Effect of Liver Fibrosis on Oral and Gut Microbiota in the Japanese General Population Determined by Evaluating the FibroScan–Aspartate Aminotransferase Score
Abstract
:1. Introduction
2. Results
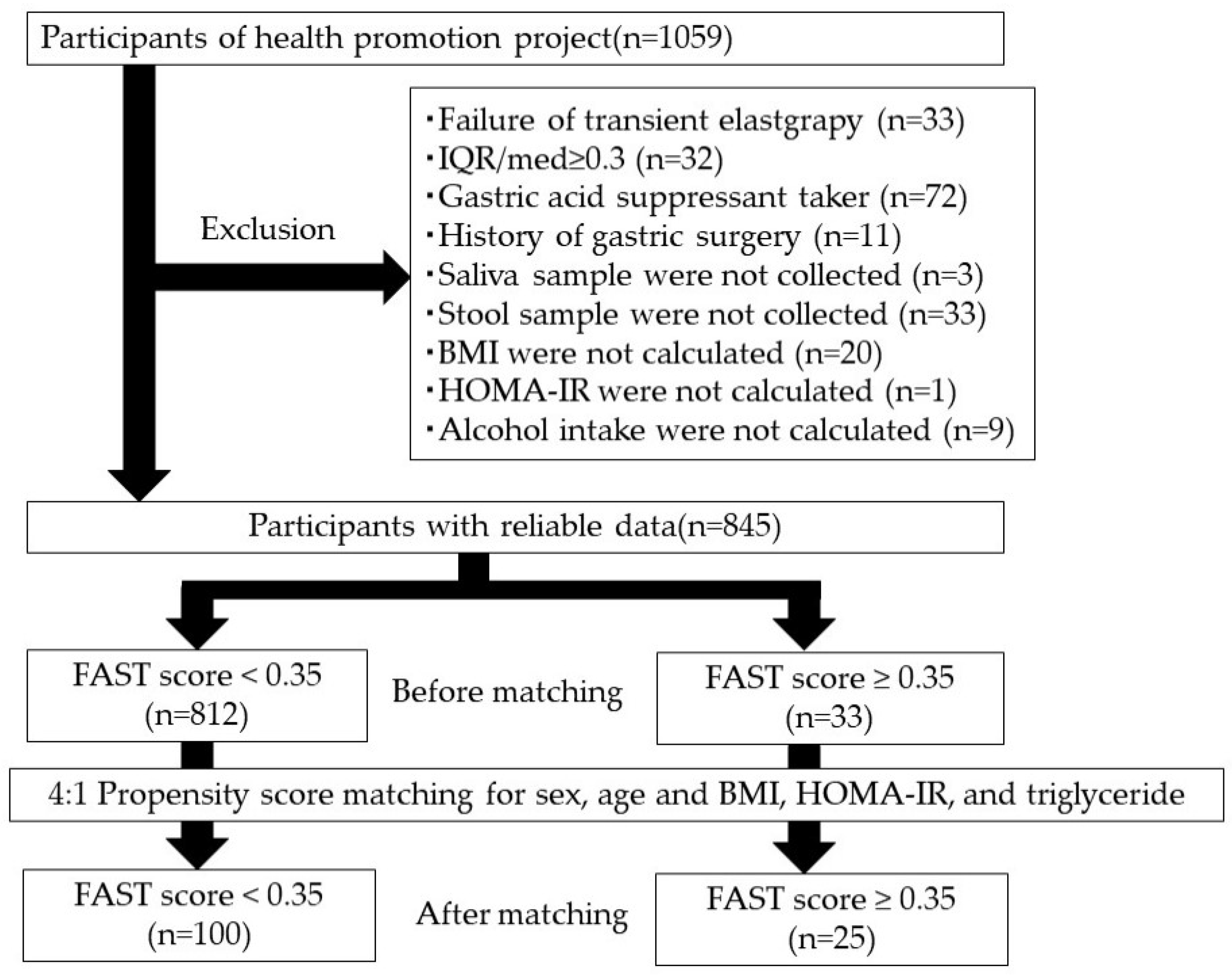
2.1. Participant Characteristics
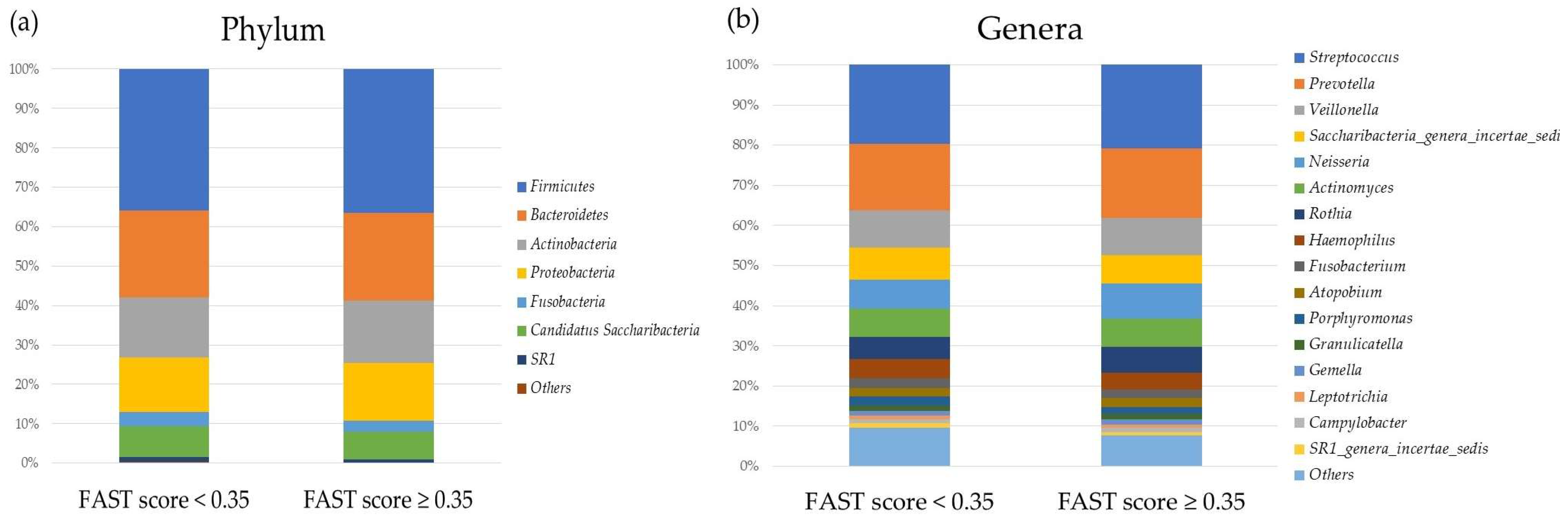
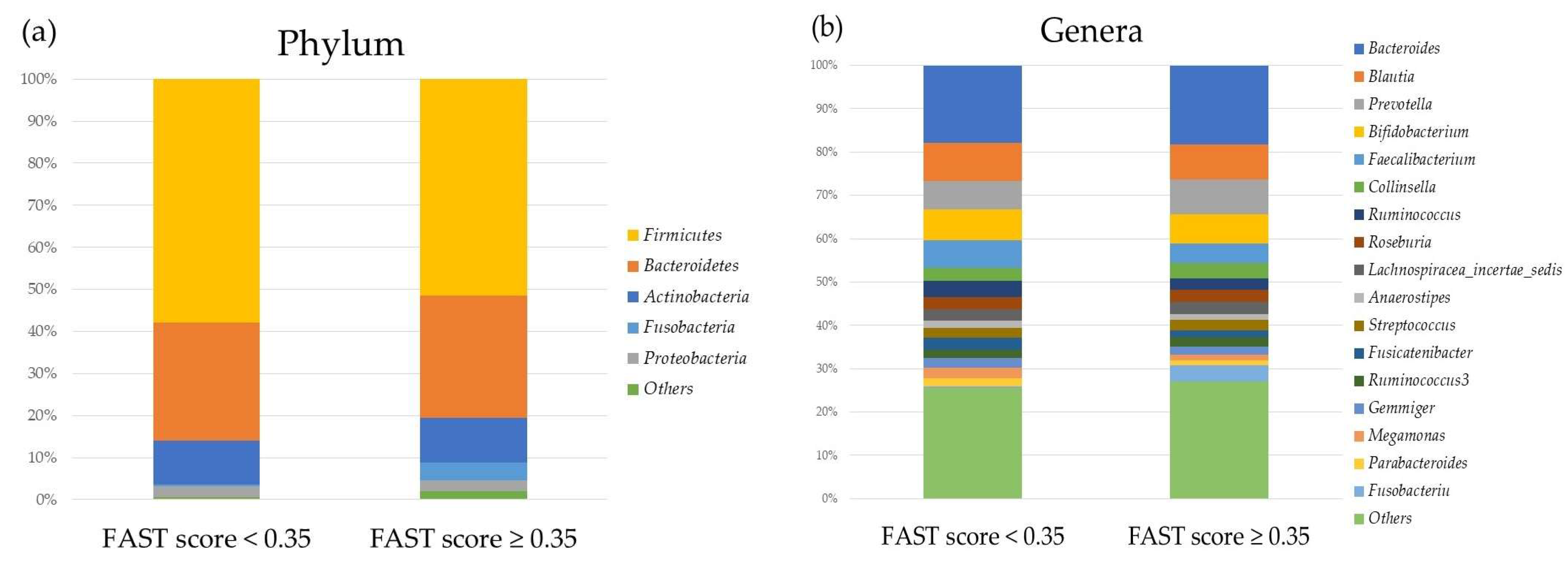
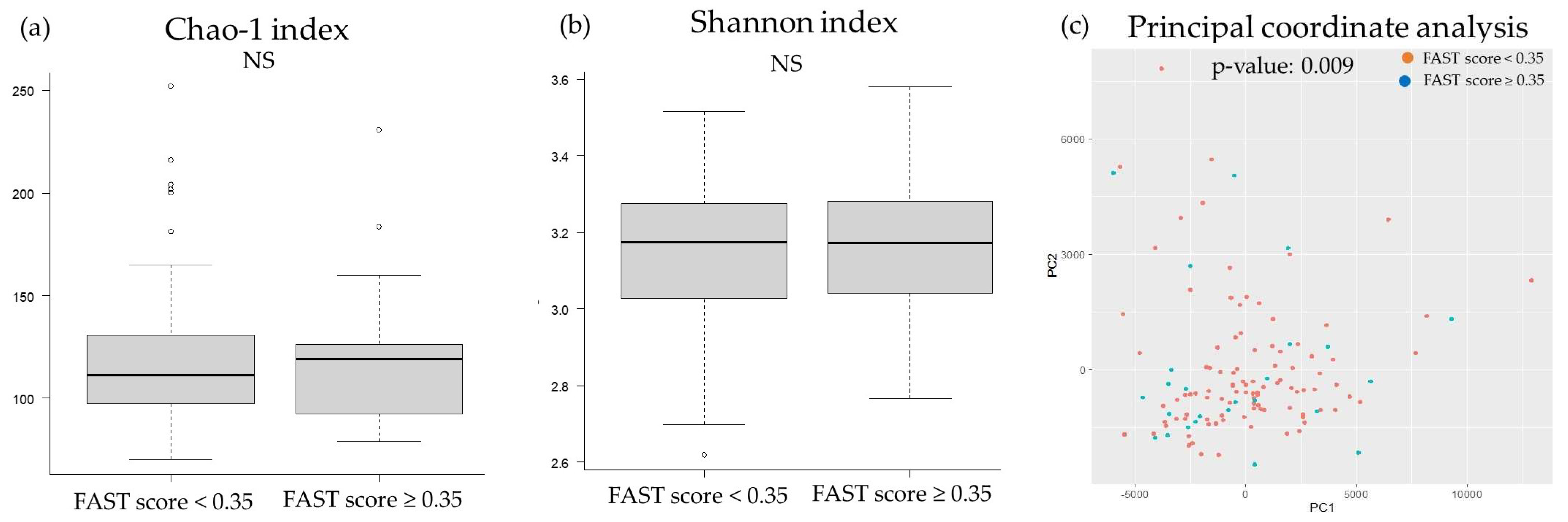
2.2. Comparison of the Oral and Gut Microbiota and Liver Fibrosis
2.3. Correlation between FAST score, FIB-4 Index, Fatty Liver Index, and Gut Microbiota
2.4. Risk Factors for Liver Fibrosis Adjusted by Fatty Liver Index
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Transient Elastography
4.3. Clinical Parameters
4.4. FAST Score
4.5. Next-Generation Sequence Analysis of Oral and Gut Microbiota
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ito, T.; Kumada, T.; Toyoda, H.; Tada, T. FIB-4 index for assessing the prognosis of hepatocellular carcinoma in patients with Child-Pugh class A liver function. J. Cancer Res. Clin. Oncol. 2015, 141, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Toyoda, H.; Kiriyama, S.; Tanikawa, M.; Hisanaga, Y.; Kanamori, A.; Kitabatake, S.; Yama, T.; Tanaka, J. Long-term prognosis of patients with chronic hepatitis C who did not receive interferon-based therapy: Causes of death and analysis based on the FIB-4 index. J. Gastroenterol. 2016, 51, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, B.; Fu, Y.; Chen, Y.; Yang, F.; Lu, H.; Chen, Y.; Xu, J.; Li, L. Changes of fecal Bifidobacterium species in adult patients with hepatitis B virus-induced chronic liver disease. Microb. Ecol. 2012, 63, 304–313. [Google Scholar] [CrossRef]
- Dubinkina, V.B.; Tyakht, A.V.; Odintsova, V.Y.; Yarygin, K.S.; Kovarsky, B.A.; Pavlenko, A.V.; Ischenko, D.S.; Popenko, A.S.; Alexeev, D.G.; Taraskina, A.Y.; et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 2017, 5, 141. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.W.; Kersten, S. Potential mediators linking gut bacteria to metabolic health: A critical view. J. Physiol. 2017, 595, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Funatsu, Y.; Ohnishi, M.; Isogawa, M.; Kawashima, K.; Tanaka, M.; Moriya, K.; Kawaratani, H.; Momoda, R.; Iio, E.; et al. Bile acid dysmetabolism in the gut-microbiota-liver axis under hepatitis C virus infection. Liver Int. 2022, 42, 124–134. [Google Scholar] [CrossRef]
- Blesl, A.; Stadlbauer, V. The gut-liver axis in cholestatic liver diseases. Nutrients 2021, 13, 1018. [Google Scholar] [CrossRef]
- Fukui, H. Leaky gut and gut-liver axis in liver cirrhosis: Clinical studies update. Gut Liver 2021, 15, 666–676. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, F.; Lu, H.; Wang, B.; Chen, Y.; Lei, D.; Wang, Y.; Zhu, B.; Li, L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011, 54, 562–572. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Ridlon, J.M.; Hylemon, P.B.; Thacker, L.R.; Heuman, D.M.; Smith, S.; Sikaroodi, M.; Gillevet, P.M. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G168–G175. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Gaborit, B.; Dutour, A.; Clement, K. Gut microbiota and non-alcoholic fatty liver disease: New insights. Clin. Microbiol. Infect. 2013, 19, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Ahmed, I.; Gillevet, P.M.; Probert, C.S.; Ratcliffe, N.M.; Smith, S.; Greenwood, R.; Sikaroodi, M.; Lam, V.; Crotty, P.; et al. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2013, 11, 868–875.e1. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Kakiyama, G.; Zhao, D.; Takei, H.; Fagan, A.; Hylemon, P.; Zhou, H.; Pandak, W.M.; Nittono, H.; Fiehn, O.; et al. Continued alcohol misuse in human cirrhosis is associated with an impaired gut-liver axis. Alcohol. Clin. Exp. Res. 2017, 41, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wu, N.; Wang, X.; Chi, Y.; Zhang, Y.; Qiu, X.; Hu, Y.; Li, J.; Liu, Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep. 2015, 5, 8096. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Zheng, R.-D.; Sun, X.-Q.; Ding, W.J.; Wang, X.Y.; Fan, J.G. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Long, W.; Zhang, C.; Liu, S.; Zhao, L.; Hamaker, B.R. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci. Rep. 2017, 7, 2594. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; White, M.B.; Monteith, P.; Noble, N.A.; Unser, A.B.; Daita, K.; Fisher, A.R.; et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J. Hepatol. 2014, 60, 940–947. [Google Scholar] [CrossRef]
- Rojkind, M.; Domínguez-Rosales, J.A.; Nieto, N.; Greenwel, P. Role of hydrogen peroxide and oxidative stress in healing responses. Cell Mol. Life Sci. 2002, 59, 1872–1891. [Google Scholar] [CrossRef]
- Friedman, S.L. The cellular basis of hepatic fibrosis--mechanisms and treatment strategies. N. Engl. J. Med. 1993, 328, 1828–1835. [Google Scholar]
- Lieber, C.S.; Baraona, E.; Hernández-Muñoz, R.; Kubota, S.; Sato, N.; Kawano, S.; Matsumura, T.; Inatomi, N. Impaired oxygen utilization. A new mechanism for the hepatotoxicity of ethanol in sub-human primates. J. Clin. Investig. 1989, 83, 1682–1690. [Google Scholar] [CrossRef]
- Kono, H.; Rusyn, I.; Uesugi, T.; Yamashina, S.; Connor, H.D.; Dikalova, A.; Mason, R.P.; Thurman, R.G. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G1005–G1012. [Google Scholar] [CrossRef] [PubMed]
- Zavaglia, D.; Normand, N.; Brewis, N.; O’Hare, P.; Favrot, M.C.; Coll, J.L. VP22-mediated and light-activated delivery of an anti-c-raf1 antisense oligonucleotide improves its activity after intratumoral injection in nude mice. Mol. Ther. 2003, 8, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Bonkovsky, H.L.; Banner, B.F.; Rothman, A.L. Iron and chronic viral hepatitis. Hepatology 1997, 25, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Moore, L.E.; Bradford, B.U.; Gao, W.; Thurman, R.G. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 1995, 108, 218–224. [Google Scholar] [CrossRef]
- Fargion, S.; Mattioli, M.; Fracanzani, A.L.; Sampietro, M.; Tavazzi, D.; Fociani, P.; Taioli, E.; Valenti, L.; Fiorelli, G. Hyperferritinemia, iron overload, and multiple metabolic alterations identify patients at risk for nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2001, 96, 2448–2455. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Betrapally, N.S.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; White, M.B.; Unser, A.; Thacker, L.R.; Sanyal, A.J.; Kang, D.J.; et al. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology 2015, 62, 1260–1271. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, X.; Cheng, Y.; Jiang, X.; Jiang, H.; Wang, Y.; Li, L. Decreased diversity of the oral microbiota of patients with hepatitis B virus-induced chronic liver disease: A pilot project. Sci. Rep. 2015, 5, 17098. [Google Scholar] [CrossRef]
- Mohammed, H.; Varoni, E.M.; Cochis, A.; Cordaro, M.; Gallenzi, P.; Patini, R.; Staderini, E.; Lajolo, C.; Rimondini, L.; Rocchetti, V. Oral dysbiosis in pancreatic cancer and liver cirrhosis: A review of the literature. Biomedicines 2018, 6, 115. [Google Scholar] [CrossRef]
- Nakahara, T.; Hyogo, H.; Ono, A.; Nagaoki, Y.; Kawaoka, T.; Miki, D.; Tsuge, M.; Hiraga, N.; Hayes, C.N.; Hiramatsu, A.; et al. Involvement of Porphyromonas gingivalis in the progression of non-alcoholic fatty liver disease. J. Gastroenterol. 2018, 53, 269–280. [Google Scholar] [CrossRef]
- Furusho, H.; Miyauchi, M.; Hyogo, H.; Inubushi, T.; Ao, M.; Ouhara, K.; Hisatune, J.; Kurihara, H.; Sugai, M.; Hayes, C.N.; et al. Dental infection of Porphyromonas gingivalis exacerbates high fat diet-induced steatohepatitis in mice. J. Gastroenterol. 2013, 48, 1259–1270. [Google Scholar] [CrossRef]
- Nagasaki, A.; Sakamoto, S.; Chea, C.; Ishida, E.; Furusho, H.; Fujii, M.; Takata, T.; Miyauchi, M. Odontogenic infection by Porphyromonas gingivalis exacerbates fibrosis in NASH via hepatic stellate cell activation. Sci. Rep. 2020, 10, 4134. [Google Scholar] [CrossRef]
- Newsome, P.N.; Sasso, M.; Deeks, J.J.; Paredes, A.; Boursier, J.; Chan, W.K.; Yilmaz, Y.; Czernichow, S.; Zheng, M.H.; Wong, V.W.; et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020, 5, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Wang, Y.; Zhang, L.; Guo, Q. Oxidative stress and liver disease. Hepatol. Res. 2012, 42, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, F.; Dajti, E.; Mantovani, A.; Newsome, P.N.; Targher, G.; Colecchia, A. Diagnostic accuracy of FibroScan-AST (FAST) score for the non-invasive identification of patients with fibrotic non-alcoholic steatohepatitis: A systematic review and meta-analysis. Gut 2023, 72, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, E.; Takayama, K.; Hiramine, S.; Hayashi, T.; Toyoda, K. Association between steatohepatitis biomarkers and hepatocellular carcinoma after hepatitis C elimination. Aliment. Pharmacol. Ther. 2020, 52, 866–876. [Google Scholar] [CrossRef]
- Fujii, H.; Fukumoto, S.; Enomoto, M.; Uchida-Kobayashi, S.; Kimura, T.; Tamori, A.; Nadatani, Y.; Takashima, S.; Nishimoto, N.; Kawada, N. The FibroScan-aspartate aminotransferase score can stratify the disease severity in a Japanese cohort with fatty liver diseases. Sci. Rep. 2021, 11, 13844. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Cusi, K.; Hilliard, M.E.; Isaacs, D.; et al. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S49–S67. [Google Scholar] [CrossRef]
- Mikami, K.; Endo, T.; Sawada, N.; Igarashi, G.; Kimura, M.; Hasegawa, T.; Iino, C.; Sawada, K.; Nakaji, S.; Ishibashi, Y.; et al. Association of bone metabolism with fatty liver disease in the elderly in Japan: A community-based study. Intern. Med. 2020, 59, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Iino, C.; Endo, T.; Mikami, K.; Hasegawa, T.; Kimura, M.; Sawada, N.; Nakaji, S.; Fukuda, S. Significant decrease in Faecalibacterium among gut microbiota in nonalcoholic fatty liver disease: A large BMI- and sex-matched population study. Hepatol. Int. 2019, 13, 748–756. [Google Scholar] [CrossRef]
- Elghannam, M.T.; Hassanien, M.H.; Ameen, Y.A.; Turky, E.A.; Elattar, G.M.; ElRay, A.A.; Eltalkawy, M.D. Oral microbiota and liver diseases. Clin. Nutr. ESPEN 2023, 54, 68–72. [Google Scholar] [CrossRef]
- Li, Z.; Ni, M.; Yu, H.; Wang, L.; Zhou, X.; Chen, T.; Liu, G.; Gao, Y. Gut microbiota and liver fibrosis: One potential biomarker for predicting liver fibrosis. BioMed Res. Int. 2020, 2020, 3905130. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.M.; Inamine, T.; Hochrath, K.; Chen, P.; Wang, L.; Llorente, C.; Bluemel, S.; Hartmann, P.; Xu, J.; Koyama, Y.; et al. Intestinal fungi contribute to development of alcoholic liver disease. J. Clin. Investig. 2017, 127, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Nakayama, J.; Moriya, K.; Kawaratani, H.; Momoda, R.; Ito, K.; Iio, E.; Nojiri, S.; Fujiwara, K.; Yoneda, M.; et al. Gut Dysbiosis Associated with Hepatitis C Virus Infection. Clin. Infect. Dis. 2018, 67, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Wei, Y.; Li, Y.; Chen, W.; Chen, H.; Wang, Q.; Yang, F.; Miao, Q.; Xiao, X.; Zhang, H.; et al. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut 2018, 67, 534–541. [Google Scholar] [CrossRef]
- Tsai, M.C.; Liu, Y.Y.; Lin, C.C.; Wang, C.C.; Wu, Y.J.; Yong, C.C.; Chen, K.D.; Chuah, S.K.; Yao, C.C.; Huang, P.Y.; et al. Gut microbiota dysbiosis in patients with biopsy-proven nonalcoholic fatty liver disease: A cross-sectional study in Taiwan. Nutrients 2020, 12, 820. [Google Scholar] [CrossRef]
- Wong, V.W.; Tse, C.H.; Lam, T.T.; Wong, G.L.; Chim, A.M.; Chu, W.C.; Yeung, D.K.; Law, P.T.; Kwan, H.S.; Yu, J.; et al. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis—A longitudinal study. PLoS ONE 2013, 8, e62885. [Google Scholar] [CrossRef]
- Fukui, H. Role of gut dysbiosis in liver diseases: What have we learned so far? Diseases 2019, 7, 58. [Google Scholar] [CrossRef]
- Lin, R.S.; Lee, F.Y.; Lee, S.D.; Tsai, Y.T.; Lin, H.C.; Lu, R.H.; Hsu, W.C.; Huang, C.C.; Wang, S.S.; Lo, K.J. Endotoxemia in patients with chronic liver diseases: Relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J. Hepatol. 1995, 22, 165–172. [Google Scholar] [CrossRef]
- Seki, E.; De Minicis, S.; Osterreicher, C.H.; Kluwe, J.; Osawa, Y.; Brenner, D.A.; Schwabe, R.F. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat. Med. 2007, 13, 1324–1332. [Google Scholar] [CrossRef]
- Sarkar, S.; Kimono, D.; Albadrani, M.; Seth, R.K.; Busbee, P.; Alghetaa, H.; Porter, D.E.; Scott, G.I.; Brooks, B.; Nagarkatti, M.; et al. Environmental microcystin targets the microbiome and increases the risk of intestinal inflammatory pathology via NOX2 in underlying murine model of Nonalcoholic Fatty Liver Disease. Sci. Rep. 2019, 9, 8742. [Google Scholar] [CrossRef]
- Del Ben, M.; Polimeni, L.; Carnevale, R.; Bartimoccia, S.; Nocella, C.; Baratta, F.; Loffredo, L.; Pignatelli, P.; Violi, F.; Angelico, F. NOX2-generated oxidative stress is associated with severity of ultrasound liver steatosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2014, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Ferolla, S.M.; Armiliato, G.N.; Couto, C.A.; Ferrari, T.C. The role of intestinal bacteria overgrowth in obesity-related nonalcoholic fatty liver disease. Nutrients 2014, 6, 5583–5599. [Google Scholar] [CrossRef]
- Liu, J.; Fu, Y.; Zhang, H.; Wang, J.; Zhu, J.; Wang, Y.; Guo, Y.; Wang, G.; Xu, T.; Chu, M.; et al. The hepatoprotective effect of the probiotic Clostridium butyricum against carbon tetrachloride-induced acute liver damage in mice. Food Funct. 2017, 8, 4042–4052. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Te, B.; Wang, L.; Gao, Y.; He, Q.; Yan, Z.; Wang, H.; Shen, G. Combination of sodium butyrate and probiotics ameliorates severe burn-induced intestinal injury by inhibiting oxidative stress and inflammatory response. Burns 2022, 48, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Kuwabara, R.; de Haan, B.J.; Smink, A.M.; de Vos, P. Acetate and Butyrate Improve β-cell Metabolism and Mitochondrial Respiration under Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 1542. [Google Scholar] [CrossRef] [PubMed]
- Oka, A.; Sartor, R.B. Microbial-based and microbial-targeted therapies for inflammatory bowel diseases. Dig. Dis. Sci. 2020, 65, 757–788. [Google Scholar] [CrossRef]
- Takeshita, K.; Mizuno, S.; Mikami, Y.; Sujino, T.; Saigusa, K.; Matsuoka, K.; Naganuma, M.; Sato, T.; Takada, T.; Tsuji, H.; et al. A single species of clostridium subcluster XIVa decreased in ulcerative colitis patients. Inflamm. Bowel Dis. 2016, 22, 2802–2810. [Google Scholar] [CrossRef]
- Adams, L.A.; Lymp, J.F.; St Sauver, J.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef]
- Targher, G.; Bertolini, L.; Rodella, S.; Tessari, R.; Zenari, L.; Lippi, G.; Arcaro, G. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care 2007, 30, 2119–2121. [Google Scholar] [CrossRef]
- Tutunchi, H.; Naeini, F.; Mobasseri, M.; Ostadrahimi, A. Triglyceride glucose (TyG) index and the progression of liver fibrosis: A cross-sectional study. Clin. Nutr. ESPEN 2021, 44, 483–487. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. eBiomedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Furet, J.P.; Kong, L.C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Zadvornova, Y.; Heuman, D.M.; Hafeezullah, M.; Hoffmann, R.G.; Sanyal, A.J.; Saeian, K. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am. J. Gastroenterol. 2009, 104, 1130–1134. [Google Scholar] [CrossRef]
- Tsai, C.F.; Chen, M.H.; Wang, Y.P.; Chu, C.J.; Huang, Y.H.; Lin, H.C.; Hou, M.C.; Lee, F.Y.; Su, T.P.; Lu, C.L. Proton pump inhibitors increase risk for hepatic encephalopathy in patients with cirrhosis in A population study. Gastroenterology 2017, 152, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Iino, C.; Shimoyama, T.; Chinda, D.; Sakuraba, H.; Fukuda, S.; Nakaji, S. Influence of Helicobacter pylori infection and atrophic gastritis on the gut microbiota in a Japanese population. Digestion 2020, 101, 422–432. [Google Scholar] [CrossRef]
- Komiya, Y.; Shimomura, Y.; Higurashi, T.; Sugi, Y.; Arimoto, J.; Umezawa, S.; Uchiyama, S.; Matsumoto, M.; Nakajima, A. Patients with colorectal cancer have identical strains of Fusobacterium nucleatum in their colorectal cancer and oral cavity. Gut 2019, 68, 1335–1337. [Google Scholar] [CrossRef]
- Albhaisi, S.; Chowdhury, A.; Sanyal, A.J. Non-alcoholic fatty liver disease in lean individuals. JHEP Rep. 2019, 1, 329–341. [Google Scholar] [CrossRef]
- Seto, W.K.; Yuen, M.F. Nonalcoholic fatty liver disease in Asia: Emerging perspectives. J. Gastroenterol. 2017, 52, 164–174. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]

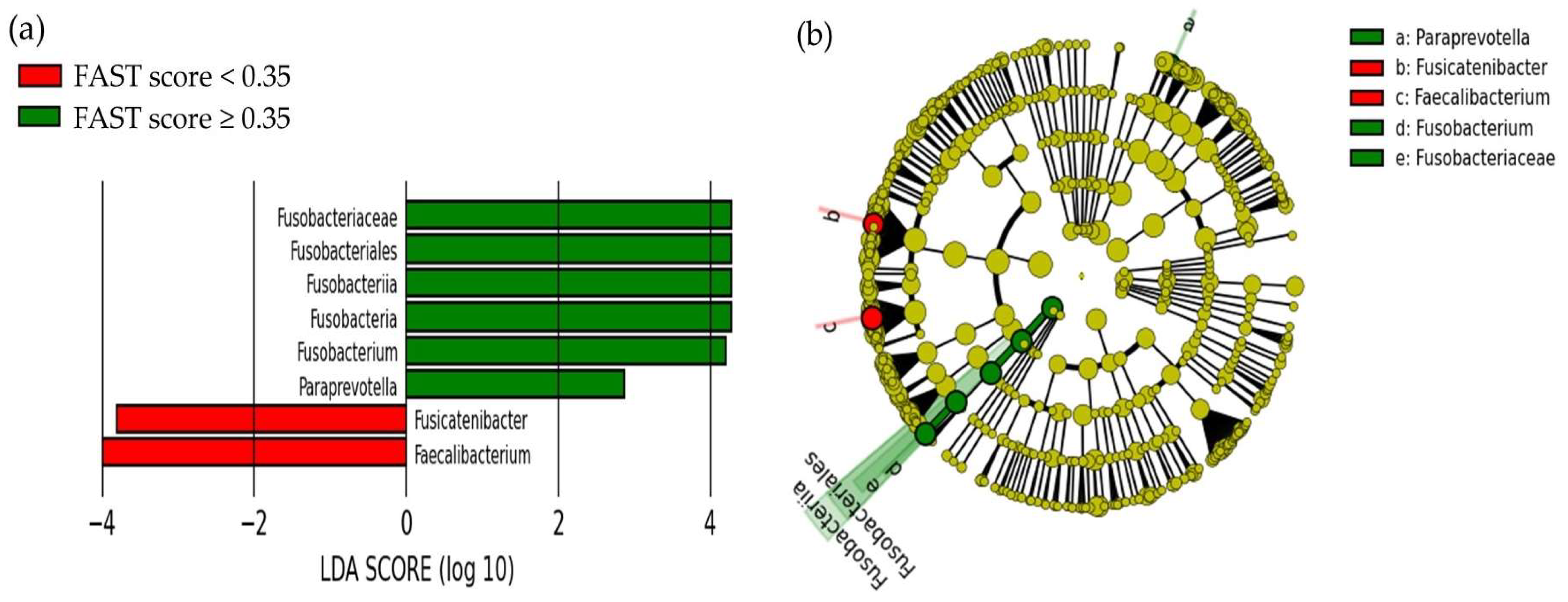
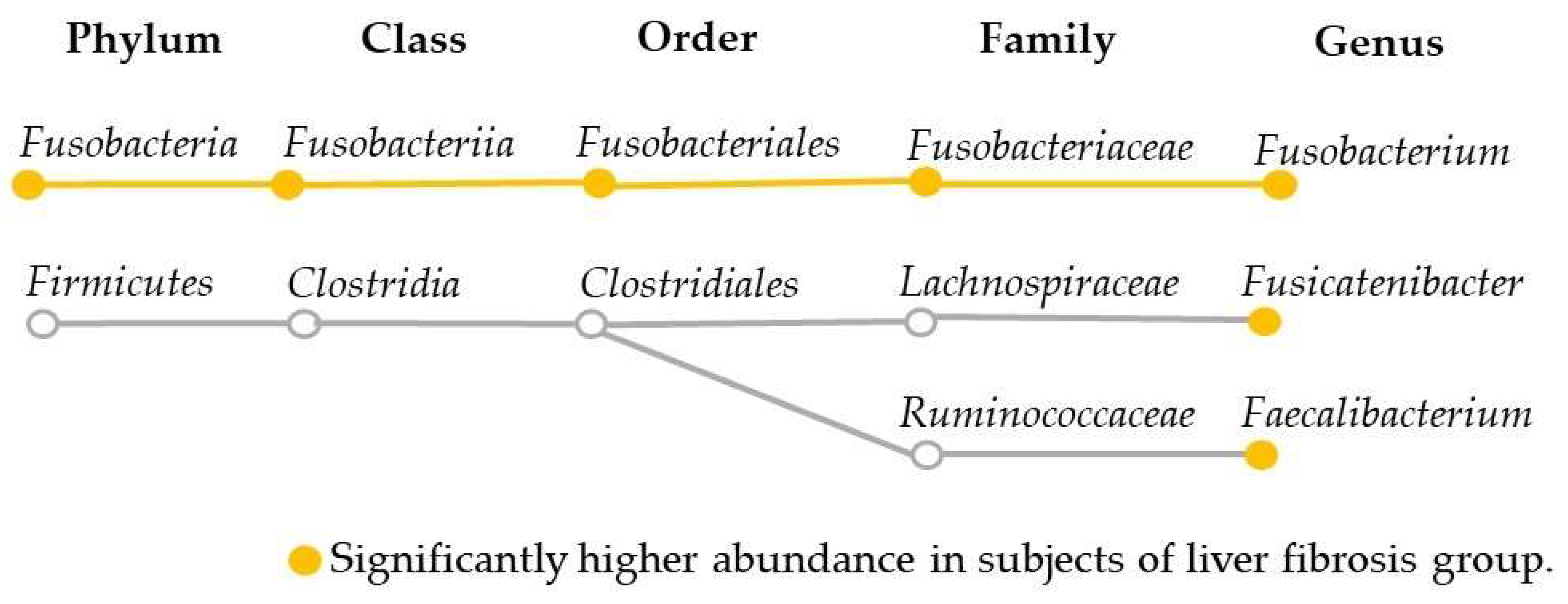
| FAST < 0.35 (n = 100) | FAST ≥ 0.35 (n = 25) | p-Value | |
|---|---|---|---|
| Sex (male/female) | 55:45 | 16:9 | 0.557 |
| Age (years) | 58.0 (45.8–68.0) | 64.0 (44.0–68.0) | 0.765 |
| BMI (kg/m2) | 25.3 (24.0–27.1) | 25.0 (24.1–25.6) | 0.412 |
| AST (U/L) | 21.0 (17.0–25.0) | 46.0 (40.0–55.0) | <0.001 |
| ALT (U/L) | 20.0 (15.8–26.0) | 63.0 (43.0–76.0) | <0.001 |
| γGTP (U/L) | 26.0 (18.0–40.0) | 87.0 (53.0–116.0) | <0.001 |
| HOMA-IR | 1.36 (1.06–2.10) | 2.13 (1.07–3.38) | 0.082 |
| Triglyceride (mg/dL) | 111.0 (66.5–173.8) | 114.0 (86.0–154.0) | 0.648 |
| HDL cholesterol (mg/dL) | 56.0 (47.0–64.0) | 57.0 (49.0–69.0) | 0.314 |
| LDL cholesterol (mg/dL) | 131.0 (110.0–144.3) | 125.0 (104.0–138.0) | 0.426 |
| FAST score | 0.07 (0.04–0.14) | 0.47 (0.41–0.59) | <0.001 |
| FIB-4 index | 0.94 (0.73–1.43) | 1.71 (0.97–1.96) | 0.001 |
| Fatty liver index | 39.6 (21.0–58.1) | 56.1 (35.5–71.1) | 0.037 |
| LS (kPa) | 4.75 (3.90–5.83) | 8.50 (5.80–11.0) | <0.001 |
| CAP (dB/m) | 252.5 (219.8–291.3) | 298.0 (257.0–331.0) | 0.003 |
| MAFLD (n) | 52 (52.0%) | 17 (68.0%) | 0.225 |
| MASLD (n) | 37 (37.0%) | 16 (64.0%) | 0.027 |
| Heavy alcohol drinker (n) | 7 (7.0%) | 4 (16.0%) | 0.305 |
| Positive HBs antigen (n) | 2 (2.0%) | 0 (0.0%) | 1.000 |
| Positive HCV antibody (n) | 0 (0.0%) | 0 (0.0%) | - |
| FAST Score | FIB-4 Index | Fatty Liver Index | ||||
|---|---|---|---|---|---|---|
| ρ | p-Value | ρ | p-Value | ρ | p-Value | |
| Phylum | ||||||
| Fusobacteria | 0.276 | 0.002 | 0.095 | 0.291 | 0.121 | 0.178 |
| Class | ||||||
| Fusobacteriia | 0.276 | 0.002 | 0.095 | 0.291 | 0.121 | 0.178 |
| Order | ||||||
| Fusobacteriales | 0.276 | 0.002 | 0.095 | 0.291 | 0.121 | 0.178 |
| Family | ||||||
| Fusobacteriaceae | 0.272 | 0.002 | 0.096 | 0.288 | 0.116 | 0.197 |
| Genus | ||||||
| Fusobacterium | 0.221 | 0.013 | 0.040 | 0.659 | 0.074 | 0.415 |
| Faecalibacterium | −0.191 | 0.033 | 0.074 | 0.413 | −0.218 | 0.015 |
| Fusicatenibacter | −0.160 | 0.074 | −0.113 | 0.208 | 0.063 | 0.485 |
| OR | 95% CI | p-Value | ||
|---|---|---|---|---|
| Fusobacterium | 1.20 | 1.01 | 1.43 | 0.033 |
| Faecalibacterium | 0.92 | 0.83 | 1.03 | 0.136 |
| Fusicatenibacter | 0.77 | 0.61 | 0.98 | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, S.; Iino, C.; Chinda, D.; Sasada, T.; Tateda, T.; Kaizuka, M.; Nomiya, H.; Igarashi, G.; Sawada, K.; Mikami, T.; et al. Effect of Liver Fibrosis on Oral and Gut Microbiota in the Japanese General Population Determined by Evaluating the FibroScan–Aspartate Aminotransferase Score. Int. J. Mol. Sci. 2023, 24, 13470. https://doi.org/10.3390/ijms241713470
Sato S, Iino C, Chinda D, Sasada T, Tateda T, Kaizuka M, Nomiya H, Igarashi G, Sawada K, Mikami T, et al. Effect of Liver Fibrosis on Oral and Gut Microbiota in the Japanese General Population Determined by Evaluating the FibroScan–Aspartate Aminotransferase Score. International Journal of Molecular Sciences. 2023; 24(17):13470. https://doi.org/10.3390/ijms241713470
Chicago/Turabian StyleSato, Satoshi, Chikara Iino, Daisuke Chinda, Takafumi Sasada, Tetsuyuki Tateda, Masatoshi Kaizuka, Hiroki Nomiya, Go Igarashi, Kaori Sawada, Tatsuya Mikami, and et al. 2023. "Effect of Liver Fibrosis on Oral and Gut Microbiota in the Japanese General Population Determined by Evaluating the FibroScan–Aspartate Aminotransferase Score" International Journal of Molecular Sciences 24, no. 17: 13470. https://doi.org/10.3390/ijms241713470






