The Rationale for Sulforaphane Favourably Influencing Gut Homeostasis and Gut–Organ Dysfunction: A Clinician’s Hypothesis
Abstract
:1. Introduction
Author’s Preamble
2. Background to the Hypothesis—The Case for Sulforaphane
The Hypothesis
3. The Emerging Role of the Gut Microbiome in Human Health
3.1. The Growing Issue of Food Intolerance
3.2. Lessons from Nature’s Inbuilt Cellular Mechanisms
4. The Evolution of the Hypothesis
4.1. The Evolution of Strategies for Addressing the Unanswered Questions
4.2. Shifting the Emphasis from the Microbe towards the Host
5. Focusing on Sulforaphane’s Clinically Relevant Properties
5.1. Unravelling the Mechanisms of Action
5.2. Nutrigenomics in Action—Enter Nrf2
5.3. Nrf2 and the Concept of Upstream Effects
5.4. SFN as a Nature-Compatible Strategy for Harnessing the Power of Nutrigenomics
5.5. Sulforaphane—A Potent Multifunctional Phytonutrient
5.6. Collaborative Contributions of SFN and the Microbiota to Gut Homeostasis
5.7. The Role of Sulforaphane in Cellular Defence Mechanisms
5.8. Harnessing-Compatible Cellular Defence Mechanisms
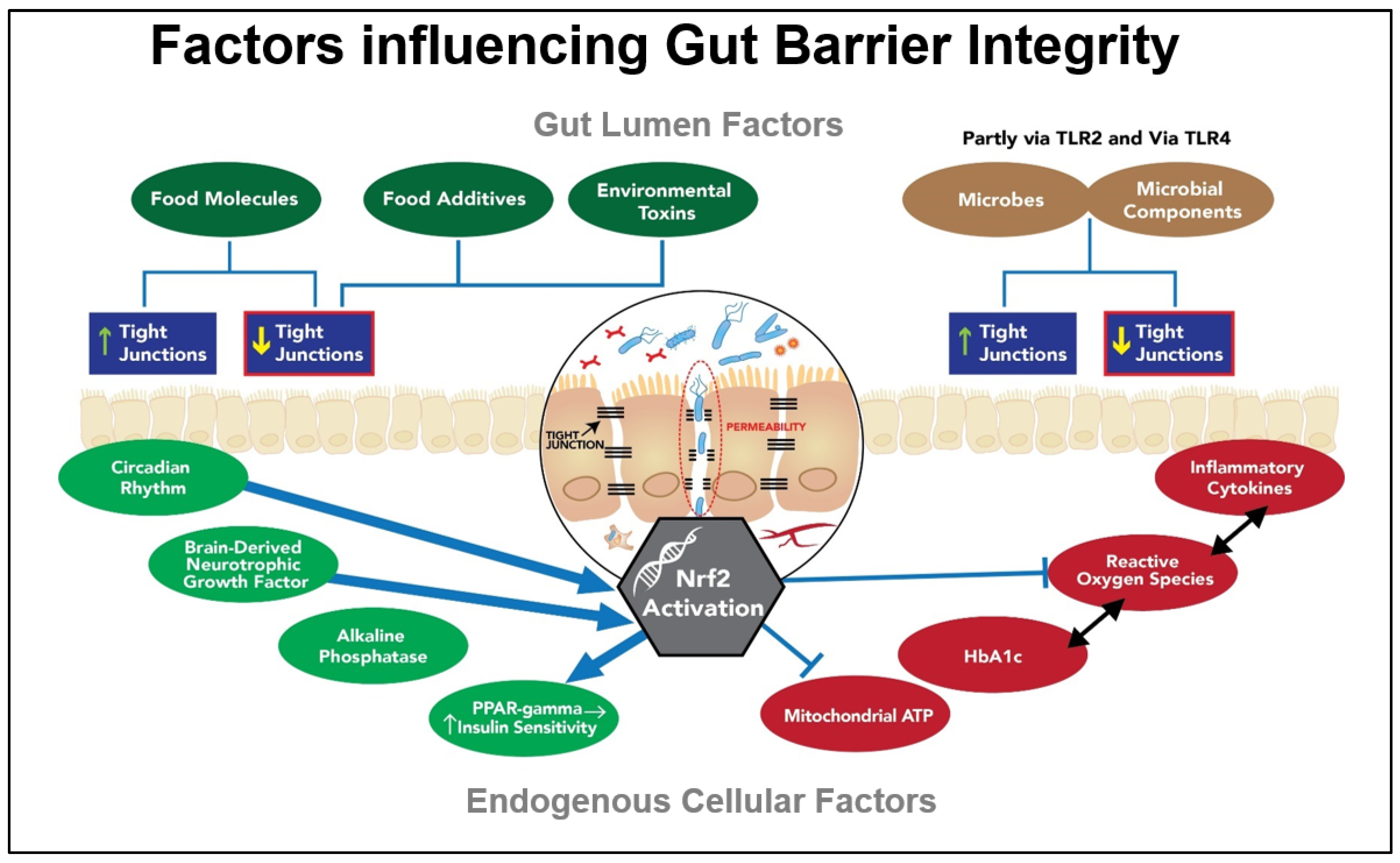
6. The Gut Barrier
6.1. Tight Junctions as Critical Components of the Gut Barrier
6.2. Exogenous Factors Impacting the Tight Junctions
6.3. Endogenous Factors Impacting the Gut Barrier and Beyond
7. Restoring Homeostasis to the Gut Ecosystem
7.1. Probiotics—Longstanding Therapy or Recent Innovation?
7.2. Symbiosis between the Host and Luminal Microbes
8. Determining an Effective Gut Repair Strategy
8.1. The Potential Impact of Sulforaphane on Restoring Gut Homeostasis
8.2. Relevant Mechanisms
- SFN INHIBITS GRAM-NEGATIVE BACTERIAL LPS BINDING: SFN inhibits the action of LPS in binding to the epithelial receptor, TLR4, thereby reducing the signalling cascade that leads to the induction of pro-inflammatory mediators via Nf-kB. This is one of several ways in which SFN can downregulate uncontrolled inflammation.
- ENHANCED CYTOPROTECTION: SFN activates epithelial cell Nrf2, thereby inducing around 200 cytoprotective genes; the effects of these include stabilising the gut barrier. Activating the Nrf2 pathway reduces oxidative stress and uncontrolled inflammation while simultaneously downregulating the pro-inflammatory transcription factor NF-kB. In so doing, SFN helps to restore gut immune homeostasis.
- NORMALISED GASTRIC MOTILITY (suppression of gastroparesis): Loss of antioxidant gene expression has been shown to contribute to the development of gastroparesis, leading to Nrf2 being considered to be a potential therapeutic target [106].
- STABLISATION OF GUT BARRIER: SFN may beneficially impact one or more of the endogenous factors that contribute to dysfunctional gut barriers. Of significance are the imbalances in inflammation-redox status and elevated HbA1c.
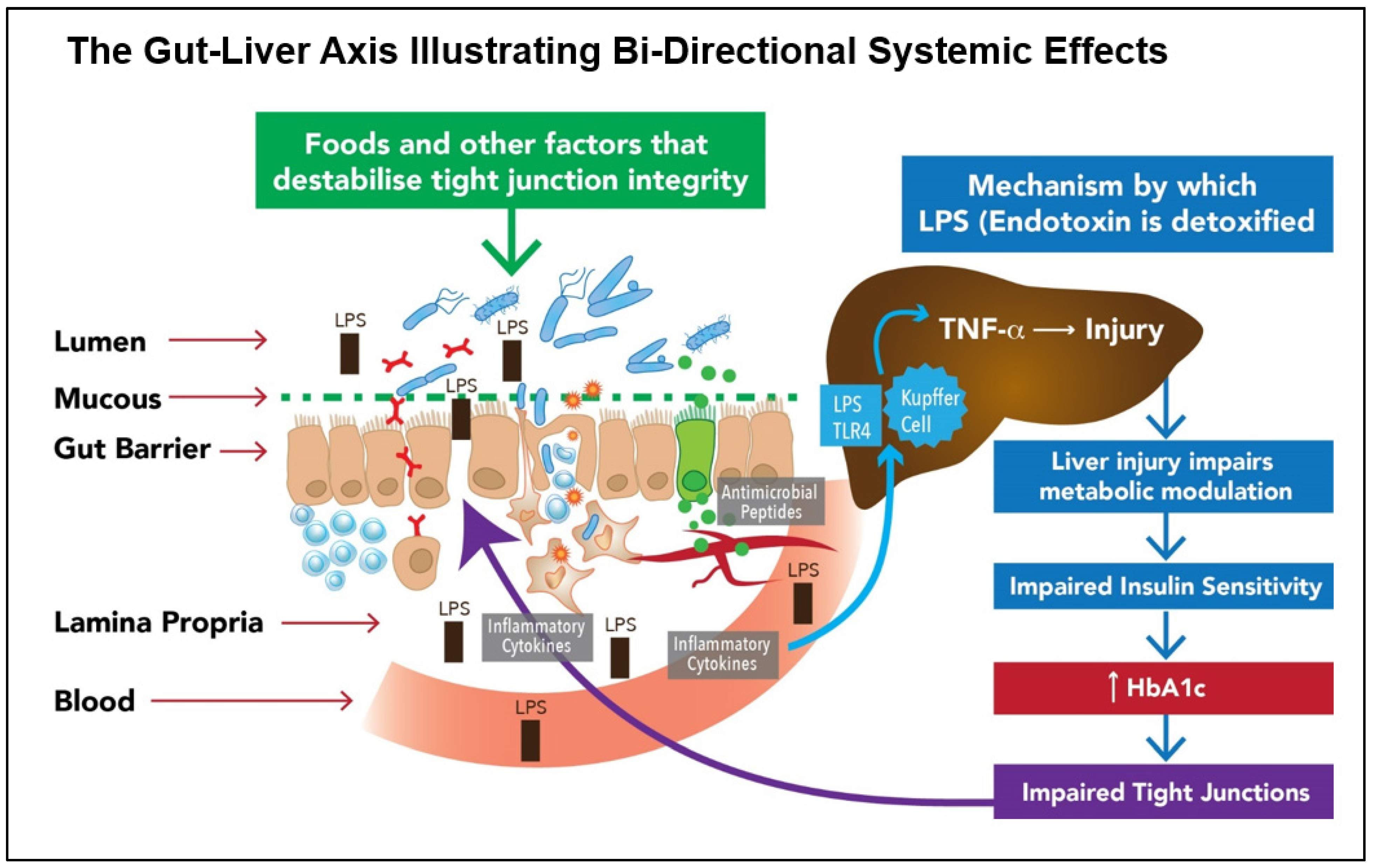
- SYSTEMIC EFFECTS: Where bacterial die-off may occur in dysbiotic individuals with impaired barrier function, potentially toxic molecules may travel via the portal circulation to the liver, where they must be detoxified. If this process is too rapid, unpleasant systemic symptoms may result. (Reducing the dose and frequency of SFN was observed by the author to ameliorate this effect.)
- ANTIMICROBIAL EFFECT: Of the Nrf2 target genes, the expression of antimicrobial beta-defensin is relevant. Endogenously synthesised antimicrobials that include beta-defensin can selectively target pathobionts or other undesirable microbes without adversely affecting the commensals [107].
- QUORUM SENSING: Biofilm degradation: In vitro studies have shown that SFN can degrade periodontal biofilms that can prevent the resolution of infections, thereby exposing the microbes to attack from elements of both the innate and adaptive immune system. Mucosal biofilm communities are also known to inhabit the human intestinal tract [108], with the potential for SFN to disrupt these biofilms. In so doing, a significant population of microbes is released into the intestinal mucosa, upregulating and potentially overloading detoxification pathways [60,109,110]. We hypothesise that this may partially explain why guided introduction of SFN is important in individuals suspected of harbouring a dysbiotic population of gut microbes.
- UREASE INHIBITION: SFN is a urease inhibitor and has been shown to block the ability of H. pylori to produce urease, the enzyme responsible for the development of gastric inflammation and potential gastric tumour development. Many other pathogens/pathobionts are urease positive, including Klebsiella, Staphlococcus aureas, E. coli, Morganella, Pseudomonas, and many others. Mycobacteria (mould) are also urease positive. It is not known whether urease-positive organisms other than H. pylori are responsive to SFN [60,110,111].

9. Case Studies
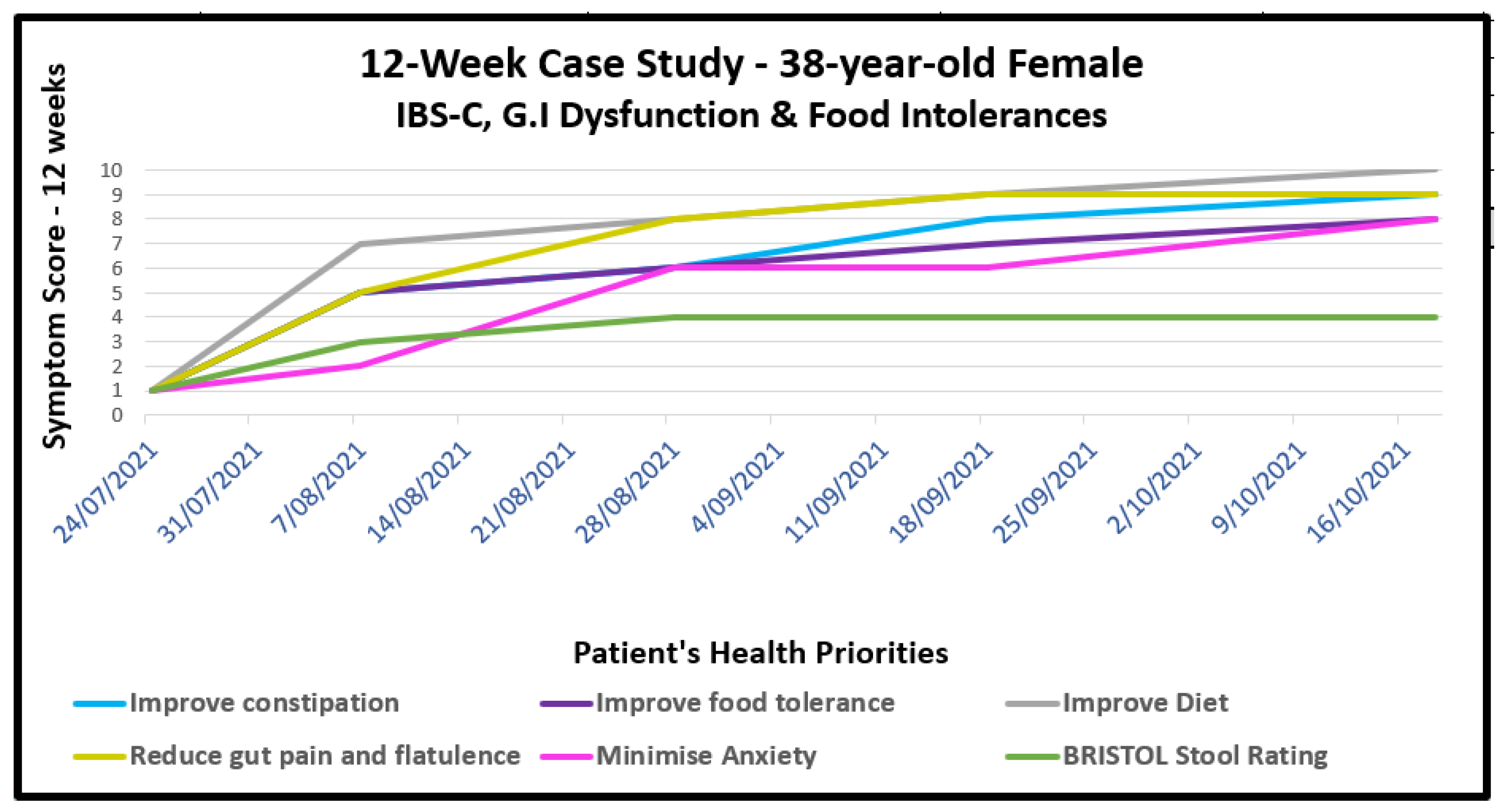
9.1. Gastrointestinal Dysfunction with Food Intolerances
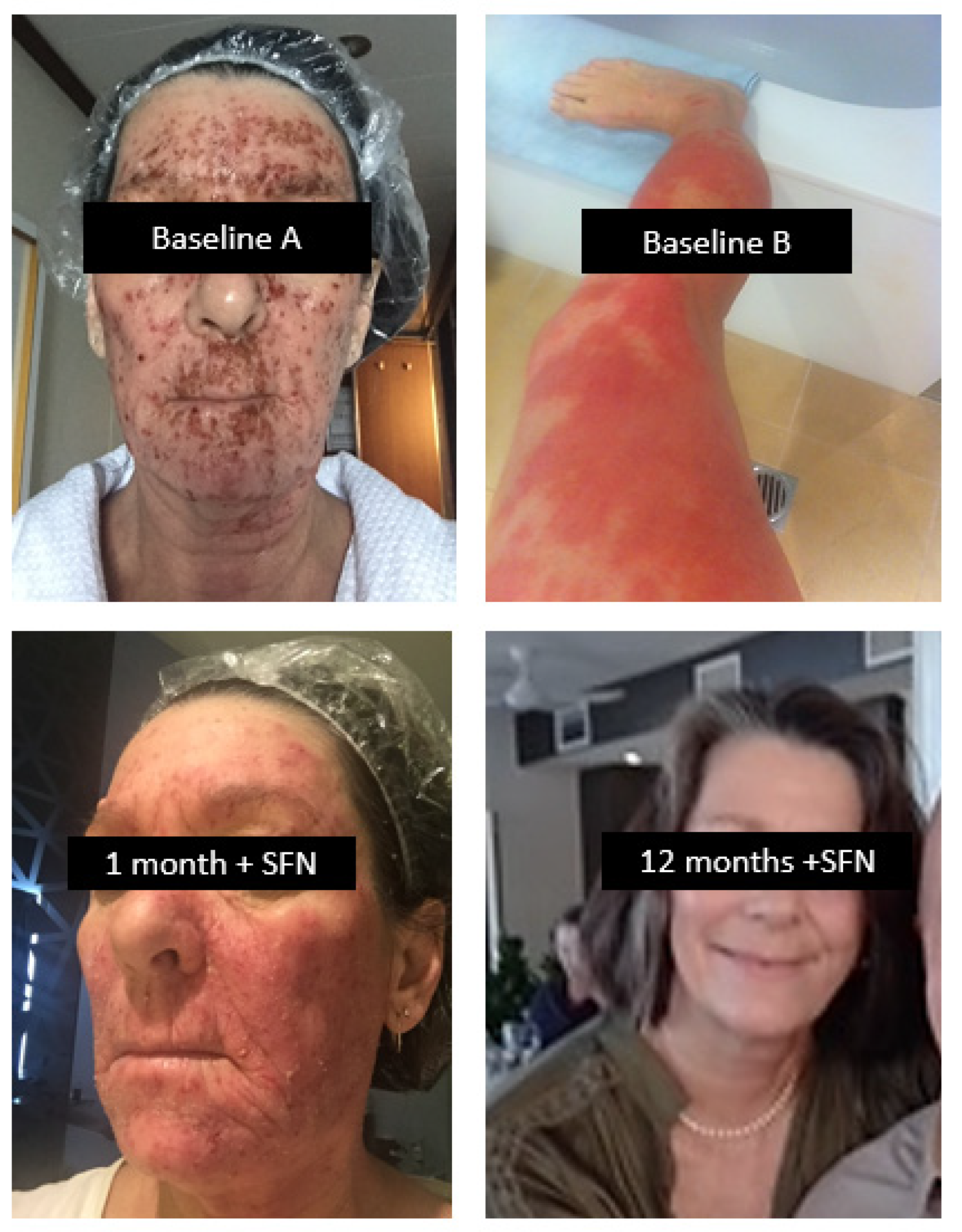
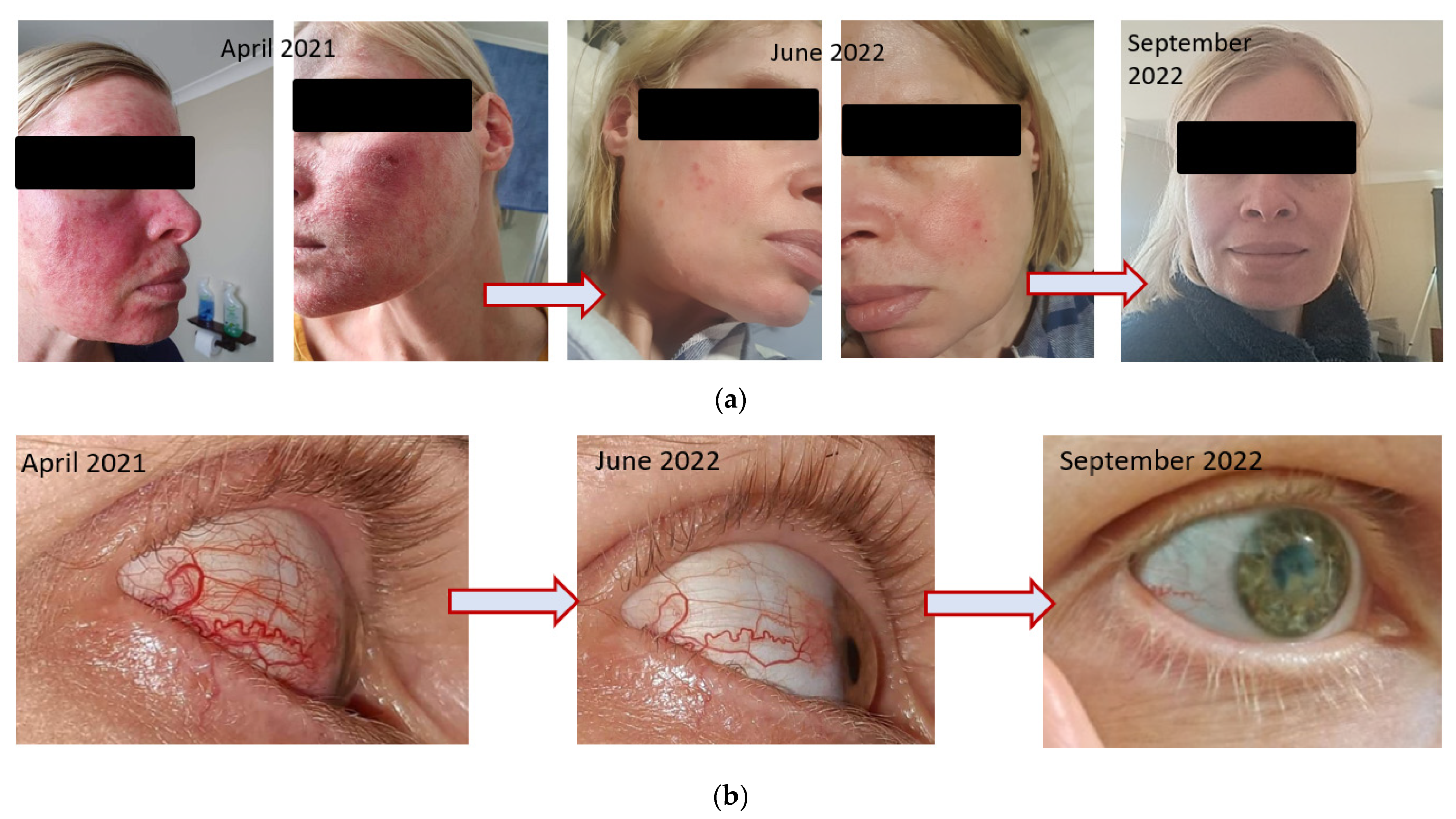
9.2. Dermatological Conditions with and without Comorbidities
10. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meldrum, J.M. What is nutritional medicine? Nutr. Health 1993, 9, 130–150. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Dumitrascu, D.; Fukudo, S.; Gerson, C.; Ghoshal, U.C.; Gwee, K.A.; Hungin, A.P.S.; Kang, J.-Y.; Minhu, C.; Schmulson, M.; et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: A Rome Foundation working team literature review. Gut 2017, 66, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Soncini, M.; Stasi, C.; Satta, P.U.; Milazzo, G.; Bianco, M.; Leandro, G.; Montalbano, L.M.; Muscatiello, N.; Monica, F.; Galeazzi, F.; et al. IBS clinical management in Italy: The AIGO survey. Dig. Liver Dis. 2019, 51, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Hawrelak, J.A.; Cattley, T.; Myers, S.P. Essential oils in the treatment of intestinal dysbiosis: A preliminary in vitro study. Altern. Med. Rev. 2009, 14, 380–384. [Google Scholar] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, T.A.; Fahey, J.W.; Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Stephenson, K.K.; Wade, K.L.; Ye, L.; Talalay, P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: A clinical phase I study. Nutr. Cancer 2006, 55, 53–62. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane: Translational research from laboratory bench to clinic. Nutr. Rev. 2013, 71, 709–726. [Google Scholar] [CrossRef]
- Ahlawat, S.; Asha; Sharma, K. Gut–organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Leech, B.; Schloss, J.; Steel, A. Treatment Interventions for the Management of Intestinal Permeability: A Cross-Sectional Survey of Complementary and Integrative Medicine Practitioners. J. Altern. Complement Med. 2019, 25, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Petersen, F.C.; Shekhar, S. Commensal Bacteria: An Emerging Player in Defense Against Respiratory Pathogens. Front. Immunol. 2019, 10, 1203. [Google Scholar] [CrossRef]
- Maier, L.; Goemans, C.V.; Wirbel, J.; Kuhn, M.; Eberl, C.; Pruteanu, M.; Müller, P.; Garcia-Santamarina, S.; Cacace, E.; Zhang, B.; et al. Unravelling the collateral damage of antibiotics on gut bacteria. Nature 2021, 599, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Losa, R.; Zweifel, B.; Wallace, R.J. Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology 2012, 158 Pt 11, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Sasso, J.M.; Ammar, R.M.; Tenchov, R.; Lemmel, S.; Kelber, O.; Grieswelle, M.; Zhou, Q.A. Gut Microbiome-Brain Alliance: A Landscape View into Mental and Gastrointestinal Health and Disorders. ACS Chem. Neurosci. 2023, 14, 1717–1763. [Google Scholar] [CrossRef]
- Shulpekova, Y.O.; Nechaev, V.M.; Popova, I.R.; Deeva, T.A.; Kopylov, A.T.; Malsagova, K.A.; Kaysheva, A.L.; Ivashkin, V.T. Food Intolerance: The Role of Histamine. Nutrients 2021, 13, 3207. [Google Scholar] [CrossRef] [PubMed]
- Panacer, K.; Whorwell, P.J. Dietary Lectin exclusion: The next big food trend? World J. Gastroenterol 2019, 25, 2973–2976. [Google Scholar] [CrossRef]
- Gray, P.E.; Mehr, S.; Katelaris, C.H.; Wainstein, B.K.; Star, A.; Campbell, D.; Joshi, P.; Wong, M.; Campbell, D.; Joshi, P.; et al. Salicylate elimination diets in children: Is food restriction supported by the evidence? Med. J. Aust. 2013, 198, 600–602. [Google Scholar] [CrossRef]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients 2020, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Petroski, W.; Minich, D.M. Is There Such a Thing as "Anti-Nutrients"? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef]
- Vojdani, A.; Afar, D.; Vojdani, E. Reaction of Lectin-Specific Antibody with Human Tissue: Possible Contributions to Autoimmunity. J. Immunol. Res. 2020, 2020, 1438957. [Google Scholar] [CrossRef] [PubMed]
- Hrubisko, M.; Danis, R.; Huorka, M.; Wawruch, M. Histamine Intolerance-The More We Know the Less We Know. A Review. Nutrients 2021, 13, 2228. [Google Scholar] [CrossRef]
- Ermer, T.; Nazzal, L.; Tio, M.C.; Waikar, S.; Aronson, P.S.; Knauf, F. Oxalate homeostasis. Nat. Rev. Nephrol. 2023, 19, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Tan, H.; Kaiko, G.E. Role of the Intestinal Epithelium and Its Interaction with the Microbiota in Food Allergy. Front. Immunol. 2020, 11, 604054. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.-Y.; Ko, H.-J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxidative Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef]
- Houghton, C.A. Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxidative Med. Cell. Longev. 2019, 2019, 2716870. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.; Merrifield, C.A.; Hutkins, R. Probiotics for human use. Nutr. Bull. 2018, 43, 212–225. [Google Scholar] [CrossRef]
- Grazul, H.; Kanda, L.L.; Gondek, D. Impact of probiotic supplements on microbiome diversity following antibiotic treatment of mice. Gut Microbes 2016, 7, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Bezkorovainy, A. Probiotics: Determinants of survival and growth in the gut. Am. J. Clin. Nutr. 2001, 73, 399s–405s. [Google Scholar] [CrossRef]
- Zangara, M.T.; McDonald, C. How diet and the microbiome shape health or contribute to disease: A mini-review of current models and clinical studies. Exp. Biol. Med. 2019, 244, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.-Z.; Federici, S.; et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics Improved by Autologous FMT. Cell 2018, 174, 1406–1423. [Google Scholar] [CrossRef]
- Walter, J. Ecological role of lactobacilli in the gastrointestinal tract: Implications for fundamental and biomedical research. Appl. Environ. Microbiol. 2008, 74, 4985–4996. [Google Scholar] [CrossRef] [PubMed]
- Canny, G.; Swidsinski, A.; McCormick, B.A. Interactions of intestinal epithelial cells with bacteria and immune cells: Methods to characterize microflora and functional consequences. Methods Mol. Biol. 2006, 341, 17–35. [Google Scholar]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Baumler, A.J. Colonocyte metabolism shapes the gut microbiota. Science 2018, 362, eaat9076. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef]
- Abreu, M.T. Toll-like receptor signalling in the intestinal epithelium: How bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 2010, 10, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shu, X.-O.; Xiang, Y.-B.; Yang, G.; Li, H.; Gao, J.; Cai, H.; Gao, Y.-T.; Zheng, W. Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality. Am. J. Clin. Nutr. 2011, 94, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Talalay, P.; Fahey, J.W. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J. Nutr. 2001, 131 (Suppl. S11), 3027S–3033S. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, Y.; Gatbonton-Schwager, T.N.; McCallum, M.L.; Kensler, T.W. Current Landscape of NRF2 Biomarkers in Clinical Trials. Antioxidants 2020, 9, 716. [Google Scholar] [CrossRef]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef] [PubMed]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Hosseinpanah, F.; Hedayati, M.; Hosseinpour-Niazi, S.; Azizi, F. Broccoli sprouts reduce oxidative stress in type 2 diabetes: A randomized double-blind clinical trial. Eur. J. Clin. Nutr. 2011, 65, 972–977. [Google Scholar] [CrossRef]
- Surh, Y.J.; Kundu, J.K.; Na, H.K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008, 74, 1526–1539. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, T.T.; Monteleone, G. Immunity, inflammation, and allergy in the gut. Science 2005, 307, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Thimmulappa, R.K.; Mai, K.H.; Srisuma, S.; Kensler, T.W.; Yamamoto, M.; Biswal, S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002, 62, 5196–5203. [Google Scholar] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Kirpich, I.A.; Parajuli, D.; McClain, C.J. The gut microbiome in NAFLD and ALD. Clin. Liver Dis. 2015, 6, 55–58. [Google Scholar] [CrossRef]
- Velloso, L.A.; Folli, F.; Saad, M.J. TLR4 at the Crossroads of Nutrients, Gut Microbiota, and Metabolic Inflammation. Endocr. Rev. 2015, 36, 245–271. [Google Scholar] [CrossRef]
- Eren, E.; Tufekci, K.U.; Isci, K.B.; Tastan, B.; Genc, K.; Genc, S. Sulforaphane Inhibits Lipopolysaccharide-Induced Inflammation, Cytotoxicity, Oxidative Stress, and miR-155 Expression and Switches to Mox Phenotype through Activating Extracellular Signal-Regulated Kinase 1/2-Nuclear Factor Erythroid 2-Related Factor 2/Antioxidant Response Element Pathway in Murine Microglial Cells. Front. Immunol. 2018, 9, 36. [Google Scholar] [PubMed]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-kappaB Inhibition and Nrf2/HO-1 Activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Stephenson, K.K.; Wade, K.L.; Talalay, P. Urease from Helicobacter pylori is inactivated by sulforaphane and other isothiocyanates. Biochem. Biophys. Res. Commun. 2013, 435, 1–7. [Google Scholar] [CrossRef]
- Tobin, I.; Zhang, G. Regulation of Host Defense Peptide Synthesis by Polyphenols. Antibiotics 2023, 12, 660. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.S.; Kim, Y.S.; Park, Z.Y.; Kim, S.Y.; Choi, N.Y.; Joung, S.M.; Seo, G.A.; Lim, K.-M.; Kwak, M.K.; Hwang, D.H.; et al. Sulforaphane suppresses oligomerization of TLR4 in a thiol-dependent manner. J. Immunol. 2010, 184, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Lee, J.; Lee, H.K.; Cho, S.; Lim, J.-H.; Choi, Y.; Pak, S.; Jeong, H.-J. Sulforaphane mitigates mast cell-mediated allergic inflammatory reactions in in silico simulation and in vitro models. Immunopharmacol. Immunotoxicol. 2020, 42, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Jadkauskaite, L.; Bahri, R.; Farjo, N.; Farjo, B.; Jenkins, G.; Bhogal, R.; Haslam, I.; Bulfone-Paus, S.; Paus, R. Nuclear factor (erythroid-derived 2)-like-2 pathway modulates substance P-induced human mast cell activation and degranulation in the hair follicle. J. Allergy Clin. Immunol. 2018, 142, 1331–1333. [Google Scholar] [CrossRef]
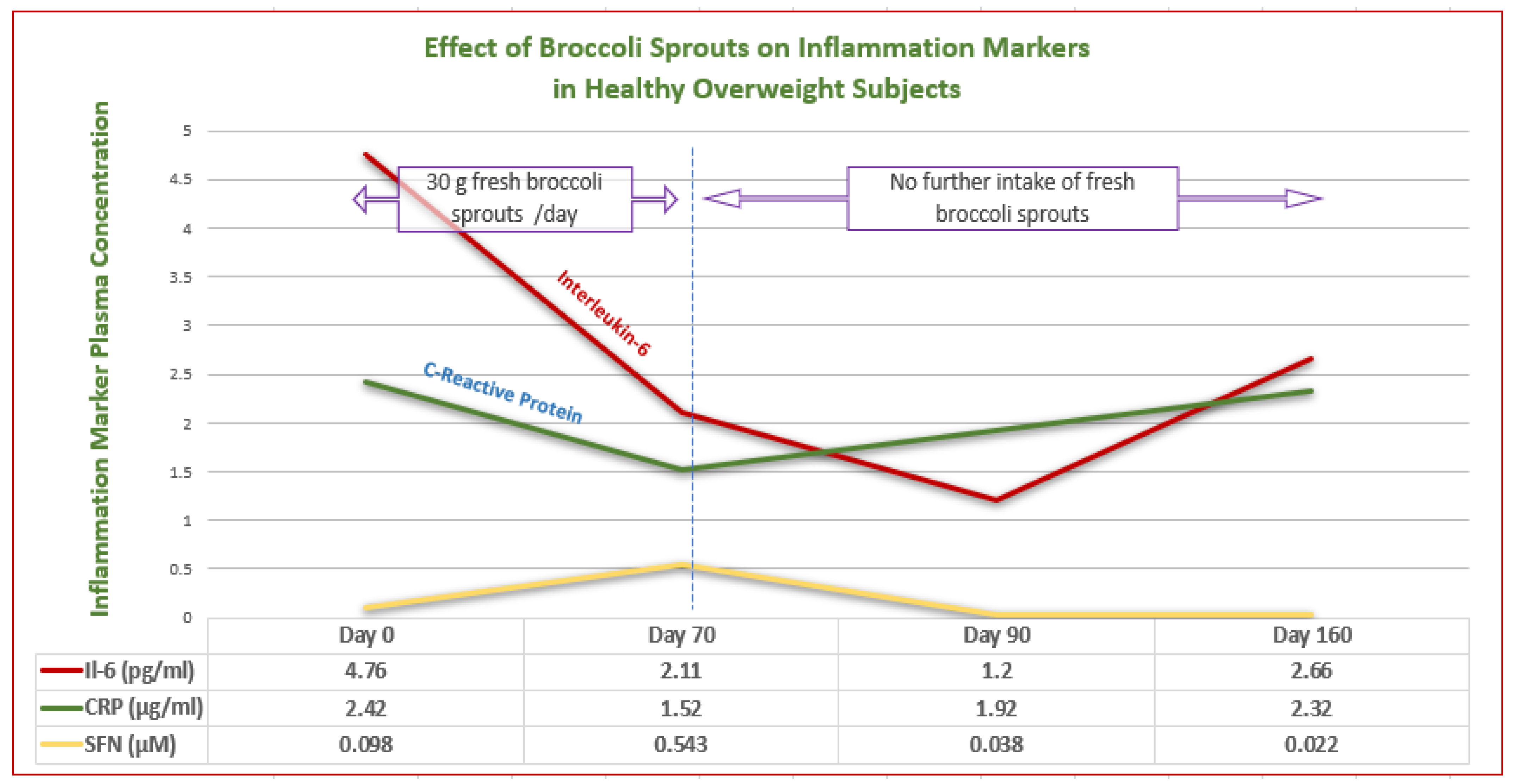
- López-Chillón, M.T.; Carazo-Díaz, C.; Prieto-Merino, D.; Zafrilla, P.; Moreno, D.A.; Villaño, D. Effects of long-term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clin. Nutr. 2019, 38, 745–752. [Google Scholar] [CrossRef]
- Hanlon, N.; Coldham, N.; Gielbert, A.; Kuhnert, N.; Sauer, M.J.; King, L.J.; Ioannides, C. Absolute bioavailability and dose-dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in rat. Br. J. Nutrition. 2007, 99, 559–564. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665. [Google Scholar] [CrossRef]
- Barrett, K.E.; Minor, J.R.; Metcalfe, D.D. Histamine secretion induced by N-acetyl cysteine. Agents Actions 1985, 16, 144–146. [Google Scholar] [CrossRef]
- Leitner, R.; Zoernpfenning, E.; Missbichler, A. Evaluation of the inhibitory effect of various drugs/active ingredients on the activity of human diamine oxidase in vitro. Clin. Transl. Allergy 2014, 4 (Suppl. S3). [Google Scholar] [CrossRef]
- Hermsdorff, H.H.; Zulet, M.A.; Puchau, B.; Martinez, J.A. Fruit and vegetable consumption and proinflammatory gene expression from peripheral blood mononuclear cells in young adults: A translational study. Nutr. Metab. 2010, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.S.; Yu, S.; Kong, A.N. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Swierczynski, M.; Fichna, J.; Piechota-Polanczyk, A. The Nrf2 in the pathophysiology of the intestine: Molecular mechanisms and therapeutic implications for inflammatory bowel diseases. Pharmacol. Res. 2021, 163, 105243. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Leech, B.; McIntyre, E.; Steel, A.; Sibbritt, D. Risk factors associated with intestinal permeability in an adult population: A systematic review. Int. J. Clin. Pract. 2019, 73, e13385. [Google Scholar] [CrossRef]
- De Santis, S.; Cavalcanti, E.; Mastronardi, M.; Jirillo, E.; Chieppa, M. Nutritional Keys for Intestinal Barrier Modulation. Front. Immunol. 2015, 6, 612. [Google Scholar] [CrossRef]
- Davis, W. Lose the Wheat, Lose the Weight: Banish Your Wheat Belly, Feel Better Than Ever, and Turbocharge Your Health; Emmaus, P.A., Ed.; Rodale: Emmaus, PA, USA, 2012; Volume xii, p. 356. [Google Scholar]
- Choung, R.S.; Unalp-Arida, A.; Ruhl, C.E.; Brantner, T.L.; Everhart, J.E.; Murray, J.A. Less Hidden Celiac Disease But Increased Gluten Avoidance Without a Diagnosis in the United States: Findings From the National Health and Nutrition Examination Surveys From 2009 to 2014. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Golley, S.; Corsini, N.; Topping, D.; Morell, M.; Mohr, P. Motivations for avoiding wheat consumption in Australia: Results from a population survey. Public. Health Nutr. 2015, 18, 490–499. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef]
- Houghton, C.A. Chapter 14-The gut microbiome: Its role in brain health. In Nutraceuticals in Brain Health and Beyond; Ghosh, D., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 193–212. [Google Scholar]
- Cantorna, M.T.; Snyder, L.; Arora, J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 184–192. [Google Scholar] [CrossRef]
- Gleeson, J.P. Diet, food components and the intestinal barrier. Nutr. Bull. 2017, 42, 123–131. [Google Scholar] [CrossRef]
- El Khoury, D.; Balfour-Ducharme, S.; Joye, I.J. A Review on the Gluten-Free Diet: Technological and Nutritional Challenges. Nutrients 2018, 10, 1410. [Google Scholar] [CrossRef]
- Lerner, A.; Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 2015, 14, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Moonwiriyakit, A.; Pathomthongtaweechai, N.; Steinhagen, P.R.; Chantawichitwong, P.; Satianrapapong, W.; Pongkorpsakol, P. Tight junctions: From molecules to gastrointestinal diseases. Tissue Barriers 2023, 11, 2077620. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, S.; Ivanov, A.I. Disruption of the epithelial barrier during intestinal inflammation: Quest for new molecules and mechanisms. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Segre, J.A. Signaling in Host-Associated Microbial Communities. Cell 2016, 164, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
- Sanlier, N.; Gokcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar]
- Beck, B.R.; Park, G.S.; Lee, Y.H.; Im, S.; Jeong, D.Y.; Kang, J. Whole Genome Analysis of Lactobacillus plantarum Strains Isolated from Kimchi and Determination of Probiotic Properties to Treat Mucosal Infections by Candida albicans and Gardnerella vaginalis. Front. Microbiol. 2019, 10, 433. [Google Scholar] [CrossRef]
- Pascal, M.; Perez-Gordo, M.; Caballero, T.; Escribese, M.M.; Longo, M.N.L.; Luengo, O.; Manso, L.; Matheu, V.; Seoane, E.; Zamorano, M.; et al. Microbiome and Allergic Diseases. Front. Immunol. 2018, 9, 1584. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Malard, F.; Dore, J.; Gaugler, B.; Mohty, M. Introduction to host microbiome symbiosis in health and disease. Mucosal Immunol. 2021, 14, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Burgueño, J.F.; Abreu, M.T. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Hug, H.; Mohajeri, M.H.; La Fata, G. Toll-Like Receptors: Regulators of the Immune Response in the Human Gut. Nutrients 2018, 10, 203. [Google Scholar] [CrossRef]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V.; Karakan, T.; Karatas, A. Strain-specific and outcome-specific efficacy of probiotics for the treatment of irritable bowel syndrome: A systematic review and meta-analysis. EClinicalMed. 2021, 41, 101154. [Google Scholar] [CrossRef] [PubMed]
- Boyle, R.J.; Robins-Browne, R.M.; Tang, M.L. Probiotic use in clinical practice: What are the risks? Am. J. Clin. Nutr. 2006, 83, 1256–1264. [Google Scholar] [CrossRef]
- Khalesi, S.; Bellissimo, N.; Vandelanotte, C.; Williams, S.; Stanley, D.; Irwin, C. A review of probiotic supplementation in healthy adults: Helpful or hype? Eur. J. Clin. Nutr. 2019, 73, 24–37. [Google Scholar] [CrossRef]
- Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Yoshikai, Y.; Tsuru, T. Daily intake of heat-killed Lactobacillus plantarum L-137 augments acquired immunity in healthy adults. J. Nutr. 2006, 136, 3069–3073. [Google Scholar] [CrossRef]
- Hirose, Y.; Yamamoto, Y.; Yoshikai, Y.; Murosaki, S. Oral intake of heat-killed Lactobacillus plantarum L-137 decreases the incidence of upper respiratory tract infection in healthy subjects with high levels of psychological stress. J. Nutr. Sci. 2013, 2, e39. [Google Scholar] [CrossRef]
- Pique, N.; Berlanga, M.; Minana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Johnston, D.G.; Corr, S.C. Toll-Like Receptor Signalling and the Control of Intestinal Barrier Function. Methods Mol. Biol. 2016, 1390, 287–300. [Google Scholar] [PubMed]
- Mukhopadhyay, S.; Sekhar, K.R.; Hale, A.B.; Channon, K.M.; Farrugia, G.; Freeman, M.L.; Gangula, P.R. Loss of NRF2 impairs gastric nitrergic stimulation and function. Free Radic. Biol. Med. 2011, 51, 619–625. [Google Scholar] [CrossRef]
- Brandl, K.; Kumar, V.; Eckmann, L. Gut-liver axis at the frontier of host-microbial interactions. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G413–G419. [Google Scholar] [CrossRef]
- Macfarlane, S.; Bahrami, B.; Macfarlane, G.T. Mucosal biofilm communities in the human intestinal tract. Adv. Appl. Microbiol. 2011, 75, 111–143. [Google Scholar]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef]
- Haristoy, X.; Fahey, J.W.; Scholtus, I.; Lozniewski, A. Evaluation of the antimicrobial effects of several isothiocyanates on Helicobacter pylori. Planta Med. 2005, 71, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Yanaka, A.; Fahey, J.W.; Fukumoto, A.; Nakayama, M.; Inoue, S.; Zhang, S.; Tauchi, M.; Suzuki, H.; Hyodo, I.; Yamamoto, M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev. Res. 2009, 2, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.; Shukla, R.; Srivastava, D.; Ghoshal, U.C. Irritable Bowel Syndrome, Particularly the Constipation-Predominant Form, Involves an Increase in Methanobrevibacter smithii, Which Is Associated with Higher Methane Production. Gut Liver 2016, 10, 932–938. [Google Scholar] [CrossRef]
- Inoue, R.; Sakaue, Y.; Kawada, Y.; Tamaki, R.; Yasukawa, Z.; Ozeki, M.; Ueba, S.; Sawai, C.; Nonomura, K.; Tsukahara, T.; et al. Dietary supplementation with partially hydrolyzed guar gum helps improve constipation and gut dysbiosis symptoms and behavioral irritability in children with autism spectrum disorder. J. Clin. Biochem. Nutr. 2019, 64, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Helmick, C.G.; Lee-Han, H.; Hirsch, S.C.; Baird, T.L.; Bartlett, C.L. Prevalence of psoriasis among adults in the U.S.: 2003–2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am. J. Prev. Med. 2014, 47, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Mde, F.; Rocha Bde, O.; Duarte, G.V. Psoriasis: Classical and emerging comorbidities. Bras. Dermatol. 2015, 90, 9–20. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houghton, C.A. The Rationale for Sulforaphane Favourably Influencing Gut Homeostasis and Gut–Organ Dysfunction: A Clinician’s Hypothesis. Int. J. Mol. Sci. 2023, 24, 13448. https://doi.org/10.3390/ijms241713448
Houghton CA. The Rationale for Sulforaphane Favourably Influencing Gut Homeostasis and Gut–Organ Dysfunction: A Clinician’s Hypothesis. International Journal of Molecular Sciences. 2023; 24(17):13448. https://doi.org/10.3390/ijms241713448
Chicago/Turabian StyleHoughton, Christine A. 2023. "The Rationale for Sulforaphane Favourably Influencing Gut Homeostasis and Gut–Organ Dysfunction: A Clinician’s Hypothesis" International Journal of Molecular Sciences 24, no. 17: 13448. https://doi.org/10.3390/ijms241713448




