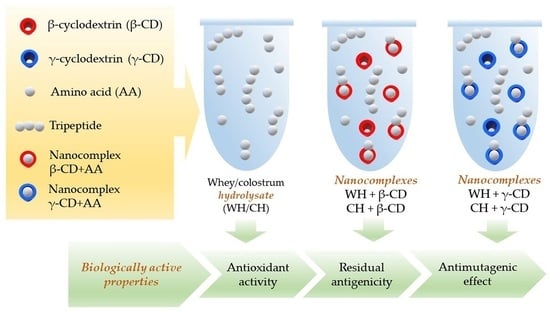
Influence of Complexation with β- and γ-Cyclodextrin on Bioactivity of Whey and Colostrum Peptides
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Protein and Peptide Composition of Whey and Colostrum Hydrolysates
2.1.1. High-Performance Liquid Chromatography (HPLC) Analysis of Hydrolysates and Their Ultrafiltrates
2.1.2. Mass Spectrometric (MS) Study of Peptide Fractions
2.1.3. Determination of Molecular Weight Distribution of Hydrolysates by Dynamic Light Scattering (DLS)
2.2. Characterization of Cyclodextrins Complexes with Dairy Peptides
2.2.1. Thermal Degradation Parameters of Hydrolysates and Their Inclusion Complexes
2.2.2. Fluorescence Emission Spectrometry of β/γ-CD Complexes
2.2.3. Determination of Molecular Weight Distribution of Cyclodextrin Complexes with Whey Peptides According to DLS
2.3. Effect of Complex Formation on Antioxidant Properties of Cleaved Dairy Proteins
2.4. Antigenic Properties of Peptide Fractions and Their Complexes with Cyclodextrins
2.5. Complexation Effect on Antimutagenic Action of Dairy Peptides
3. Discussion
4. Materials and Methods
4.1. Preparation of Whey and Colostrum Protein Hydrolysates and Determination of Their Protein and Peptide Composition
4.1.1. Enzymatic Hydrolysis and Fractionation of Proteolysis Products
4.1.2. High-Performance Liquid Chromatography of Hydrolysates
4.1.3. Mass Spectrometric Analysis of Hydrolysates
4.2. Preparation of β- and γ-Cyclodextrin Complexes with Whey and Colostrum Protein Hydrolysates and Their Characteristics
4.2.1. Incubation of Cyclodextrins with Whey and Colostrum Peptides
4.2.2. Fluorescence Emission Spectrometry (Fluorescence Emission Spectra of Tryptophan Residues)
4.2.3. Analysis of Hydrolysates/Complexes by Dynamic Light Scattering
- (1)
- WH–5 kDa (1.0 mg/mL);
- (2)
- CH–5 kDa (1.0 mg/mL);
- (3)
- β-CD (0.5/1.0/2.0 mg/mL);
- (4)
- γ-CD (0.5/1.0/2.0 mg/mL);
- (5)
- β-CD:WH–5 kDa = 0.5:1.0 (0.5 mg/mL of β-CD and 1.0 mg/mL WH–5 kDa);
- (6)
- β-CD:WH–5 kDa = 1.0:1.0 (1.0 mg/mL of β-CD and 1.0 mg/mL WH–5 kDa);
- (7)
- β-CD:WH–5 kDa = 2.0:1.0 (2.0 mg/mL of β-CD and 1.0 mg/mL WH–5 kDa);
- (8)
- γ-CD:WH–5 kDa = 0.5:1.0 (0.5 mg/mL of γ-CD and 1.0 mg/mL WH–5 kDa);
- (9)
- γ-CD:WH–5 kDa = 1.0:1.0 (1.0 mg/mL of γ-CD and 1.0 mg/mL WH–5 kDa);
- (10)
- γ-CD:WH–5 kDa = 2.0:1.0 (2.0 mg/mL of γ-CD and 1.0 mg/mL WH–5 kDa).
4.2.4. Thermogravimetric Analysis and Differential Scanning Calorimetry of Inclusion Complexes
4.3. Evaluation of Antioxidant Activity of Enzymatic Hydrolysates and Cyclodextrin Complexes with Peptides
4.4. Antigenicity Determination of Dairy Peptides and their Complexes with Cyclodextrins
4.5. Evaluation of Antimutagenic Effect of Native Hydrolysates and in Complexes with Cyclodextrins
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, A.G.; Carpena, M.; García Oliveira, P.; Mejuto, J.C.; Prieto, M.A.; Simal Gandara, J. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host–Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhang, Y.; Niu, Y.; Ke, Q.; Kou, X. Cyclodextrins as Carriers for Volatile Aroma Compounds: A Review. Carbohydr. Polym. 2021, 269, 118292. [Google Scholar] [CrossRef] [PubMed]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins Inclusion Complex: Preparation Methods, Analytical Techniques and Food Industry Applications. Food Chem. 2022, 84, 132467. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Shen, J.; Matai, I.; Sachdev, A.; Boy, R.; Tonelli, A.E. Cyclodextrin-Based Nanostructures. Prog. Mater. Sci. 2022, 124, 100869. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, Physicochemical Properties and Pharmaceutical Applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Loftsson, T. γ-Cyclodextrin. Int. J. Pharm. 2017, 516, 278–292. [Google Scholar] [CrossRef]
- Astray, G.; Mejuto, J.C.; Simal-Gandara, J. Latest Developments in the Application of Cyclodextrin Host-Guest Complexes in Beverage Technology Processes. Food Hydrocoll. 2020, 106, 105882. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of Cyclodextrins in Food Science. A Review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Liu, J.; Ding, X.; Fu, Y.; Xiang, C.; Yuan, Y.; Zhang, Y.; Yu, P. Cyclodextrins Based Delivery Systems for Macro Biomolecules. Eur. J. Med. Chem. 2021, 212, 113105. [Google Scholar] [CrossRef]
- Fu, Y.; Amin, M.S.; Li, Q.; Bak, K.H.; Lametsch, R. Applications in nutrition: Peptides as taste enhancers. In Biologically Active Peptides; Toldrá, F., Wu, J., Eds.; Academic Press: New York, NY, USA, 2021; Volume 23, pp. 569–580. [Google Scholar] [CrossRef]
- Sun, X.; Okagu, O.D.; Udenigwe, C.C. Encapsulation Technology for Protection and Delivery of Bioactive Peptides. In Biologically Active Peptides; Toldrá, F., Wu, J., Eds.; Academic Press: New York, NY, USA, 2021; Volume 15, pp. 331–356. [Google Scholar] [CrossRef]
- Höhme, L.; Fischer, C.; Kleinschmidt, T. Characterization of Bitter Peptides in Casein Hydrolysates Using Comprehensive Two-Dimensional Liquid Chromatography. Food Chem. 2023, 404, 134527. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Geng, S.; Liu, B.; Wang, H.; Liang, G. Self-Assembled Mechanism of Hydrophobic Amino Acids and β-Cyclodextrin Based on Experimental and Computational Methods. Food Res. Int. 2018, 112, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Linde, G.A.; Junior, A.L.; de Faria, E.V.; Colauto, N.B.; de Moraes, F.F.; Zanin, G.M. The Use of 2D NMR to study β-Cyclodextrin Complexation and Debittering of Amino Acids and Peptides. Food Res. Int. 2010, 43, 187–192. [Google Scholar] [CrossRef]
- Tamura, M.; Mori, N.; Miyoshi, T.; Koyama, S.; Kohri, H.; Okai, H. Practical Debittering Using Model Peptides and Related Compounds. Agric. Biol. Chem. 1990, 54, 41–51. [Google Scholar] [CrossRef]
- Linde, G.A.; Junior, A.L.; de Faria, E.V.; Colauto, N.B.; de Moraes, F.F.; Zanin, G.M. Taste Modification of Amino Acids and Protein Hydrolysate by α-Cyclodextrin. Food Res. Int. 2009, 42, 814–818. [Google Scholar] [CrossRef]
- Monge Neto, A.Á.; Ströher, R.; Assenha, H.B.R.; Scagion, V.P.; Correa, D.S.; Zanin, G.M. Interaction of Peptides Obtained from the Enzymatic Hydrolysis of Soybean Meal with Cyclodextrins: An Evaluation of Bitterness Reduction. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 59–69. [Google Scholar] [CrossRef]
- Rudolph, S.; Riedel, E.; Henle, T. Studies on the Interaction of the Aromatic Amino Acids Tryptophan, Tyrosine and Phenylalanine as Well as Tryptophan-Containing Dipeptides with Cyclodextrins. Eur. Food Res. Technol. 2018, 244, 1511–1519. [Google Scholar] [CrossRef]
- Singh, A.; Temitope Idowu, A.; Benjakul, S.; Kishimura, H.; Aluko, R.E.; Kumagai, Y. Debittering Salmon (Salmo salar) Frame Protein Hydrolysate Using 2-Butanol in Combination with β-Cyclodextrin: Impact on Some Physicochemical Characteristics and Antioxidant Activities. Food Chem. 2020, 321, 126686. [Google Scholar] [CrossRef]
- Xia, Y.; Zhu, L.; Wu, G.; Liu, T.; Li, X.; Wang, X.; Zhang, H. Comparative Study of Various Methods Used for Bitterness Reduction from Pea (Pisum sativum L.) Protein Hydrolysates. LWT 2022, 159, 113228. [Google Scholar] [CrossRef]
- Rukluarh, S.; Kanjanapongkul, K.; Panchan, N.; Niumnuy, C. Effect of Inclusion Conditions on Characteristics of Spray Dried Whey Protein Hydrolysate/γ-Cyclodextrin Complexes. J. Food Sci. Agric. Technol. 2019, 5, 5–12. [Google Scholar]
- Halavach, T.N.; Kurchenko, V.P.; Zhygankov, V.G.; Evdokimov, I.A. Determination of Physicochemical, Immunochemical and Antioxidant Properties, Toxicological and Hygienic Assessment of Whey Protein Concentrate and Its Hydrolysate. Foods Raw Mater. 2015, 3, 105–114. [Google Scholar] [CrossRef]
- Golovach, T.N.; Dudchik, N.V.; Veremeenko, E.G.; Tsygankou, V.G.; Bondaruk, A.M.; Filonyuk, V.A.; Shevlyakov, V.V.; Ushkov, A.A.; Sobol, Y.A.; Erm, G.I.; et al. Evaluation of Antimutagenic and Antifungal Properties, Parameters of Acute Toxicity and Sensitizing Activity of Enzymatic Whey Protein Hydrolysate. Foods Raw Mater. 2016, 4, 38–47. [Google Scholar] [CrossRef]
- Halavach, T.M.; Dudchik, N.V.; Tarun, E.I.; Zhygankov, V.G.; Kurchenko, V.P.; Romanovich, R.V.; Khartitonov, V.D.; Asafov, V.A. Biologically Active Properties of Hydrolysed and Fermented Milk Proteins. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 714–720. [Google Scholar] [CrossRef]
- Halavach, T.M.; Savchuk, E.S.; Bobovich, A.S.; Dudchik, N.V.; Tsygankow, V.G.; Tarun, E.I.; Kharitonov, V.D.; Asafov, V.A. Antimutagenic and Antibacterial Activity of β-Cyclodextrin Clathrates with Extensive Hydrolysates of colostrum and Whey. Biointerface Res. Appl. Chem. 2021, 11, 8626–8638. [Google Scholar] [CrossRef]
- Halavach, T.M.; Kurchenko, V.P.; Tsygankow, V.G.; Bondaruk, A.M.; Tarun, E.I.; Asafov, V.A. β-Cyclodextrin Nanocomplexes with Biologically Active Peptides from Hydrolysed Bovine Whey and Colostrum. Biointerface Res. Appl. Chem. 2022, 12, 8502–8514. [Google Scholar] [CrossRef]
- Kurchenko, V.P.; Halavach, T.M.; Sushynskaya, N.V.; Tarun, E.I.; Dudchik, N.V.; Tsygankow, V.G.; Lodygin, A.D.; Evdokimov, I.A. Multicomponent Composites of Cyclodextrin Nanocomplexes with Biologically Active Substances for Functional Foods. Food Process. Tech. Technol. 2022, 52, 375–389. (In Russian) [Google Scholar] [CrossRef]
- De Castro, R.J.S.; Bagagli, M.P.; Sato, H.H. Improving the Functional Properties of Milk Proteins: Focus on the Specificities of Proteolytic Enzymes. Curr. Opin. Food Sci. 2015, 1, 64–69. [Google Scholar] [CrossRef]
- Agyei, D.; Ongkudon, C.M.; Wei, C.Y.; Chan, A.S.; Danquah, M.K. Bioprocess Challenges to the Isolation and Purification of Bioactive Peptides. Food Bioprod. Process. 2016, 98, 244–256. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Karami, Z.; Pateiro, M.; Lorenzo, J.M. A Review on Health-Promoting, Biological, and Functional Aspects of Bioactive Peptides in Food Applications. Biomolecules 2021, 11, 631. [Google Scholar] [CrossRef]
- Aluko, R.E. Food protein-derived peptides: Production, Isolation, and Purification. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2018; Volume 15, pp. 389–412. [Google Scholar] [CrossRef]
- Tavano, O.L.; Berenguer-Murcia, A.; Secundo, F.; Fernandez-Lafuente, R. Biotechnological Applications of Proteases in Food Technology. Compr. Rev. Food Sci. Food Saf. 2018, 17, 412–436. [Google Scholar] [CrossRef]
- Dos Santos Aguilar, J.G.; Sato, H.H. Microbial Proteases: Production and Application in Obtaining Protein Hydrolysates. Food Res. Int. 2018, 103, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.-H.; Tavano, O.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Use of Alcalase in the Production of Bioactive Peptides: A Review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [CrossRef]
- Dullius, A.; Goettert, M.I.; de Souza, C.F.V. Whey Protein Hydrolysates as a Source of Bioactive Peptides for Functional Foods—Biotechnological Facilitation of Industrial Scale-up. J. Funct. Foods 2018, 42, 58–74. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Ksiażyk, J.; Luna, M.S.; Migacheva, N.; Picaud, J.-C.; Ramenghi, L.A.; Singhal, A.; Wabitsch, M. Partial Hydrolyzed Protein as a Protein Source for Infant Feeding: Do or Don’t? Nutrients 2022, 14, 1720. [Google Scholar] [CrossRef] [PubMed]
- Agyei, D.; Danquah, M.K.; Sarethy, I.P.; Pan, S. Antioxidative Peptides Derived from Food Proteins. In Free Radicals in Human Health and Disease; Rani, V., Yadav, U.C.S., Eds.; Springer: New Dehli, India, 2015; pp. 417–430. [Google Scholar] [CrossRef]
- Kumar, N.; Raghavendra, M.; Tokas, J.; Singal, H.R. Milk Proteins: Precursors of Antioxidative Peptides and Their Health Benefits. In Dairy in Human Health and Disease Across the Lifespan; Watson, R.R., Collier, R.J., Preedy, V.R., Eds.; Academic Press: New York, NY, USA, 2017; Volume 24, pp. 313–323. [Google Scholar] [CrossRef]
- Mann, B.; Kumari, A.; Kumar, R.; Sharma, R.; Prajapati, K.; Mahboob, S.; Athira, S. Antioxidant Activity of Whey Protein Hydrolysates in Milk Beverage System. J. Food Sci. Technol. 2014, 52, 3235–3241. [Google Scholar] [CrossRef]
- da Rosa, L.O.L.; Santana, M.C.; Avezedo, T.L.; Brígida, A.I.S.; Godoy, R.; Pacheco, S.; Mellinger-Silva, C.; Cabral, L.M.C. A Comparison of Dual-Functional Whey Hydrolysates by the Use of Commercial Proteases. Food Sci. Technol. 2018, 38, 31–36. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Buckin, V.; Kelly, P.M.; Giblin, L. Invited Review: Whey Proteins as Antioxidants and Promoters of Cellular Antioxidant Pathways. J. Dairy Sci. 2018, 101, 4747–4761. [Google Scholar] [CrossRef]
- Aree, T. Understanding Structure and Thermodynamics of β-Cyclodextrin Encapsulation of Chlorogenic, Caffeic and Quinic Acids: Implications for Enriching Antioxidant Capacity and Masking Bitterness in Coffee. Food Chem. 2019, 293, 550–560. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Millao, S.; Luzardo-Ocampo, I.; Campos-Vega, R.; Acevedo, F.; Shene, C.; Rubilar, M. Inclusion of Piperine in β-Cyclodextrin Complexes Improves Their Bioaccessibility and in Vitro Antioxidant Capacity. Food Hydrocoll. 2019, 91, 143–152. [Google Scholar] [CrossRef]
- Song, S.; Gao, K.; Niu, R.; Wang, J.; Zhang, J.; Gao, C.; Yang, B.; Liao, X. Inclusion Complexes Between Chrysin and Amino-Appended β-Cyclodextrins (ACDs): Binding Behavior, Water Solubility, in Vitro Antioxidant Activity and Cytotoxicity. Mater. Sci. Eng. 2020, 106, 110161. [Google Scholar] [CrossRef]
- Reddy, C.K.; Jung, E.S.; Son, S.Y.; Lee, C.H. Inclusion Complexation of Catechins-Rich Green Tea Extract by β-Cyclodextrin: Preparation, Physicochemical, Thermal, and Antioxidant Properties. LWT 2020, 131, 109723. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, R.; Sun, X.; Yu, X.; Xiao, Y.; Wang, L.; Hu, W.; Zhong, T. The p-Anisaldehyde/β-cyclodextrin inclusion Complexes as a Sustained Release Agent: Characterization, Storage Stability, Antibacterial and Antioxidant Activity. Food Control 2022, 132, 108561. [Google Scholar] [CrossRef]
- Mantovan, M.J.; Marim, B.M.; Giraldo, G.A.G.J.; Pereira, F.; Kishima, J.O.F.; Zuluaga, M.Y.A.; Resta, V.G. Nanomaterials for Nutraceuticals and Preservative Agents. In Research and Technological Advances in Food Science; Prakash, B., Ed.; Academic Press: New York, NY, USA, 2022; Volume 17, pp. 425–445. [Google Scholar] [CrossRef]
- Szajewska, H.; Horvath, A. A Partially Hydrolyzed 100% Whey Formula and the Risk of Eczema and Any Allergy: An Updated Meta-Analysis. World Allergy Organ. J. 2017, 10, 27. [Google Scholar] [CrossRef]
- Kiewiet, M.; Faas, M.; de Vos, P. Immunomodulatory Protein Hydrolysates and Their Application. Nutrients 2018, 10, 904. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Munasir, Z.; Hegar, B.; Kumarawati, D.; Suryawan, A.; Kadim, M.; Djais, J.T.; Basrowi, R.W.; Krisnamurti, D. A Perspective on Partially Hydrolyzed Protein Infant Formula in Nonexclusively Breastfed Infants. Korean J. Pediatr. 2019, 62, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Meyer, R.; Chouraqui, J.-P.; Dupont, C.; Fiocchi, A.; Salvatore, S.; Shamir, R.; Szajewska, H.; Thapar, N.; Venter, C.; et al. The Role of Milk Feeds and Other Dietary Supplementary Interventions in Preventing Allergic Disease in Infants: Fact or Fiction? Clin. Nutr. 2021, 40, 358–371. [Google Scholar] [CrossRef]
- Liang, X.; Qian, G.; Sun, J.; Yang, M.; Shi, X.; Yang, H.; Wu, J.; Wang, Z.; Zheng, Y.; Yue, X. Evaluation of Antigenicity and Nutritional Properties of Enzymatically Hydrolyzed Cow Milk. Sci. Rep. 2021, 11, 18623. [Google Scholar] [CrossRef]
- Pessato, T.B.; de Carvalho, N.C.; Tavano, O.L.; Fernandes, L.G.R.; Zollner, R.d.L.; Netto, F.M. Whey Protein Isolate Hydrolysates Obtained with Free and Immobilized Alcalase: Characterization and Detection of Residual Allergens. Food Res. Int. 2016, 83, 112–120. [Google Scholar] [CrossRef]
- Sorva, R.; Makinenkiljunen, S.; Juntunenbackman, K. β-Lactoglobulin Secretion in Human Milk Varies Widely After Cow’s Milk Ingestion in Mothers of Infants with Cow’s Milk Allergy. J. Allergy Clin. Immunol. 1994, 93, 787–792. [Google Scholar] [CrossRef]
- Järvinen, K.-M.; Chatchatee, P.; Bardina, L.; Beyer, K.; Sampson, H.A. IgE and IgG Binding Epitopes on α-Lactalbumin and β-Lactoglobulin in Cow’s Milk Allergy. Int. Arch. Allergy Immunol. 2001, 126, 111–118. [Google Scholar] [CrossRef]
- Fritsché, R.; Adel-Patient, K.; Bernard, H.; Martin-Paschoud, C.; Schwarz, C.; Ah-Leung, S.; Wal, J.-M. IgE-Mediated Rat Mast Cell Triggering with Tryptic and Synthetic Peptides of Bovine β-Lactoglobulin. Int. Arch. Allergy Immunol. 2005, 138, 291–297. [Google Scholar] [CrossRef]
- Kamphorst, A.; Mendes de Sá, I.; Faria, A.M.; Sinisterra, R. Association Complexes Between Ovalbumin and Cyclodextrins Have No Effect on the Immunological Properties of Ovalbumin. Eur. J. Pharm. Biopharm. 2004, 57, 199–205. [Google Scholar] [CrossRef]
- He, M.; Zhong, C.; Hu, H.; Jin, Y.; Chen, Y.; Lou, K.; Gao, F. Cyclodextrin/Chitosan Nanoparticles for Oral Ovalbumin Delivery: Preparation, Characterization and Intestinal Mucosal Immunity in Mice. Asian J. Pharm. Sci. 2019, 14, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Heydari, S.; Hosseini, S.E.; Mortazavian, A.M.; Taheri, S. Extraction of Bioactive Peptides Produced in Probiotic Yoghurt and Determination of Their Biological Activities. Int. Dairy J. 2023, 139, 105544. [Google Scholar] [CrossRef]
- Lee, J.H.; Paik, H.-D. Anticancer and Immunomodulatory Activity of Egg Proteins and Peptides: A Review. Poult. Sci. 2019, 98, 6505–6516. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.K.; Kuwabara, Y.; Hara, K.; Watanabe, Y.; Tamai, Y. Antimutagenic Effect of Amino Acids on the Mutagenicity of N-methyl-N′-nitro-N-nitrosoguanidine (MNNG). Biosci. Biotechnol. Biochem. 2002, 66, 1400–1402. [Google Scholar] [CrossRef] [PubMed]
- Van Boekel, M.A.J.S.; Weerens, C.N.J.M.; Holstra, A.; Scheidtweiler, C.E.; Alink, G.M. Antimutagenic Effects of Casein and Its Digestion Products. Food Chem. Toxicol. 1993, 31, 731–737. [Google Scholar] [CrossRef]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- Guberti, M.; Botti, S.; Capuzzo, M.T.; Nardozi, S.; Fusco, A.; Cera, A.; Dugo, L.; Piredda, M.; De Marinis, M.G. Bovine Colostrum Applications in Sick and Healthy People: A Systematic Review. Nutrients 2021, 13, 2194. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.; Kaplan, M.; Duman, H.; Bayraktar, A.; Ertürk, M.; Henrick, B.M.; Frese, S.A.; Karav, S. Bovine Colostrum and Its Potential for Human Health and Nutrition. Front. Nutr. 2021, 8, 651721. [Google Scholar] [CrossRef]
- ISO 8968–1:2014; Milk and Milk Products. Determination of Nitrogen Content. Part 1: Kjeldahl Principle and Crude Protein Calculation. International Organization for Standardization (ISO): Geneva, Switzerland, 2014.
- Broido, A. A Simple, Sensitive Graphical Method of Treating Thermogravimetric Analysis Data. J. Polym. Sci. B Polym. Phys. 1969, 7, 1761–1773. [Google Scholar] [CrossRef]
- Chambers, J.M.; Freeny, A.; Heiberger, R.M. Analysis of Variance; Designed Experiments. In Statistical Models in S, 1st ed.; Chambers, J.M., Hastie, T.J., Eds.; Wadsworth & Brooks/Cole: Pacific Grove, CA, USA, 1992; Volume 5, pp. 145–190. [Google Scholar] [CrossRef]
- Dunnett, C.W. A Multiple Comparison Procedure for Comparing Several Treatments with A Control. J. Am. Stat. Assoc. 1955, 50, 1096–1121. [Google Scholar] [CrossRef]
- Miller, R.G. Simultaneous Statistical Inference, 2nd ed.; Springer: New York, NY, USA, 1981; pp. 189–210. [Google Scholar] [CrossRef]
- Yandell, B.S. Practical Data Analysis for Designed Experiments, 1st ed.; Chapman & Hall: New York, NY, USA, 1997; pp. 145–158. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 4 April 2023).
- Signorell, A.; Aho, K.; Alfons, A.; Anderegg, N.; Aragon, T.; Arachchige, C.; Arppe, A.; Baddeley, A.; Barton, K.; Bolker, B.; et al. DescTools: Tools for Descriptive Statistics, R Package Version 0.99.48; CRAN: Vienna, Austria, 2023. [Google Scholar]








| Sample Name | Particle Size (Radius, R), nm | MW of Particles (MW R), kDa | Range of Detected MW, kDa | Relative Polydispersity (Pd) 2, % | Peptide Length, AA |
|---|---|---|---|---|---|
| WH–5 kDa | 0.7 1 | 1.7 1 | 1.4–2.3 3 | 48.6 | 15 1/13–21 3 |
| CH–5 kDa | 0.6 1 | 0.9 1 | 0.7–1.4 3 | 41.8 | 8 1/6–13 3 |
| Sample Name | Temperature of Maximum Thermal Oxidative Degradation Speed (TVmax), °C | Maximum Thermal Oxidative Degradation Speed (Vmax), mg/°C | Activation Energy (Ea), kJ/mol |
|---|---|---|---|
| β/γ-cyclodextrin | 301.8 ± 2.0 A1/303.4 ± 2.3 A2 | 0.43 ± 0.02 A1,α/0.33 ± 0.01 A2,β | 324 ± 10 A1,α/292 ± 15 A2,β |
| Ultrafiltered whey hydrolysate (WH–5 kDa) | 266.0 ± 2.0 | 0.031 ± 0.002 | 79 ± 1 |
| Mechanical mixture (WH–5 kDa:β/γ-CD = 1:2) | 297.5 ± 2.1 B1/289.9 ± 2.5 B2 | 0.29 ± 0.01 B1/0.17 ± 0.01 B2 | 118 ± 3 B1/110 ± 1 B2 |
| Complex (WH–5 kDa:β/γ-CD = 1:2) | 305.1 ± 2.2 C1/296.6 ± 2.3 C2 | 0.15 ± 0.02 C1/0.11 ± 0.01 C2 | 105 ± 3 C1,α/97 ± 2 C2,β |
| Ultrafiltered colostrum hydrolysate (CH–5 kDa) | 285.8 ± 2.9 | 0.027 ± 0.001 | 65 ± 1 |
| Mechanical mixture (CH–5 kDa:β/γ-CD = 1:2) | 289.7 ± 2.7 B1/283.5 ± 2.3 B2 | 0.30 ± 0.02 B1/0.18 ± 0.01 B2 | 125 ± 4 B1/114 ± 3 B2 |
| Complex (CH–5 kDa:β/γ-CD = 1:2) | 289.7 ± 3.5 B1/294.3 ± 2.8 C2 | 0.14 ± 0.01 C1/0.15 ± 0.01 C2 | 107 ± 2 C1,α/102 ± 1 C2,β |
| Sample Name | Particle Size (Radius, R), nm | Molecular Weight of Particles (MW R), kDa | Relative Polydispersity (Pd) 2, % |
|---|---|---|---|
| WH–5 kDa | 0.7 1 | 1.7 1/1.4–2.3 | 48.6 |
| β-CD | 0.7 | 1.5 | 81.8 |
| γ-CD | 0.8 | 2.1 | 37.4 |
| WH–5 kDa:β-CD = 1:0.5, incubation time | |||
| 15 min | 0.7–1.0 | 1.5–3.1 | 30.7–58.0 |
| 30 min | 0.6–1.2 | 1.2–5.2 | 16.9–42.8 |
| 60 min | 0.6–1.3 | 1.0–6.7 | 24.6–42.5 |
| WH–5 kDa:β-CD = 1:1, incubation time | |||
| 15 min | 0.7–1.0 | 1.5–3.6 | 34.3–51.2 |
| 30 min | 0.7–1.4 | 1.6–7.2 | 17.5–45.4 |
| 60 min | 0.7–1.1 | 1.7–4.5 | 31.4–53.0 |
| WH–5 kDa:β-CD = 1:2, incubation time | |||
| 15 min | 0.7–1.1 | 1.2–4.5 | 15.6–42.8 |
| 30 min | 0.7–0.8 | 1.6–1.8 | 32.8–39.1 |
| 60 min | 0.6–0.7 | 0.9–1.5 | 35.2–45.6 |
| WH–5 kDa:γ-CD = 1:0.5, incubation time | |||
| 15 min | 0.8–1.1 | 2.1–4.6 | 27.7–33.5 |
| 30 min | 0.6–1.0 | 1.1–3.5 | 19.5–28.7 |
| 60 min | 0.8 | 1.9–2.1 | 32.7–34.4 |
| WH–5 kDa:γ-CD = 1:1, incubation time | |||
| 15 min | 0.8–1.1 | 2.0–3.9 | 19.3–36.2 |
| 30 min | 0.8 | 1.9–2.2 | 16.0–46.7 |
| 60 min | 0.6–1.0 | 1.1–3.2 | 32.0–59.7 |
| WH–5 kDa:γ-CD = 1:2, incubation time | |||
| 15 min | 0.6–0.8 | 1.1–1.9 | 32.8–45.5 |
| 30 min | 0.7–0.8 | 1.5–2.1 | 26.7–34.7 |
| 60 min | 0.7 | 1.3–1.7 | 32.9–38.7 |
| Sample Name | IC50, μg of Protein/mL | IC50 (Native Protein)/ IC50 (Hydrolysate) | IC50 (Hydrolysate)/ IC50 (Complex) |
|---|---|---|---|
| WP | 81.6 ± 2.1 A | 1.0 A | – |
| WH | 32.1 ± 1.6 B | 2.54 ± 0.18 B | – |
| WH–5 kDa | 13.8 ± 0.7 C | 5.91 ± 0.16 C | 1.0 A |
| β-CD + WH–5 kDa | 6.36 ± 0.35 D | 12.9 ± 1.0 D,α | 2.18 ± 0.23 B,α |
| γ-CD + WH–5 kDa | 4.97 ± 0.17 E | 16.4 ± 0.2 E,I | 2.78 ± 0.06 C,I |
| CP | 56.9 ± 0.9 a | 1.0 a | – |
| CH | 17.3 ± 0.4 b | 3.29 ± 0.05 b | – |
| CH–5 kDa | 8.60 ± 0.24 c | 6.62 ± 0.22 c | 1.0 a |
| β-CD + CH–5 kDa | 5.95 ± 0.38 d | 9.60 ± 0.69 d,β | 1.45 ± 0.09 b,β |
| γ-CD + CH–5 kDa | 4.01 ± 0.08 e | 14.2 ± 0.4 e,II | 2.14 ± 0.13 c,II |
| β-CD | 106.5 ± 0.7 F/f | – | – |
| γ-CD | 76.4 ± 4.0 G/g | – | – |
| Sample Name | Residual Antigenicity (RA), % | RA (Hydrolysate)/ RA (Complex) |
|---|---|---|
| WP | 100 A | – |
| WH | 5.8 ± 0.2 B | – |
| WH–5 kDa | 0.047 ± 0.002 C | 1.0 A |
| β-CD + WH–5 kDa | 0.0083 ± 0.0003 D | 5.62 ± 0.01 B |
| γ-CD + WH–5 kDa | 0.0089 ± 0.0003 D | 5.28 ± 0.08 B |
| CP | 100 a | – |
| CH | 0.68 ± 0.05 b | – |
| CH–5 kDa | 0 | – |
| β-CD + CH–5 kDa | 0 | – |
| γ-CD + CH–5 kDa | 0 | – |
| β/γ-ЦД | 0 | – |
| Sample Name | Level of Mutation Reducing (%) (0.03–0.5 mg of Protein Per Plate) | |
|---|---|---|
| S. typhimurium TA 98 | S. typhimurium TA 100 | |
| WH–5 kDa | 14.10–25.93 A,α,I | 12.05–20.19 A,α,II |
| β-CD + WH–5 kDa | 14.07–28.37 A,I | 12.34–21.61 A,I |
| γ-CD + WH–5 kDa | 16.98–29.86 A,I | 13.92–22.97 A,II |
| CH–5 kDa | 9.15–23.91 a,α,I | 8.48–19.36 a,α,I |
| β-CD + CH–5 kDa | 10.00–25.25 a,I | 13.69–21.21 a,I |
| γ-CD + CH–5 kDa | 7.91–21.28 a,I | 9.95–19.47 a,I |
| β/γ-ЦД | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halavach, T.M.; Kurchenko, V.P.; Tarun, E.I.; Dudchik, N.V.; Yatskou, M.M.; Lodygin, A.D.; Alieva, L.R.; Evdokimov, I.A.; Ulrih, N.P. Influence of Complexation with β- and γ-Cyclodextrin on Bioactivity of Whey and Colostrum Peptides. Int. J. Mol. Sci. 2023, 24, 13987. https://doi.org/10.3390/ijms241813987
Halavach TM, Kurchenko VP, Tarun EI, Dudchik NV, Yatskou MM, Lodygin AD, Alieva LR, Evdokimov IA, Ulrih NP. Influence of Complexation with β- and γ-Cyclodextrin on Bioactivity of Whey and Colostrum Peptides. International Journal of Molecular Sciences. 2023; 24(18):13987. https://doi.org/10.3390/ijms241813987
Chicago/Turabian StyleHalavach, Tatsiana M., Vladimir P. Kurchenko, Ekaterina I. Tarun, Natalia V. Dudchik, Mikalai M. Yatskou, Aleksey D. Lodygin, Ludmila R. Alieva, Ivan A. Evdokimov, and Natasa Poklar Ulrih. 2023. "Influence of Complexation with β- and γ-Cyclodextrin on Bioactivity of Whey and Colostrum Peptides" International Journal of Molecular Sciences 24, no. 18: 13987. https://doi.org/10.3390/ijms241813987