendon-derived cells with different initial seeding densities: An in vitro study in mouse flexor tendon cells. Stem Cells Dev. 2020, 29, 1016–1025. [Google Scholar] [CrossRef]
- Tan, Q.; Lui, P.P.; Rui, Y.F. Effect of in vitro passaging on the stem cell-related properties of tendon-derived stem cells-implications in tissue engineering. Stem Cells Dev. 2012, 21, 790–800. [Google Scholar] [CrossRef] [Green Version]
- Colter, D.C.; Class, R.; DiGirolamo, C.M.; Prockop, D.J. Rapid expansion of recycling stem cells in cultures of plasticadherent cells from human bone marrow. Proc. Natl. Acad. Sci. USA 2000, 97, 3213. [Google Scholar] [CrossRef]
- Dezawa, M.; Ishikawa, H.; Itokazu, Y.; Yoshihara, T.; Hoshino, M.; Takeda, S.; Ide, C.; Nabeshima, Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 2005, 309, 314. [Google Scholar] [CrossRef]
- Kawabata, M.; Imamura, T.; Miyazono, K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998, 9, 49–61. [Google Scholar] [CrossRef]
- Anada, T.; Araseki, A.; Matsukawa, S.; Yamasaki, T.; Kamakura, S.; Suzuki, O. Effect of octacalcium phosphate ionic dissolution products on osteoblastic cell differentiation. Key. Eng. Mater. 2008, 361–363, 31–34. [Google Scholar]
- Walia, B.; Huang, A.H. Tendon stem progenitor cells: Understanding the biology to inform therapeutic strategies for tendon repair. J. Orthop. Res. 2019, 37, 1270–1280. [Google Scholar] [CrossRef]
- Qin, S.; Wang, W.; Liu, Z.; Hua, X.; Fu, S.; Dong, F.; Li, A.; Liu, Z.; Wang, P.; Dai, L.; et al. Fibrochondrogenic differentiation potential of tendon-derived stem/progenitor cells from human patellar tendon. J. Orthop. Translat. 2019, 22, 101–108. [Google Scholar] [CrossRef]
- Chen, S.; Feng, J.; Bao, Q.; Li, A.; Zhang, B.; Shen, Y.; Zhao, Y.; Guo, Q.; Jing, J.; Lin, S.; et al. Adverse effects of osteocytic constitutive activation of ß-catenin on bone strength and bone growth. J. Bone Miner. Res. 2015, 30, 1184–1194. [Google Scholar] [CrossRef]
- Day, T.F.; Guo, X.; Garrett-Beal, L.; Yang, Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 2005, 8, 739–750. [Google Scholar] [CrossRef] [Green Version]
- Kolpakova, E.; Olsen, B.R. Wnt/beta-catenin−A canonical tale of cell-fate choice in the vertebrate skeleton. Dev. Cell 2005, 8, 626–627. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, H.; Chen, Y.; Ao, X.; Chen, C.; Zhang, W.; He, F.; Liao, X.; Jiang, X.; Li, T.; et al. Baicalein accelerates tendon-bone healing via activation of Wnt/β-catenin signaling pathway in rats. Biomed. Res. Int. 2018, 2018, 3849760. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, O.; Yagishita, H.; Yamazaki, M.; Amano, T.; Aoba, T. Adsorption of bovine serum albumin onto octacalcium phosphate and its hydrolyzates. Cells Mater. 1995, 5, 45–54. [Google Scholar]
- Aoba, T.; Fukae, M.; Tanabe, T.; Shimizu, M.; Moreno, E.C. Selective adsorption of porcine-amelogenins onto hydroxyapatite and their inhibitory activity on hydroxyapatite growth in supersaturated solutions. Calcif. Tissue Int. 1987, 41, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.C.; Aoba, T. Comparative solubility study of human dental enamel, dentin, and hydroxyapatite. Calcif. Tissue Int. 1991, 49, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.C.; Aoba, T. Calcium bonding in enamel fluid and driving force for enamel mineralization in the secretory stage of amelogenesis. Adv. Dent. Res. 1987, 1, 245–251. [Google Scholar] [CrossRef]
- Moreno, E.C.; Kresak, M.; Zahradnik, R.T. Fluoridated hydroxyapatite solubility and caries formation. Nature 1974, 247, 64–65. [Google Scholar] [CrossRef]
- Tung, M.S.; Eidelman, N.; Sieck, B.; Brown, W.E. Octacalcium phosphate solubility product from 4 to 37 °C. J. Res. Natl. Bur. Stand. 1988, 93, 613–624. [Google Scholar] [CrossRef]
- Moreno, E.C.; Brown, W.E.; Osborn, G. Solubility of dicalcium phosphate dihydrate in aqueous systems. Soil. Sci. Soc. Am. J. 1960, 24, 94–98. [Google Scholar] [CrossRef]





| Period (Days) | Ca2+ (mM) | Pi (mM) | pH | DS at pH (Each) and 37 °C | |||
|---|---|---|---|---|---|---|---|
| HA | OCP | DCPD | |||||
| DMEM | 0 | 1.85 | 1.12 | 7.59 | 4.16 × 1012 | 5.24 × 103 | 6.83 × 10−1 |
| Control | 3 | 1.93 | 1.88 | 7.65 | 3.87 × 1013 | 3.64 × 103 | 1.18 × 100 |
| OCP | 3 | 1.20 | 1.65 | 7.66 | 3.03 × 1012 | 4.34 × 103 | 6.69 × 10−1 |
| CDHA | 3 | 1.50 | 1.47 | 7.68 | 7.94 × 1012 | 8.24 × 103 | 7.44 × 10−1 |
| Control | 9 | 2.10 | 1.92 | 7.62 | 4.55 × 1013 | 4.52 × 104 | 1.29 × 100 |
| OCP | 9 | 1.43 | 1.59 | 7.63 | 4.76 × 1012 | 6.55 × 103 | 7.57 × 10−1 |
| CDHA | 9 | 1.61 | 1.44 | 7.65 | 7.84 × 1012 | 8.69 × 103 | 7.75 × 10−1 |
| Control | 15 | 2.12 | 1.90 | 7.68 | 8.39 × 1013 | 6.25 × 104 | 1.31 × 100 |
| OCP | 15 | 1.52 | 1.54 | 7.69 | 1.06 × 1013 | 1.04 × 104 | 7.90 × 10−1 |
| CDHA | 15 | 1.63 | 1.40 | 7.70 | 1.26 × 1013 | 1.10 × 104 | 7.72 × 10−1 |
| Gene | Primer Sequences | Universal Probe |
|---|---|---|
| CD44 | F 5′-GGATGACGCCTTCTTTATTGG-3′ R 5′-TGTTGCATGGCTTTTTGAGT-3′ | #49 |
| CD90 | F 5′-CCACAAGCTCCAATAAAACTATCAA-3′ R 5′-AGCAGCCAGGAAGTGTTTTG-3′ | #40 |
| Nanog | F 5′-TGCACTCAAGGATAGGTTTCAG-3′ R 5′-TTTGGAACCAGGTCTTCACC-3′ | #67 |
| Col1a1 | F 5′-TCCTGGCAAGAACGGAGAT-3′ R 5′-CAGGAGGTCCACGCTCAC-3′ | #60 |
| OCN | F 5′-ATAGACTCCGGCGCTACCTC-3′ R 5′-CCAGGGGATCTGGGTAGG-3′ | #125 |
| OPN | F 5′-GAGTTTGGCAGCTCAGAGGA-3′ R 5′-TCTGCTTCTGAGATGGGTCA-3′ | #41 |
| RUNX2 | F 5′-CCACAGAGCTATTAAAGTGACAGTG-3′ R 5′-AACAAACTAGGTTTAGAGTCATCAAGC-3′ | #98 |
| GAPDH | F 5′-TGGGAAGCTGGTCATCAAC-3′ R 5′-GCATCACCCCATTTGATGTT-3′ | #9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Shiwaku, Y.; Hamai, R.; Tsuchiya, K.; Takahashi, T.; Suzuki, O. Effect of Octacalcium Phosphate Crystals on the Osteogenic Differentiation of Tendon Stem/Progenitor Cells In Vitro. Int. J. Mol. Sci. 2023, 24, 1235. https://doi.org/10.3390/ijms24021235
Liu X, Shiwaku Y, Hamai R, Tsuchiya K, Takahashi T, Suzuki O. Effect of Octacalcium Phosphate Crystals on the Osteogenic Differentiation of Tendon Stem/Progenitor Cells In Vitro. International Journal of Molecular Sciences. 2023; 24(2):1235. https://doi.org/10.3390/ijms24021235
Chicago/Turabian StyleLiu, Xianchen, Yukari Shiwaku, Ryo Hamai, Kaori Tsuchiya, Tetsu Takahashi, and Osamu Suzuki. 2023. "Effect of Octacalcium Phosphate Crystals on the Osteogenic Differentiation of Tendon Stem/Progenitor Cells In Vitro" International Journal of Molecular Sciences 24, no. 2: 1235. https://doi.org/10.3390/ijms24021235





