Sustainable Solution for Plastic Pollution: Upcycling Waste Polypropylene Masks for Effective Oil-Spill Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterizations
2.2. Synthesis of Sorbent
2.3. Porosity Measurement
2.4. Contact Angle Measurement
3. Results
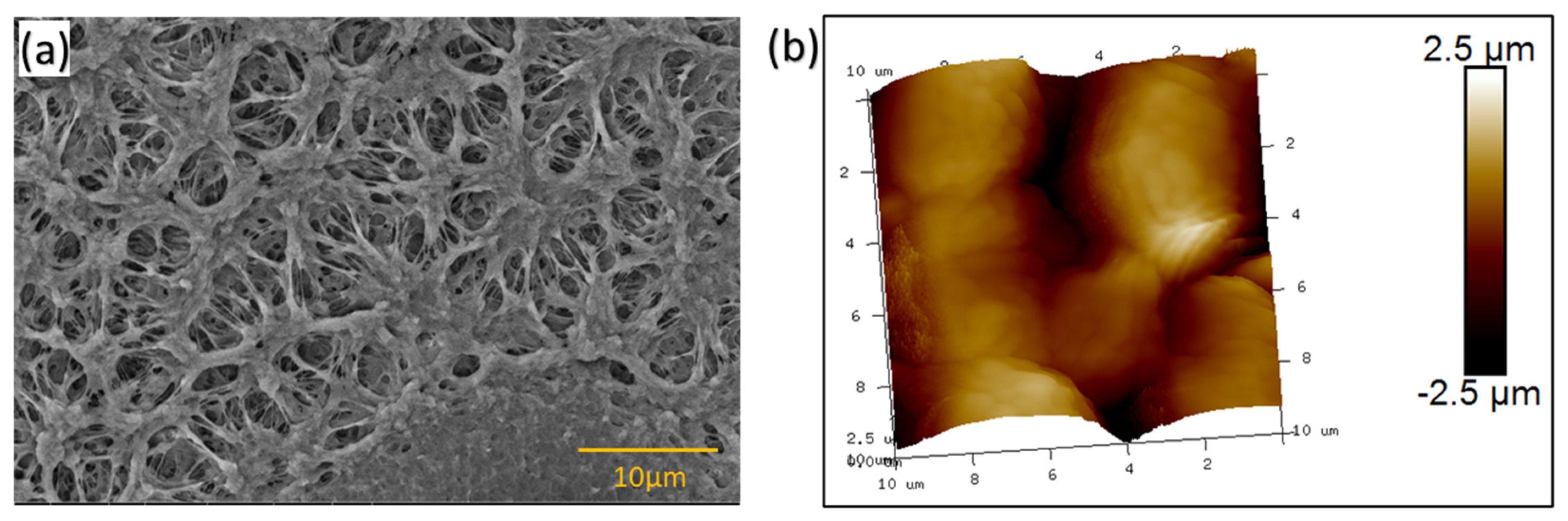
3.1. SEM and AFM Analysis
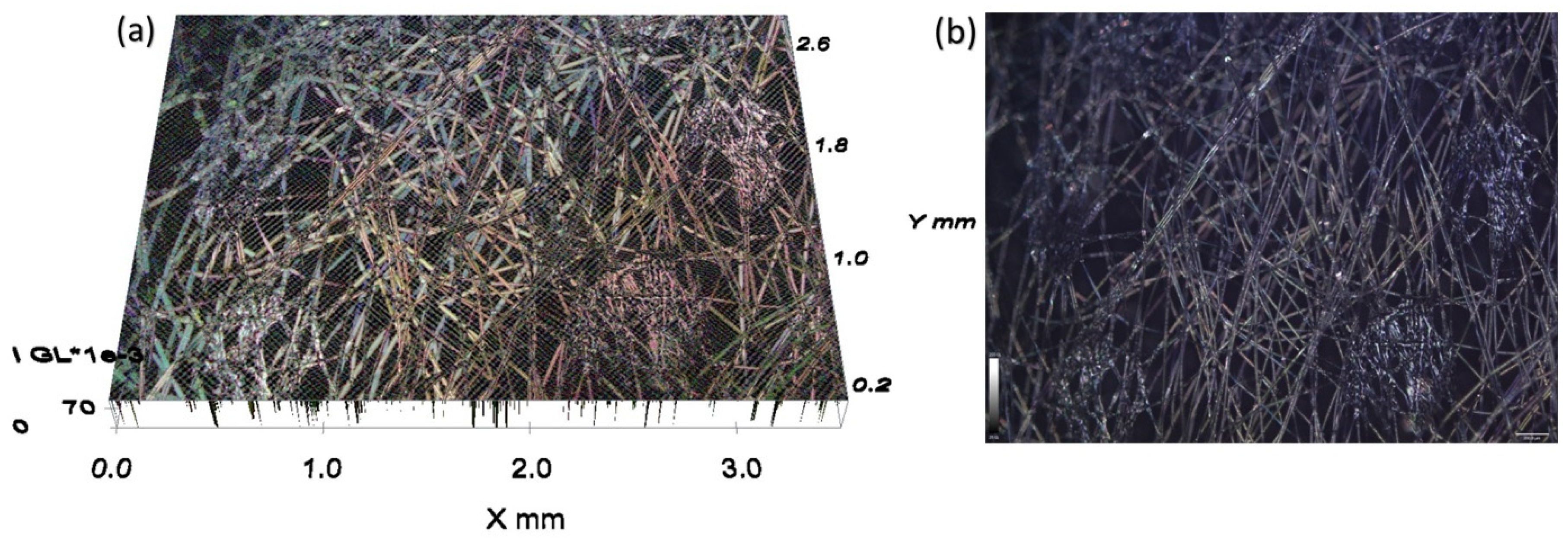
3.2. Microscopy Images
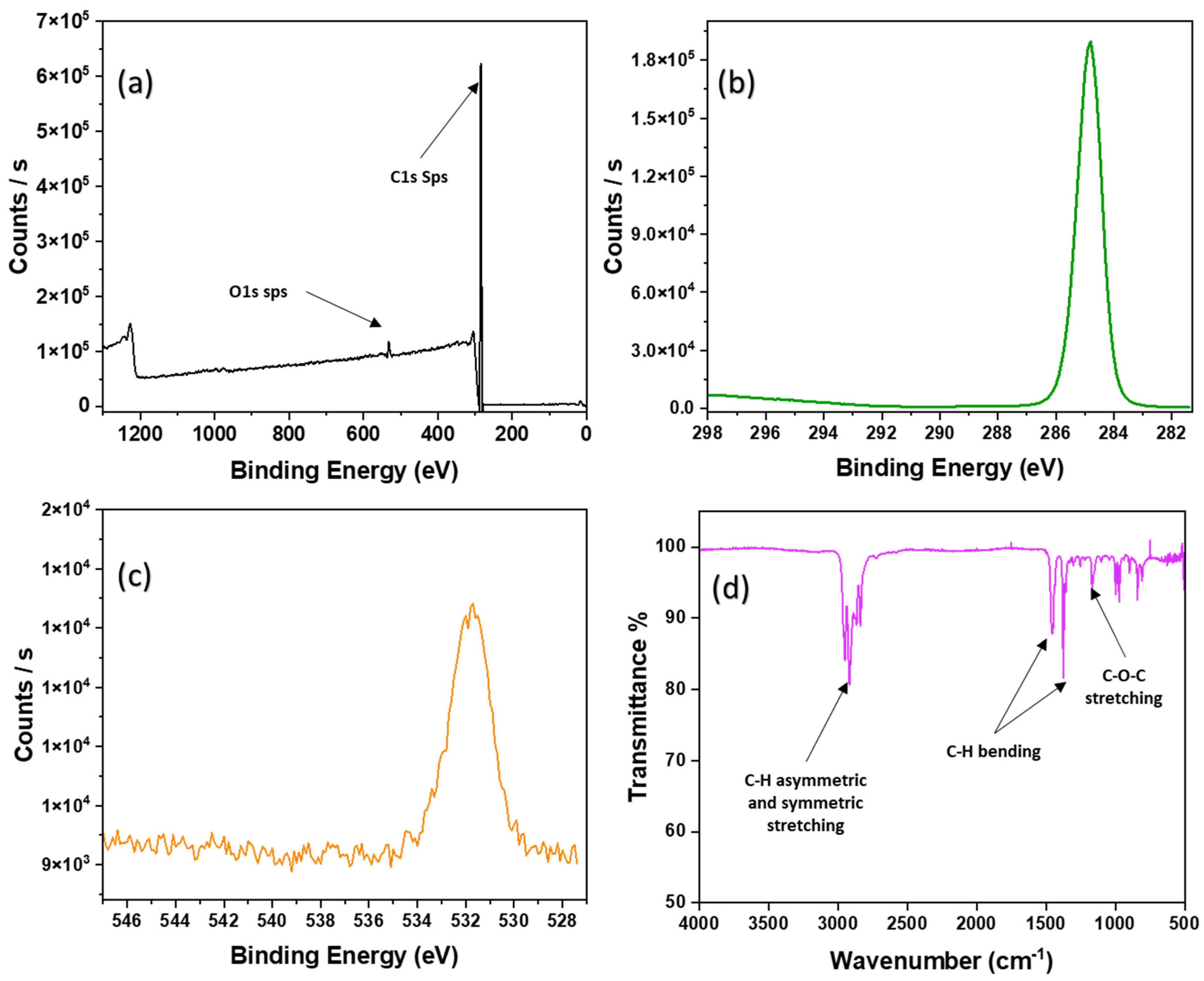
3.3. XPS Analysis
3.4. FT-IR Analysis
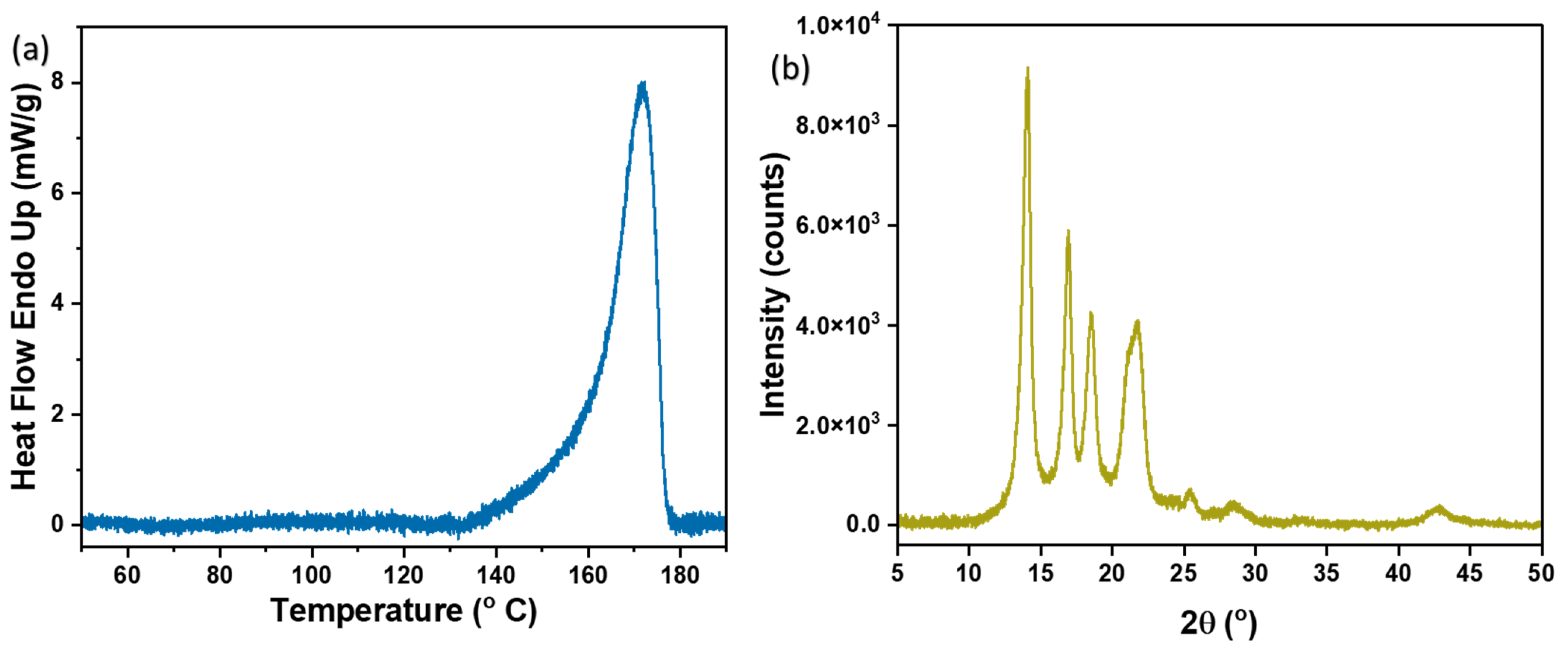
3.5. DSC and XRD Analysis
3.6. Contact Angle
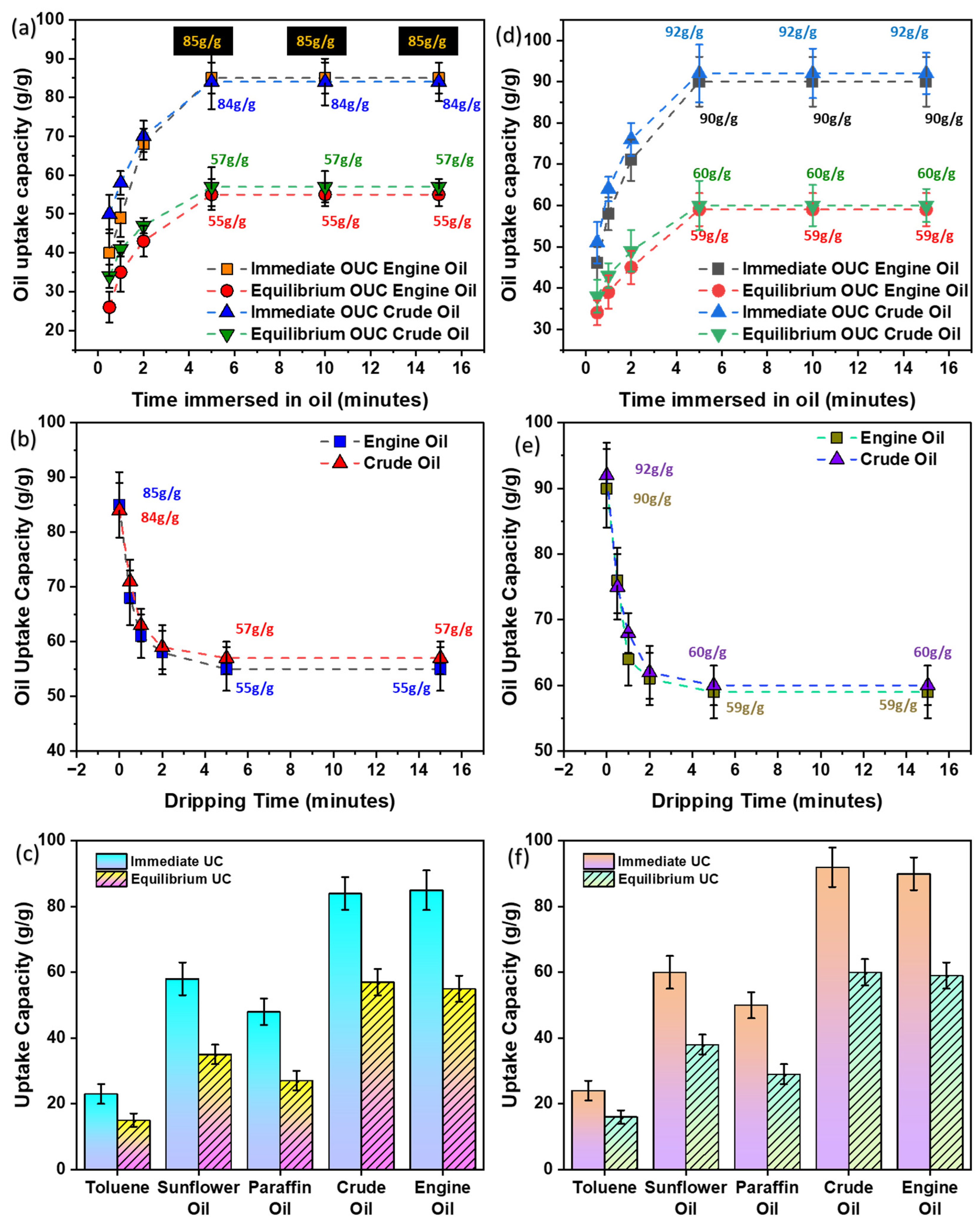
3.7. Comparison of Oil-Sorption Capacity Using Thin Films with and without Pouch
3.7.1. Oil Saturation
3.7.2. Oil Dripping
3.8. Comparison with Different Oils
3.9. Oil–Water Separation
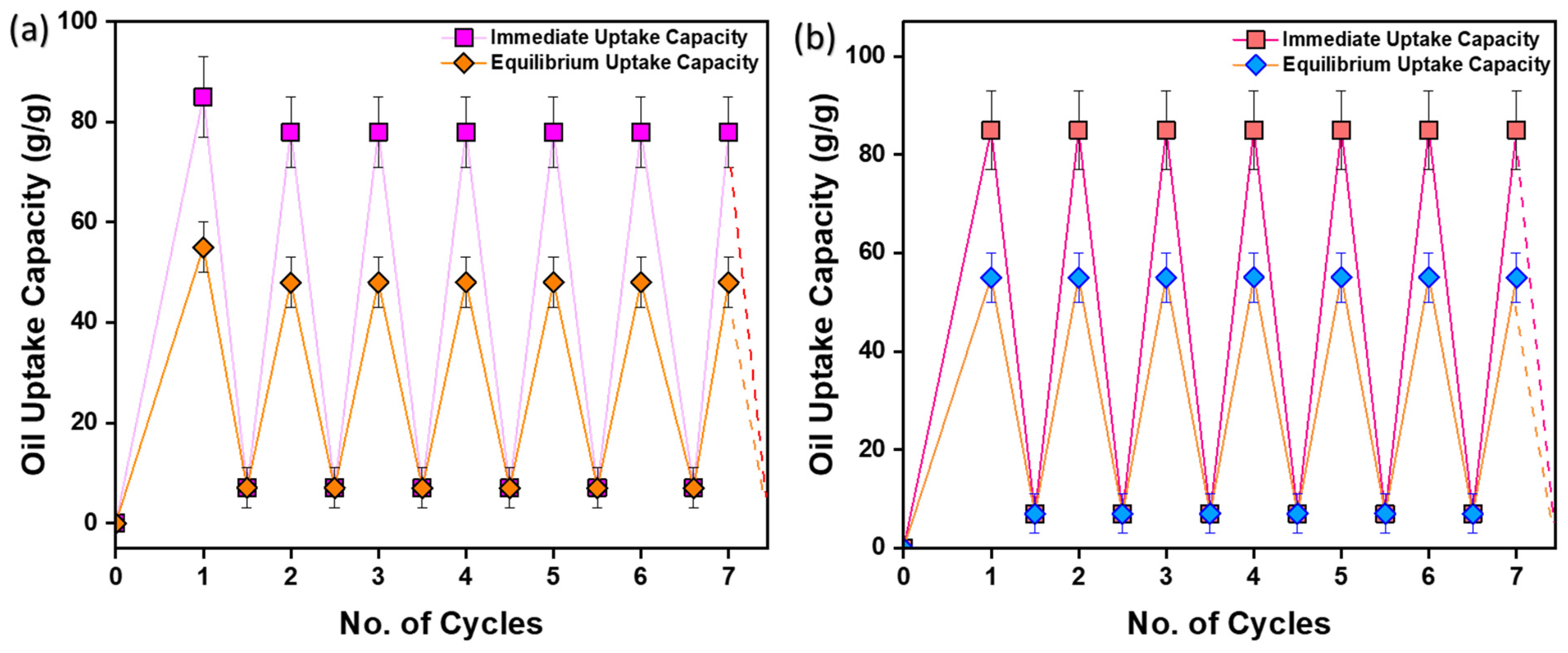
3.10. Recyclability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| PP | Polypropylene |
| COVID-19 | Coronavirus Disease of 2019 |
| PS | Polystyrene |
| PE | Polyethylene |
| PET | Polyethylene terephthalate |
| PVC | Polyvinyl chloride |
| PPE | Personal-protective equipment |
| SUP | Single-use plastic |
| RT-PCR | Real-time polymerase chain reaction |
| CO2 | Carbon dioxide |
| PU | Polyurethane |
| PVDF | |
| polyvinylidene fluoride | |
| PC | Polycarbonate |
| HDPE | High-density polyethylene |
| MAF-6 | Metal–azolate framework |
| SEM | Scanning electron microscopy |
| AFM | Atomic force microscopy |
| RMS | Root mean square surface roughness |
| XPS | X-ray photoelectron spectroscopy |
| C1s | Carbon 1 species |
| O1s | Oxygen 1 species |
| FT-IR | Fourier transform infrared |
| DSC | Differential scanning calorimetry |
| XRD | X-ray diffraction |
| S.No. | Serial number |
| UC | Uptake capacity |
| OUC | Oil-uptake capacity |
References
- Dey, A.; Dhumal, C.V.; Sengupta, P.; Kumar, A.; Pramanik, N.K.; Alam, T. Challenges and possible solutions to mitigate the problems of single-use plastics used for packaging food items: A review. J. Food Sci. Technol. 2021, 58, 3251–3269. [Google Scholar] [CrossRef] [PubMed]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shi, K.; Zhang, X.; Yu, K.; Zhang, H.; He, J.; Ju, Y.; Liu, J. From plastic waste to wealth using chemical recycling: A review. J. Environ. Chem. Eng. 2022, 10, 106867. [Google Scholar] [CrossRef]
- Zheng, J.; Suh, S. Strategies to reduce the global carbon footprint of plastics. Nat. Clim. Chang. 2019, 9, 374–378. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, E.; Mishra, R.; Kumar, A.; Caucci, S. Utilization of plastic wastes for sustainable environmental management: A review. ChemSusChem 2021, 14, 3985–4006. [Google Scholar] [CrossRef]
- Kedzierski, M.; Frère, D.; Le Maguer, G.; Bruzaud, S. Why is there plastic packaging in the natural environment? Understanding the roots of our individual plastic waste management behaviours. Sci. Total Environ. 2020, 740, 139985. [Google Scholar] [CrossRef]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of microplastics and nanoplastics in mammalian systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Awasthi, A.K.; Wei, F.; Tan, Q.; Li, J. Single-use plastics: Production, usage, disposal, and adverse impacts. Sci. Total Environ. 2021, 752, 141772. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Kurniawan, W.; Aziz, M. Technological review on thermochemical conversion of COVID-19-related medical wastes. Resour. Conserv. Recycl. 2021, 167, 105429. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Mekonnen, B.A. Current plastics pollution threats due to COVID-19 and its possible mitigation techniques: A waste-to-energy conversion via Pyrolysis. Environ. Syst. Res. 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Harussani, M.M.; Sapuan, S.M.; Rashid, U.; Khalina, A.; Ilyas, R.A. Pyrolysis of polypropylene plastic waste into carbonaceous char: Priority of plastic waste management amidst COVID-19 pandemic. Sci. Total Environ. 2022, 803, 149911. [Google Scholar] [CrossRef]
- Friese, C.R.; Veenema, T.G.; Johnson, J.S.; Jayaraman, S.; Chang, J.C.; Clever, L.H. Respiratory Protection Considerations for Healthcare Workers during the COVID-19 Pandemic. Health Secur. 2020, 18, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Bruinen de Bruin, Y.; Lequarre, A.S.; McCourt, J.; Clevestig, P.; Pigazzani, F.; Zare Jeddi, M.; Colosio, C.; Goulart, M. Initial impacts of global risk mitigation measures taken during the combatting of the COVID-19 pandemic. Saf. Sci. 2020, 128, 104773. [Google Scholar] [CrossRef]
- Cheng, V.C.C.; Wong, S.C.; Chuang, V.W.M.; So, S.Y.C.; Chen, J.H.K.; Sridhar, S.; To, K.K.W.; Chan, J.F.W.; Hung, I.F.N.; Ho, P.L.; et al. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J. Infect. 2020, 81, 107–114. [Google Scholar] [CrossRef]
- Yanase, S.; Sugimori, H. Prevalence of COVID-19 and the Continued Citizen-Based Control in Japan. Adv. Exp. Med. Biol. 2021, 1327, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Silva, A.L.P.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. COVID-19 Pandemic Repercussions on the Use and Management of Plastics. Environ. Sci. Technol. 2020, 54, 7760–7765. [Google Scholar] [CrossRef] [PubMed]
- Delgado, D.; Quintana, F.W.; Perez, G.; Liprandi, A.S.; Ponte-Negretti, C.; Mendoza, I.; Baranchuk, A. Personal safety during the COVID-19 pandemic: Realities and perspectives of healthcare workers in Latin America. Int. J. Environ. Res. Public Health 2020, 17, 2798. [Google Scholar] [CrossRef] [Green Version]
- Benson, N.U.; Bassey, D.E.; Palanisami, T. COVID pollution: Impact of COVID-19 pandemic on global plastic waste footprint. Heliyon 2021, 7, e06343. [Google Scholar] [CrossRef]
- Chowdhury, H.; Chowdhury, T.; Sait, S.M. Estimating marine plastic pollution from COVID-19 face masks in coastal regions. Mar. Pollut. Bull. 2021, 168, 112419. [Google Scholar] [CrossRef] [PubMed]
- Shams, M.; Alam, I.; Mahbub, M.S. Plastic Pollution During COVID-19: Plastic Waste Directives and Its Long-term Impact on the Environment. Environ. Adv. 2021, 5, 100119. [Google Scholar] [CrossRef]
- Parashar, N.; Hait, S. Plastics in the time of COVID-19 pandemic: Protector or polluter? Sci. Total Environ. 2021, 759, 144274. [Google Scholar] [CrossRef] [PubMed]
- Prasittisopin, L.; Ferdous, W.; Kamchoom, V. Microplastics in construction and built environment. Dev. Built Environ. 2023, 15, 100188. [Google Scholar] [CrossRef]
- Paul, B.; Dynes, J.J.; Chang, W. Modified zeolite adsorbents for the remediation of potash brine-impacted groundwater: Built-in dual functions for desalination and pH neutralization. Desalination 2017, 419, 141–151. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, P.; Schartup, A.T.; Zhang, Y. Plastic waste release caused by COVID-19 and its fate in the global ocean. Proc. Natl. Acad. Sci. USA 2021, 118, e2111530118. [Google Scholar] [CrossRef]
- Sastri, V.R. Commodity Thermoplastics: Polyvinyl Chloride, Polyolefins, Cycloolefins and Polystyrene. In Plastics in Medical Devices; Winovia LLC.: Chicago, IL, USA, 2022; pp. 113–166. [Google Scholar] [CrossRef]
- Gopanna, A.; Rajan, K.P.; Thomas, S.P.; Chavali, M. Polyethylene and polypropylene matrix composites for biomedical applications. In Materials for Biomedical Engineering Thermoset and Thermoplastic Polymers; Elsevier: Amsterdam, The Netherlands, 2019; pp. 175–216. [Google Scholar] [CrossRef]
- Kurzweil, P.; Müller, A.; Wahler, S. The ecological footprint of covid-19 mrna vaccines: Estimating greenhouse gas emissions in Germany. Int. J. Environ. Res. Public Health 2021, 18, 7425. [Google Scholar] [CrossRef]
- Karim, N.; Afroj, S.; Lloyd, K.; Oaten, L.C.; Andreeva, D.V.; Carr, C.; Farmery, A.D.; Kim, I.D.; Novoselov, K.S. Sustainable personal protective clothing for healthcare applications: A review. ACS Nano 2020, 14, 12313–12340. [Google Scholar] [CrossRef]
- NCT04373096 Enhanced Hood PPE to Minimize COVID-19 Transmission to Front-Line Health Care Workers. 2020. Available online: https://clinicaltrials.gov/show/NCT04373096 (accessed on 10 March 2023).
- Celis, J.E.; Espejo, W.; Paredes-Osses, E.; Contreras, S.A.; Chiang, G.; Bahamonde, P. Plastic residues produced with confirmatory testing for COVID-19: Classification, quantification, fate, and impacts on human health. Sci. Total Environ. 2021, 760, 144167. [Google Scholar] [CrossRef]
- Rahimi, A.R.; Garciá, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 1–11. [Google Scholar] [CrossRef]
- Garcia, J.M.; Robertson, M.L. The future of plastics recycling. Science 2017, 358, 870–872. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Yan, P.; Hao, X.; Xu, B.; Wang, W.; Aurangzeib, M. Non-biodegradable microplastics in soils: A brief review and challenge. J. Hazard. Mater. 2021, 409, 124525. [Google Scholar] [CrossRef]
- Patrício Silva, A.L.; Prata, J.C.; Duarte, A.C.; Barcelò, D.; Rocha-Santos, T. An urgent call to think globally and act locally on landfill disposable plastics under and after covid-19 pandemic: Pollution prevention and technological (Bio) remediation solutions. Chem. Eng. J. 2021, 426, 131201. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, G.; Garg, A. Single-Use Plastics: A Roadmap for Sustainability? Supremo Amic. 2021, 24, 585. [Google Scholar] [CrossRef]
- Issac, M.N.; Kandasubramanian, B. Effect of microplastics in water and aquatic systems. Environ. Sci. Pollut. Res. 2021, 28, 19544–19562. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Dong, X.; Zhao, Y.; Wang, D.J. Recycling and chemical upcycling of waste disposable medical masks. Acta Polym. Sin. 2020, 51, 1295–1306. [Google Scholar] [CrossRef]
- Thwe Win, T.; Jongvivatsakul, P.; Jirawattanasomkul, T.; Prasittisopin, L.; Likitlersuang, S. Use of polypropylene fibers extracted from recycled surgical face masks in cement mortar. Constr. Build. Mater. 2023, 391, 131845. [Google Scholar] [CrossRef]
- Damayanti, D.; Saputri, D.R.; Marpaung, D.S.S.; Yusupandi, F.; Sanjaya, A.; Simbolon, Y.M.; Asmarani, W.; Ulfa, M.; Wu, H.S. Current Prospects for Plastic Waste Treatment. Polymers 2022, 14, 3133. [Google Scholar] [CrossRef]
- Battegazzore, D.; Cravero, F.; Frache, A. Is it possible to mechanical recycle the materials of the disposable filtering masks? Polymers 2020, 12, 2726. [Google Scholar] [CrossRef]
- Xiang, M.; Yang, Z.; Yang, J.; Lu, T.; Wu, D.; Liu, Z.; Xue, R.; Dong, S. Conductive polymer composites fabricated by disposable face masks and multi-walled carbon nanotubes: Crystalline structure and enhancement effect. J. Renew. Mater. 2022, 10, 821–831. [Google Scholar] [CrossRef]
- Xiang, M.; Yang, Z.; Zhou, S.; Lu, T.; Zhang, S.; Sun, L.; Dong, S. Polymer Composites Completely Derived from Waste: The Crystalline Structure and the Mechanical Enhancement Effect. ACS Appl. Polym. Mater. 2021, 3, 3679–3684. [Google Scholar] [CrossRef]
- Pulikkalparambil, H.; Nandi, D.; Rangappa, S.M.; Prasanth, S.; Siengchin, S. Polymer composites from natural fibers and recycled waste surgical masks during COVID-19 pandemic. Polym. Compos. 2022, 43, 3944–3950. [Google Scholar] [CrossRef]
- Kheirabadi, S.; Sheikhi, A. Recent advances and challenges in recycling and reusing biomedical materials. Curr. Opin. Green Sustain. Chem. 2022, 38, 100695. [Google Scholar] [CrossRef] [PubMed]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef] [PubMed]
- Simatupang, M.M.; Veronika, E. Hazard risk identification from the used masks in Jabodetabek. IOP Conf. Ser. Earth Environ. Sci. 2022, 1027, 12003. [Google Scholar] [CrossRef]
- Supriyanto; Ylitervo, P.; Richards, T. Gaseous products from primary reactions of fast plastic pyrolysis. J. Anal. Appl. Pyrolysis 2021, 158, 105248. [Google Scholar] [CrossRef]
- Alston, S.M.; Clark, A.D.; Arnold, J.C.; Stein, B.K. Environmental impact of pyrolysis of mixed WEEE plastics Part 1: Experimental pyrolysis data. Environ. Sci. Technol. 2011, 45, 9380–9385. [Google Scholar] [CrossRef]
- Harussani, M.M.; Salit, M.S.; Rashid, U.; Abdan, K. Plastic Waste Conversion into Electrical, Thermal and Fuel Energy via Incinerations and Pyrolysis amidst COVID-19 Pandemic. In Proceedings of the AIUE 2nd Energy and Human Habitat Conference, Cape Town, South Africa, 26 July 2021; pp. 1–9. [Google Scholar]
- Pan, R.; Ferreira Martins, M.; Debenest, G. Pyrolysis of waste polyethylene in a semi-batch reactor to produce liquid fuel: Optimization of operating conditions. Energy Convers. Manag. 2021, 237, 114114. [Google Scholar] [CrossRef]
- Pan, R.; Martins, M.F.; Debenest, G. Optimization of oil production through ex-situ catalytic pyrolysis of waste polyethylene with activated carbon. Energy 2022, 248, 123514. [Google Scholar] [CrossRef]
- Pourebrahimi, S. Upcycling face mask wastes generated during COVID-19 into value-added engineering materials: A review. Sci. Total Environ. 2022, 851, 158396. [Google Scholar] [CrossRef]
- Lee, G.; Eui Lee, M.; Kim, S.S.; Joh, H.I.; Lee, S. Efficient upcycling of polypropylene-based waste disposable masks into hard carbons for anodes in sodium ion batteries. J. Ind. Eng. Chem. 2022, 105, 268–277. [Google Scholar] [CrossRef]
- Yuwen, C.; Liu, B.; Rong, Q.; Zhang, L.; Guo, S. Porous carbon materials derived from discarded COVID-19 masks via microwave solvothermal method for lithium-sulfur batteries. Sci. Total Environ. 2022, 817, 152995. [Google Scholar] [CrossRef]
- Mendoza, R.; Oliva, J.; Padmasree, K.P.; Oliva, A.I.; Mtz-Enriquez, A.I.; Zakhidov, A. Highly efficient textile supercapacitors made with face masks waste and thermoelectric Ca3Co4O9-δ oxide. J. Energy Storage 2022, 46, 103818. [Google Scholar] [CrossRef]
- Serafin, J.; Sreńscek-Nazzal, J.; Kamińska, A.; Paszkiewicz, O.; Michalkiewicz, B. Management of surgical mask waste to activated carbons for CO2 capture. J. CO2 Util. 2022, 59, 101970. [Google Scholar] [CrossRef]
- Varghese, P.J.G.; David, D.A.; Karuth, A.; Manamkeri Jafferali, J.F.; Sabura, S.B.; George, J.J.; Rasulev, B.; Raghavan, P. Experimental and Simulation Studies on Nonwoven Polypropylene-Nitrile Rubber Blend: Recycling of Medical Face Masks to an Engineering Product. ACS Omega 2022, 7, 4791–4803. [Google Scholar] [CrossRef]
- Cavalcante, J.; Hardian, R.; Szekely, G. Antipathogenic upcycling of face mask waste into separation materials using green solvents. Sustain. Mater. Technol. 2022, 32, e00448. [Google Scholar] [CrossRef]
- Wu, D.; Fang, L.; Qin, Y.; Wu, W.; Mao, C.; Zhu, H. Oil sorbents with high sorption capacity, oil/water selectivity and reusability for oil spill cleanup. Mar. Pollut. Bull. 2014, 84, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, P.; Mavinkere Rangappa, S.; Siengchin, S.; Puttegowda, M.; Thiagamani, S.M.K.; Hemath Kumar, M.; Oladijo, O.P.; Fiore, V.; Moure Cuadrado, M.M. Sustainable recycling technologies for thermoplastic polymers and their composites: A review of the state of the art. Polym. Compos. 2022, 43, 5831–5862. [Google Scholar] [CrossRef]
- Saleem, J.; Ning, C.; Barford, J.; McKay, G. Combating oil spill problem using plastic waste. Waste Manag. 2015, 44, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Saleem, J.; Adil Riaz, M.; McKay, G. Oil sorbents from plastic wastes and polymers: A review. J. Hazard. Mater. 2018, 341, 424–437. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Wang, J.; Ren, Y.; Xuan, C.; Liu, C.; Shen, C. Superhydrophobic and superoleophilic porous reduced graphene oxide/polycarbonate monoliths for high-efficiency oil/water separation. J. Hazard. Mater. 2018, 344, 849–856. [Google Scholar] [CrossRef]
- Lang, X.H.; Zhu, T.Y.; Zou, L.; Prakashan, K.; Zhang, Z.X. Fabrication and characterization of polypropylene aerogel material and aerogel coated hybrid materials for oil-water separation applications. Prog. Org. Coatings 2019, 137, 105370. [Google Scholar] [CrossRef]
- Chen, X.; Liang, Y.N.; Tang, X.-Z.; Shen, W.; Hu, X. Additive-free poly (vinylidene fluoride) aerogel for oil/water separation and rapid oil absorption. Chem. Eng. J. 2017, 308, 18–26. [Google Scholar] [CrossRef]
- Zhu, Q.; Chu, Y.; Wang, Z.; Chen, N.; Lin, L.; Liu, F.; Pan, Q. Robust superhydrophobic polyurethane sponge as a highly reusable oil-absorption material. J. Mater. Chem. A 2013, 1, 5386. [Google Scholar] [CrossRef]
- Zou, L.; Phule, A.D.; Sun, Y.; Zhu, T.Y.; Wen, S.; Zhang, Z.X. Superhydrophobic and superoleophilic polyethylene aerogel coated natural rubber latex foam for oil-water separation application. Polym. Test. 2020, 85, 106451. [Google Scholar] [CrossRef]
- Wu, W.; Du, M.; Shi, H.; Zheng, Q.; Bai, Z. Application of graphene aerogels in oil spill recovery: A review. Sci. Total Environ. 2023, 856, 159107. [Google Scholar] [CrossRef]
- Ge, J.; Zhao, H.Y.; Zhu, H.W.; Huang, J.; Shi, L.A.; Yu, S.H. Advanced Sorbents for Oil-Spill Cleanup: Recent Advances and Future Perspectives. Adv. Mater. 2016, 28, 10459–10490. [Google Scholar] [CrossRef]
- Kukkar, D.; Rani, A.; Kumar, V.; Younis, S.A.; Zhang, M.; Lee, S.-S.; Tsang, D.C.W.; Kim, K.-H. Recent advances in carbon nanotube sponge–based sorption technologies for mitigation of marine oil spills. J. Colloid Interface Sci. 2020, 570, 411–422. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.; Lee, W.; Nam, C. Superhydrophobic polypropylene sorbent derived from discarded face masks: A highly efficient adsorbent for oil spill sorbent. Chemosphere 2022, 303, 135186. [Google Scholar] [CrossRef]
- Guselnikova, O.; Semyonov, O.; Kirgina, M.; Ivanov, A.; Zinoviev, A.; Postnikov, P. Polymer waste surgical masks decorated by superhydrophobic metal-organic frameworks towards oil spills clean-up. J. Environ. Chem. Eng. 2022, 10, 107105. [Google Scholar] [CrossRef]
- Moelling, K.; Broecker, F. Viruses and evolution—Viruses first? A personal perspective. Front. Microbiol. 2019, 10, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balloux, F.; van Dorp, L. Q&A: What are pathogens, and what have they done to and for us? BMC Biol. 2017, 15, 91. [Google Scholar] [CrossRef]
- Roos, Y.H. Water and Pathogenic Viruses Inactivation—Food Engineering Perspectives. Food Eng. Rev. 2020, 12, 251–267. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, Q.; Zheng, Y.; He, G.; Shi, H. Plastic waste as the potential carriers of pathogens. Curr. Opin. Food Sci. 2021, 41, 224–230. [Google Scholar] [CrossRef]
- Yang, P.; Wang, X. COVID-19: A new challenge for human beings. Cell. Mol. Immunol. 2020, 17, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Saleem, J.; Tahir, F.; Baig, M.Z.K.; Al-Ansari, T.; McKay, G. Assessing the environmental footprint of recycled plastic pellets: A life-cycle assessment perspective. Environ. Technol. Innov. 2023, 32, 103289. [Google Scholar] [CrossRef]
- Erbil, H.Y.; Demirel, A.L.; Avcı, Y.; Mert, O. Transformation of a Simple Plastic into a Superhydrophobic Surface. Science 2003, 299, 1377–1380. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Gee, D.R.; Melia, T.P. Thermal properties of melt and solution crystallized isotactic polypropylene. Makromol. Chem. 1970, 132, 195–201. [Google Scholar] [CrossRef]
- Saleem, J.; Baig, M.Z.K.; Luyt, A.S.; Shakoor, R.A.; Zekri, A.; McKay, G. Free-standing polypropylene porous thin films using energy efficient coating technique. Energy Rep. 2023, 9, 31–39. [Google Scholar] [CrossRef]
- Khedr, R.F. Radiation-Grafting on Polypropylene Copolymer Membranes for Using in Cadmium Adsorption. Polymers 2023, 15, 686. [Google Scholar] [CrossRef]

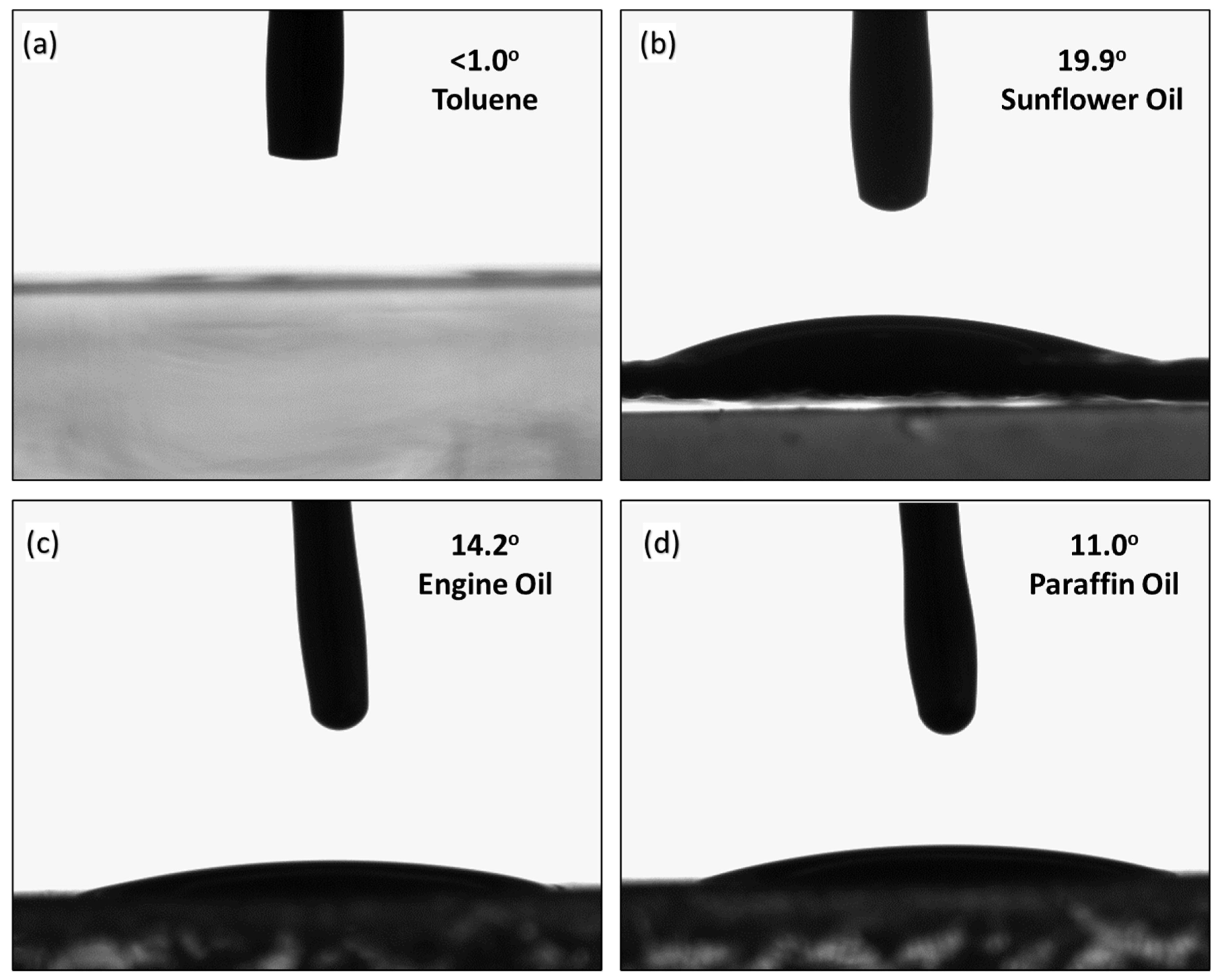

| Fluid | Contact Angle (°) |
|---|---|
| Toluene | 0.7 ± 0.2 |
| Sunflower oil | 19.9 ± 2.3 |
| Engine oil | 14.2 ± 1.3 |
| Paraffin oil | 11.0 ± 2.6 |
| S.No. | Saturation Time (min) | Immediate Oil Uptake Capacity (g/g) | Equilibrium * Oil Uptake Capacity (g/g) | ||
|---|---|---|---|---|---|
| Engine Oil | Crude Oil | Engine Oil | Crude Oil | ||
| 1 | 0.5 | 40 ± 6 | 50 ± 5 | 26 ± 4 | 34 ± 3 |
| 2 | 1 | 49 ± 5 | 58 ± 3 | 35 ± 5 | 41 ± 2 |
| 3 | 2 | 68 ± 4 | 70 ± 4 | 43 ± 4 | 47 ± 2 |
| 4 | 5 | 85 ± 4 | 84 ± 7 | 55 ± 4 | 57 ± 5 |
| 5 | 10 | 85 ± 4 | 84 ± 6 | 55 ± 3 | 57 ± 4 |
| 6 | 15 | 85 ± 4 | 84 ± 4 | 55 ± 3 | 57 ± 2 |
| S.No. | Dripping Time (min) | Oil Uptake Capacity (g/g) | |
|---|---|---|---|
| Engine Oil | Crude Oil | ||
| 1 | 0 | 85 ± 6 | 84 ± 5 |
| 2 | 0.5 | 68 ± 5 | 71 ± 3 |
| 3 | 1 | 61 ± 4 | 63 ± 3 |
| 4 | 2 | 58 ± 4 | 59 ± 4 |
| 5 | 5 | 55 ± 4 | 57 ± 3 |
| 6 | 15 | 55 ± 4 | 57 ± 1 |
| Oil | Immediate Uptake Capacity (g/g) | Equilibrium * Uptake Capacity (g/g) |
|---|---|---|
| Toluene | 23 ± 3 | 15 ± 2 |
| Sunflower Oil | 58 ± 5 | 35 ± 3 |
| Paraffin Oil | 48 ± 4 | 27 ± 3 |
| Crude Oil | 84 ± 5 | 57 ± 4 |
| Engine Oil | 85 ± 6 | 55 ± 4 |
| S. No. | Oil in Water (%) | Efficiency% |
|---|---|---|
| 1 | 1 | 99.5 ± 0.3 |
| 2 | 2 | 99.5 ± 0.4 |
| 3 | 3 | 99.5 ± 0.4 |
| 4 | 4 | 99.5 ± 0.5 |
| 5 | 5 | 99.5 ± 0.4 |
| 6 | 6 | 99.5 ± 0.3 |
| 7 | 7 | 99.6 ± 0.4 |
| 8 | 8 | 100 ± 0.3 |
| 9 | 9 | 100 ± 0.2 |
| 10 | 10 | 100 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, J.; Moghal, Z.K.B.; Shakoor, R.A.; McKay, G. Sustainable Solution for Plastic Pollution: Upcycling Waste Polypropylene Masks for Effective Oil-Spill Management. Int. J. Mol. Sci. 2023, 24, 12368. https://doi.org/10.3390/ijms241512368
Saleem J, Moghal ZKB, Shakoor RA, McKay G. Sustainable Solution for Plastic Pollution: Upcycling Waste Polypropylene Masks for Effective Oil-Spill Management. International Journal of Molecular Sciences. 2023; 24(15):12368. https://doi.org/10.3390/ijms241512368
Chicago/Turabian StyleSaleem, Junaid, Zubair Khalid Baig Moghal, Rana Abdul Shakoor, and Gordon McKay. 2023. "Sustainable Solution for Plastic Pollution: Upcycling Waste Polypropylene Masks for Effective Oil-Spill Management" International Journal of Molecular Sciences 24, no. 15: 12368. https://doi.org/10.3390/ijms241512368






