Alternations of NF-κB Signaling by Natural Compounds in Muscle-Derived Cancers
Abstract
:1. The Role of NF-κB in Carcinogenesis
2. Muscle and the Transcription Factor NF-κB
2.1. Stages of Myogenesis
2.2. NF-κB Family and Its Regulators
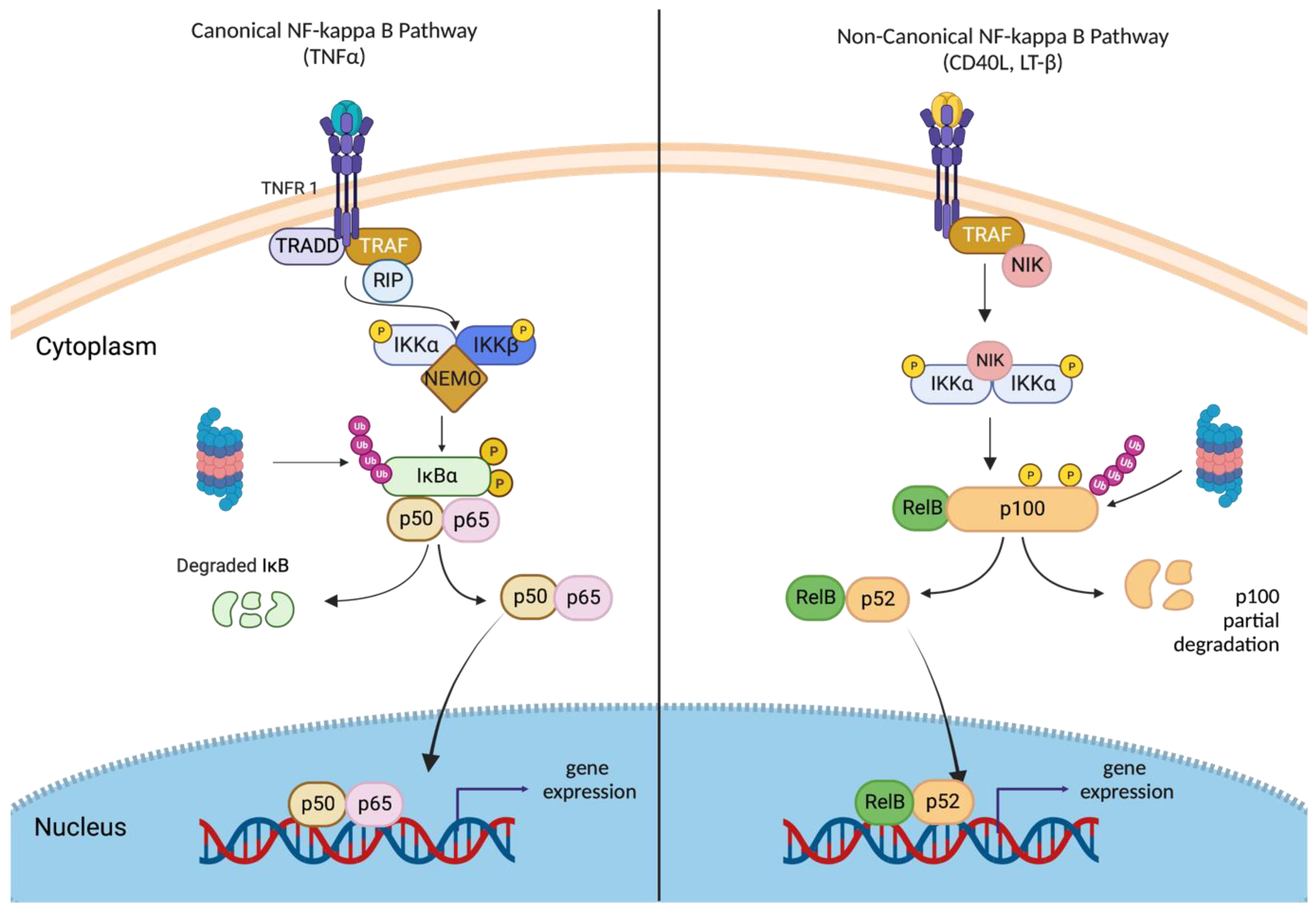
2.3. Canonical and Non-Canonical NF-κB Signaling
2.4. Dual Role of NF-κB in Myogenesis
2.5. NF-κB: Orchestrating Muscle Regeneration
2.6. The Role of NF-κB in Muscular Dystrophy
2.7. NF-κB in Myositis
3. Substances of Natural Origin Targeting NF-κB
3.1. Curcumin
3.2. Epigallocatechin-3-Gallate (EGCG)
3.3. Resveratrol
3.4. Quercetin
3.5. Berberine
3.6. Caffeic Acid Phenethyl Ester (CAPE)
3.7. Cucurbitacin E (CurE)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef]
- Kucharczak, J.; Simmons, M.J.; Fan, Y.; Gélinas, C. To be, or not to be: NF-kappaB is the answer-role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene 2003, 22, 8961–8982. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Pettaway, C.A.; Uehara, H.; Bucana, C.D.; Fidler, I.J. Blockade NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene 2001, 20, 4188–4197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Zhang, L.; Ni, Z.; Sun, G.; Gao, H.; Cheng, Z.; Xu, J.; Yin, P. Resveratrol induces AMPK-dependent MDR1 inhibition in colorectal cancer HCT116/L-OHP cells by preventing activation of NF-κB signaling and suppressing cAMP-responsive element transcriptional activity. Tumour Biol. 2015, 36, 9499–9510. [Google Scholar] [CrossRef] [PubMed]
- Suttana, W.; Mankhetkorn, S.; Poompimon, W.; Palagani, A.; Zhokhov, S.; Gerlo, S.; Haegeman, G.; Berghe, W.V. Differential chemosensitization of P-glycoprotein overexpressing K562/Adr cells by withaferin A and Siamois polyphenols. Mol. Cancer 2010, 9, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyke, S.M.; Russell, S.T.; Tisdale, M.J. Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-kappaB activation. Br. J. Cancer 2004, 91, 1742–1750. [Google Scholar] [CrossRef] [Green Version]
- Moore-Carrasco, R.; Busquets, S.; Almendro, V.; Palanki, M.; López-Soriano, F.J.; Argilés, J.M. The AP-1/NF-kappa B double inhibitor SP100030 can revert muscle wasting during experimental cancer cachexia. Int. J. Oncol. 2007, 30, 1239–1245. [Google Scholar]
- Cai, D.; Frantz, J.D.; Tawa, N.E., Jr.; Melendez, P.A.; Oh, B.-C.; Lidov, H.G.W.; Hasselgren, P.-O.; Frontera, W.R.; Lee, J.; Glass, D.J.; et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 2004, 119, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Sen, R.; Baltimore, D. Inducibility of κ immunoglobulin enhancer-binding protein NF-κB by a posttranslational mechanism. Cell 1986, 47, 921–928. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building Muscle: Molecular Regulation of Myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yablonka-Reuveni, Z. The Skeletal Muscle Satellite Cell: Still Young and Fascinating at 50. J. Histochem. Cytochem. 2011, 59, 1041–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zammit, P.S. Function of the Myogenic Regulatory Factors Myf5, MyoD, Myogenin and MRF4 in Skeletal Muscle, Satellite Cells and Regenerative Myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Yokoyama, S.; Asahara, H. The Myogenic Transcriptional Network. Cell. Mol. Life Sci. 2011, 68, 1843–1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chal, J.; Pourquié, O. Making Muscle: Skeletal Myogenesis in Vivo and in Vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef] [Green Version]
- Espín-Palazón, R.; Traver, D. The NF-ΚB Family: Key Players during Embryonic Development and HSC Emergence. Exp. Hematol. 2016, 44, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Sen, R.; Smale, S.T. Selectivity of the NF-ΚB Response. Cold Spring Harb. Perspect. Biol. 2010, 2, a000257. [Google Scholar] [CrossRef] [Green Version]
- Oeckinghaus, A.; Ghosh, S. The NF-KappaB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Hans, H.; Michel, K. Regulation and Function of IKK and IKK-Related Kinases. Sci. STKE 2006, 2006, re13. [Google Scholar]
- Wong, E.T.; Tergaonkar, V. Roles of NF-ΚB in Health and Disease: Mechanisms and Therapeutic Potential; Karin, M., Ed.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 116. [Google Scholar]
- Sun, S.C. The Non-Canonical NF-ΚB Pathway in Immunity and Inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Tubaro, C.; Arcuri, C.; Giambanco, I.; Donato, R. S100B Protein in Myoblasts Modulates Myogenic Differentiation via NF-ΚB-Dependent Inhibition of MyoD Expression. J. Cell. Physiol. 2010, 223, 270–282. [Google Scholar] [PubMed]
- Yamakawa, H.; Kusumoto, D.; Hashimoto, H.; Yuasa, S. Stem Cell Aging in Skeletal Muscle Regeneration and Disease. Int. J. Mol. Sci. 2020, 21, 1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langen, R.C.J.; Velden, J.L.J.; Schols, A.M.W.J.; Kelders, M.C.J.M.; Wouters, E.F.M.; Janssen-Heininger, Y.M.W. Tumor Necrosis Factor-Alpha Inhibits Myogenic Differentiation through MyoD Protein Destabilization. FASEB J. 2004, 18, 227–237. [Google Scholar] [CrossRef]
- Guttridge, D.C.; Albanese, C.; Reuther, J.Y.; Pestell, R.G.; Baldwin, A.S., Jr. NF-KappaB Controls Cell Growth and Differentiation through Transcriptional Regulation of Cyclin D1. Mol. Cell Biol. 1999, 19, 5785–5799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Hertlein, E.; Bakkar, N.; Sun, H.; Acharyya, S.; Wang, J.; Carathers, M.; Davuluri, R.; Guttridge, D.S. NF-kB Regulation of YY1 Inhibits Skeletal Myogenesis through Transcriptional Silencing of Myofibrillar Genes. Mol. Cell. Biol. 2007, 27, 4374–4387. [Google Scholar] [CrossRef] [Green Version]
- Bakkar, N.; Wang, J.; Ladner, K.J.; Wang, H.; Dahlman, J.M.; Carathers, M.; Acharyya, S.; Rudnicki, M.A.; Hollenbach, A.D.; Guttridge, D.C. IKK/NF-KappaB Regulates Skeletal Myogenesis via a Signaling Switch to Inhibit Differentiation and Promote Mitochondrial Biogenesis. J. Cell Biol. 2008, 180, 787–802. [Google Scholar] [CrossRef]
- Enwere, E.K.; LaCasse, E.C.; Adam, N.J.; Korneluk, R.G. Role of the TWEAK-Fn14-CIAP1-NF-ΚB Signaling Axis in the Regulation of Myogenesis and Muscle Homeostasis. Front. Immunol. 2014, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.M.; Wang, D.J.; Peterson, J.M.; Shintaku, J.; Liyanarachchi, S.; Coppola, V.; Frakes, A.E.; Kaspar, B.K.; Cornelison, D.D.; Guttridge, D.C. An NF-ΚB—EphrinA5-Dependent Communication between NG2+ Interstitial Cells and Myoblasts Promotes Muscle Growth in Neonates. Dev. Cell 2016, 36, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Sousa-Victor, P.; García-Prat, L.; Muñoz-Cánoves, P. Control of Satellite Cell Function in Muscle Regeneration and Its Disruption in Ageing. Nat. Rev. Mol. Cell. Biol. 2021, 23, 204–226. [Google Scholar] [CrossRef]
- Straughn, A.R.; Hindi, S.M.; Xiong, G.; Kumar, A. Canonical NF-ΚB Signaling Regulates Satellite Stem Cell Homeostasis and Function during Regenerative Myogenesis. J. Mol. Cell. Biol. 2019, 11, 53–66. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Weidemann, A.; Poser, C.; Bigot, A.; Maltzahn, J.V. Stimulation of Non-Canonical NF-ΚB Through Lymphotoxin-β-Receptor Impairs Myogenic Differentiation and Regeneration of Skeletal Muscle. Front. Cell Dev. Biol. 2021, 9, 721543. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.J.; Davies, K.E. Duchenne muscular dystrophy and dystrophin: Pathogenesis and opportunities for treatment: Third in molecular medicine review series. EMBO Rep. 2004, 5, 872–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, J.D.; Khanna, S.; Kaminski, H.J.; Rao, J.S.; Merriam, A.P.; Richmonds, C.R.; Leahy, P.; Li, J.; Guo, W.; Andrade, F.H. A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum. Mol. Genet. 2002, 11, 263–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.Z.; Johnson, A.P.; Rando, T.A. NF kappa B and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic. Biol. Med. 2001, 31, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Monici, M.C.; Aguennouz, M.; Mazzeo, A.; Messina, C.; Vita, G. Activation of nuclear factor-kappaB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology 2003, 60, 993–997. [Google Scholar] [CrossRef]
- Kumar, A.; Borek, A.M. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: A possible role in Duchenne muscular dystrophy. FASEB J. 2003, 17, 386–396. [Google Scholar] [CrossRef] [Green Version]
- Acharyya, S.; Villalta, S.A.; Bakkar, N.; Bupha-Intr, T.; Janssen, P.M.L.; Carathers, M.; Li, Z.; Beg, A.A.; Ghosh, S.; Sahenk, Z.; et al. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J. Clin. Investig. 2007, 117, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Baghdiguian, S.; Martin, M.; Richard, I. Calpain 3 deficiency is associated with myonuclear apoptosis and profound perturbation of the IkappaB alpha/NF-kappaB pathway in limb-girdle muscular dystrophy type 2A. Nat. Med. 1999, 5, 503–511. [Google Scholar] [CrossRef]
- Baghdiguian, S.; Richard, I.; Martin, M. Pathophysiology of limb girdle muscular dystrophy type 2A: Hypothesis and new insights into the IkappaBalpha/NF-kappaB survival pathway in skeletal muscle. J. Mol. Med. 2001, 79, 254–261. [Google Scholar] [CrossRef]
- Kramerova, I.; Beckmann, J.S.; Spencer, M.J. Molecular and cellular basis of calpainopathy (limb girdle muscular dystrophy type 2A). Biochim. Biophys. Acta 2007, 1772, 128–144. [Google Scholar] [CrossRef] [Green Version]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kappaB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackenzie, S.J.; Nicolau, S.; Connolly, A.M.; Mendell, J.R. Therapeutic Approaches for Duchenne Muscular Dystrophy: Old and New. Semin. Pediatr. Neurol. 2021, 37, 100877. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, I.E.; de Visser, M.; Werth, V.P. Classification of myositis. Nat. Rev. Rheumatol. 2018, 14, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Needham, M.; Mastaglia, F. Advances in inclusion body myositis: Genetics, pathogenesis and clinical aspects. Expert Opin. Orphan Drugs 2017, 5, 431–443. [Google Scholar] [CrossRef]
- Wynn, T. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Barca, E.; Aguennouz, M.; Mazzeo, A.; Messina, S.; Toscano, A.; Vita, G.L.; Portaro, S.; Parisi, D.; Rodolico, C. ANT1 is reduced in sporadic inclusion body myositis. Neurol. Sci. 2013, 34, 217–224. [Google Scholar] [CrossRef]
- Tournadre, A.; Lenief, V.; Miossec, P. Expression of TLR3 and TLR7 in muscle is characteristic of inflammatory myopathy and is differentially regulated by Th1 and Th17 cytokines. Arthritis Rheum. 2010, 62, 2144–2151. [Google Scholar] [CrossRef]
- Bakkar, N.; Guttridge, D.C. NF-κB Signaling: A Tale of Two Pathways in Skeletal Myogenesis. Physiol. Rev. 2010, 90, 495–511. [Google Scholar] [CrossRef] [Green Version]
- Lightfoot, A.P.; Cooper, R.G. The role of myokines in muscle health and disease. Curr. Opin. Rheumatol. 2016, 28, 661–666. [Google Scholar] [CrossRef] [Green Version]
- Creus, K.K.; De Paepe, B.; Werbrouck, B.F.; Vervaet, V.; Weis, J.; Bleecker, J.L.D. Distribution of the NF-κB Complex in the Inflammatory Exudates Characterizing the Idiopathic Inflammatory Myopathies. Ann. N. Y. Acad. Sci. 2009, 1173, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Carrero, R.; Cerrada, I.; Lledó, E.; Carrero, R.; Cerrada, I.; Lledó, E.; Dopazo, J.; García-García, F.; Rubio, M.; Trigueros, C.; et al. IL1β Induces Mesenchymal Stem Cells Migration and Leucocyte Chemotaxis through NF-κB. Stem Cell Rev. Rep. 2012, 8, 905–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, I.; Leroy, V.; Kwon, H.M.; Martin, P.-Y.; Féraille, E.; Hasler, U. Osmoprotective Transcription Factor NFAT5/TonEBP Modulates Nuclear Factor-κB Activity. Mol. Biol. Cell 2010, 21, 3459–3474. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; He, F.; Zhou, L.; Shi, M.; Li, F.; Jia, H. Activation of TLR4 induces inflammatory muscle injury via mTOR and NF-κB pathways in experimental autoimmune myositis mice. Biochem. Biophys. Res. Commun. 2022, 603, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [CrossRef] [Green Version]
- Salucci, S.; Bavelloni, A.; Stella, A.B.; Fabbri, F.; Vannini, I.; Piazzi, M.; Volkava, K.; Volkava, K.; Scotlandi, K.; Martinelli, G.; et al. The Cytotoxic Effect of Curcumin in Rhabdomyosarcoma Is Assoociated with the Modulation of AMPK, AKT/mTOR, STAT and p53 Signaling. Nutrients 2023, 15, 740. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, T.S.; Ayob, A.Z.; Myint, H.H.L.; Thiagarajah, S.; Amini, F. Targeting colorectal cancer stem cells using curcumin and curcumin analogues: Insights into the mechanism of the therapeutic efficacy. Cancer Cell Int. 2015, 15, 96. [Google Scholar] [CrossRef] [Green Version]
- Shishodia, S.; Potdar, P.; Gairola, C.G.; Aggarwal, B.B. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: Correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis 2003, 24, 1269–1279. [Google Scholar] [CrossRef]
- Jobin, C.; A Bradham, C.; Russo, M.P.; Juma, B.; Narula, A.S.; A Brenner, D.; Sartor, R.B. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J. Immunol. 1999, 163, 3474–3483. [Google Scholar] [CrossRef]
- Buhrmann, C.; Mobasheri, A.; Busch, F.; Aldinger, C.; Stahlmann, R.; Montaseri, A.; Shakibaei, M. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: Role of the phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 2011, 286, 28556–28566. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin-3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Orr, W.S.; Denbo, J.W.; Saab, K.R.; Ng, V.Y.; Wu, J.; Li, K.; Garner, J.M.; Morton, C.L.; Du, Z.; Pfeffer, L.M.; et al. Curcumin potentiates rhabdomyosarcoma radiosensitivity by suppressing NF-κB activity. PLoS ONE 2013, 8, e51309. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Wang, W. Green tea polyphenol EGCG suppresses osteosarcoma cell growth through upregulating miR-1. Tumour Biol. 2016, 37, 4373–4382. [Google Scholar] [CrossRef]
- Mayer, B.F.; Stagno, M.J.; Fuchs, J.; Warmann, S.W.; Schmid, E. Epigallocatechin Gallate Inhibits Cell Growth and Hedgehog Signalling in Human Rhabdomyosarcoma Cell Lines. Anticancer Res. 2023, 43, 1025–1030. [Google Scholar] [CrossRef]
- Ahmad, N.; Gupta, S.; Mukhtar, H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappaB in cancer cells versus normal cells. Arch. Biochem. Biophys. 2000, 376, 338–346. [Google Scholar] [CrossRef]
- Ohishi, T.; Hayakawa, S.; Miyoshi, N. Involvement of microRNA modifications in anticancer effects of major polyphenols from green tea, coffee, wine, and curry. Crit. Rev. Food Sci. Nutr. 2022, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.Y.; Song, Y.A.; Park, Y.L.; Myung, E.; Chung, C.; Park, K.; Cho, S.; Lee, W.; Kim, H.; Rew, J.; et al. Epigallocatechin-3-gallate Inhibits LPS-Induced NF-κB and MAPK Signaling Pathways in Bone Marrow-Derived Macrophages. Gut Liver 2012, 6, 188–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Wang, L.; Cui, J.; Huoc, Z.; Xue, J.; Cui, H.; Mao, Q.; Yang, R. Resveratrol inhibits NF-κB signaling through suppression of p65 and IkappaB kinase activities. Pharmazie 2013, 68, 689–694. [Google Scholar] [PubMed]
- Capiralla, H.; Vingtdeux, V.; Zhao, H.; Capiralla, H.; Vingtdeux, V.; Zhao, H.; Sankowski, R.; Al-Abed, Y.; Davies, P.; Marambaud, P. Resveratrol mitigates lipopolysaccharide- and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J. Neurochem. 2012, 120, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Kundu, J.K.; Shin, Y.K.; Kim, S.H.; Surh, Y. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcinogenesis 2006, 27, 1465–1474. [Google Scholar] [CrossRef]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Endale, M.; Park, S.-C.; Kim, S.; Kim, S.-H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yin, G.; Dai, J.; Sun, Y.Z.; Hoffman, R.M.; Yang, Z.; Fan, Y. Chemoprevention by Quercetin of Oral Squamous Cell Carcinoma by Suppression of the NF-κB Signaling Pathway in DMBA-treated Hamsters. Anticancer Res. 2017, 37, 4041–4049. [Google Scholar] [PubMed] [Green Version]
- Shrestha, R.; Mohankumar, K.; Martin, G.; Hailemariam, A.; Lee, S.-O.; Jin, U.-H.; Burghardt, R.; Safe, S. Flavonoids kaempferol and quercetin are nuclear receptor 4A1 (NR4A1, Nur77) ligands and inhibit rhabdomyosarcoma cell and tumor growth. J. Exp. Clin. Cancer Res. 2021, 40, 392. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Chung, M.-Y.; Lim, T.G.; Huh, W.B.; Lee, H.J.; Lee, K.W. Quercetin suppresses intracellular ROS formation, MMP activation, and cell motility in human fibrosarcoma cells. J. Food Sci. 2013, 78, H1464–H1469. [Google Scholar] [CrossRef]
- Lan, H.; Hong, W.; Fan, P.; Qian, D.; Zhu, J.; Bai, B. Quercetin Inhibits Cell Migration and Invasion in Human Osteosarcoma Cells. Cell Physiol. Biochem. 2017, 43, 553–567. [Google Scholar] [CrossRef]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crişan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef] [Green Version]
- Shinji, S.; Nakamura, S.; Nihashi, Y.; Umezawa, K.; Takaya, T. Berberine and palmatine inhibit the growth of human rhabdomyosarcoma cells. Biosci. Biotechnol. Biochem. 2020, 84, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Liu, J.; Song, Z.; Pan, X.; Chen, L.; Cui, X.; Wang, M. Berberine suppresses amyloid-beta-induced inflammatory response in microglia by inhibiting nuclear factor-kappaB and mitogen-activated protein kinase signalling pathways. J. Pharm. Pharmacol. 2012, 64, 1510–1521. [Google Scholar] [CrossRef]
- Jin, H.; Jin, X.; Cao, B.; Wang, W. Berberine affects osteosarcoma via downregulating the caspase-1/IL-1β signaling axis. Oncol. Rep. 2017, 37, 729–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przystupski, D.; Niemczura, M.J.; Górska, A.; Supplitt, S.; Kotowski, K.; Wawryka, P.; Rozborska, P.; Woźniak, K.; Michel, O.; Kiełbik, A.; et al. In Search of Panacea—Review of Recent Studies Concerning Nature-Derived Anticancer Agents. Nutrients 2019, 11, 1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.C.; Chu, K.H.; Liang, Y.C.; Lin, Y.L.; Chiang, B.L. Caffeic acid phenethyl ester inhibits nuclear factor-κB and protein kinase B signalling pathways and induces caspase-3 expression in primary human CD4+ T cells. Clin. Exp. Immunol. 2010, 160, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, M.M.M.; Windle, H.J.; El Homasany, B.S.; Sabra, K.; Kelleher, D. Caffeic acid phenethyl ester modulates Helicobacter pylori-induced nuclear factor-kappa B and activator protein-1 expression in gastric epithelial cells. Br. J. Pharmacol. 2005, 146, 1139–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yang, X.; Liu, Y.; Jiang, T.; Ren, S.; Chen, J.; Xiong, H.; Yuan, M.; Li, W.; Machens, H.; et al. NRF2 signalling pathway: New insights and progress in the field of wound healing. J. Cell. Mol. Med. 2021, 25, 5857–5868. [Google Scholar] [CrossRef]
- Prasad, N.R.; Karthikeyan, A.; Karthikeyan, S.; Reddy, B.V. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef]
- Chanda, J.; Biswas, S.; Kar, A.; Mukherjee, P.K. Determination of cucurbitacin E in some selected herbs of ayurvedic importance through RP-HPLC. J. Ayurveda Integr. Med. 2020, 11, 287–293. [Google Scholar] [CrossRef]
- Alghasham, A.A. Cucurbitacins—A promising target for cancer therapy. Int. J. Health Sci. 2013, 7, 77–89. [Google Scholar] [CrossRef]
- Qiao, J.; Xu, L.-H.; He, J.; Ouyang, D.-Y.; He, X.-H. Cucurbitacin E exhibits anti-inflammatory effect in RAW 264.7 cells via suppression of NF-κB nuclear translocation. Inflamm. Res. 2013, 62, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Cheng, W.; Yue, Y.; Hu, Y.; Zhang, J.; Pan, X.; Xu, Z.; Zhang, P. Cucurbitacin E inhibits TNF-α-induced inflammatory cytokine production in human synoviocyte MH7A cells via suppression of PI3K/Akt/NF-κB pathways. Int. Immunopharmacol. 2015, 29, 884–890. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, S.; Wu, Y.; Zhang, J. Cucurbitacin E inhibits osteosarcoma cells proliferation and invasion through attenuation of PI3K/AKT/mTOR signalling pathway. Biosci. Rep. 2016, 36, e00405. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Wang, M.; Guo, W.; Sun, W.; Liu, Y. Curcumin in Osteosarcoma Therapy: Combining with Immunotherapy, Chemotherapeutics, Bone Tissue Engineering Materials and Potential Synergism with Photodynamic Therapy. Front. Oncol. 2021, 11, 672490. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.W.; Murillo, G.; Yu, C.; van Breemen, R.B.; Boddie, A.W.; Pezzuto, J.M.; Das Gupta, T.K.; Mehta, R.G. Resveratrol inhibits rhabdomyosarcoma cell proliferation. Eur. J. Cancer Prev. 2005, 14, 351–356. [Google Scholar] [CrossRef]
- Harati, K.; Slodnik, P.; Chromik, A.M.; Goertz, O.; Hirsch, T.; Kapalschinski, N.; Klein-Hitpass, L.; Kolbenschlag, J.; Uhl, W.; Lehnhardt, M.; et al. Resveratrol induces apoptosis and alters gene expression in human fibrosarcoma cells. Anticancer Res. 2015, 35, 767–774. [Google Scholar] [PubMed]
- De Luca, A.; Bellavia, D.; Raimondi, L.; Carina, V.; Costa, V.; Fini, M.; Giavaresi, G. Multiple Effects of Resveratrol on Osteosarcoma Cell Lines. Pharmaceuticals 2022, 15, 342. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-G.; Jenabi, J.M.; Liu, X.F.; Reynolds, C.P.; Triche, T.J.; Sorensen, P.H. Inhibition of the insulin-like growth factor I receptor by epigallocatechin gallate blocks proliferation and induces the death of Ewing tumor cells. Mol. Cancer Ther. 2010, 9, 1396–1407. [Google Scholar] [CrossRef] [Green Version]

| Compound | Cancer | Stimulated Pathway | References |
|---|---|---|---|
| Curcumin | Fibrosarcoma | Inducing apoptosis by downregulating NF-κB, IL-6, and IL-11. | [57,59,60,61,93,94] |
| Osteosarcoma | Apoptosis suppresses the proliferation, invasion, and metastasis. | ||
| Rhabdomyosarcoma | Suppressing NF-κB activity, modulation of AMPK, AKT/mTOR, STAT, and p53 signaling. | ||
| Resveratrol | Rhabdomyosarcoma | Inhibition of cell proliferation, induces arrest of the S/G2 phase of the cell cycle and reduction of cyclin B expression. | [95,96,97] |
| Fibrosarcoma | Induces apoptosis, inhibition of cell proliferation, expression of apoptosis-associated genes was altered. | ||
| Osteosarcoma | Inhibition of cell growth through the involvement of the AKT and caspase-3 pathways, inhibition of cell migration and a decrease in the level of IL-8 secretion. | ||
| Epigallocatechin-3-gallate (EGCG) | Rhabdomyosarcoma | Reducing cell proliferation and downregulating the HH signaling pathway. | [64,65,66,67,68,98] |
| Osteosarcoma | Inhibition of cell proliferation by upregulation of miR-1. | ||
| Ewing sarcoma | Induction of apoptosis, inhibition of cell proliferation, and inhibition of activation of the IGF-1R pathway. | ||
| Quercetin | Rhabdomyosarcoma | NR4A1 protein binding and inhibition of NR4A1-dependent transactivation in cells, regulating the pro-oncogenic genes PAX3-FOXO1 and G9a. | [76,77,78] |
| Fibrosarcoma | Inhibition of cell motility by inhibiting the activation of MMPs, attenuating the formation of ROS. | ||
| Osteosarcoma | Attenuation of cell migration and invasion, downregulation of expression levels of mRNA and proteins HIF-1α, VEGF, MMP2, and MMP9. | ||
| Berberine | Rhabdomyosarcoma | Inhibited the cell cycle of all RMS cells at the G1 phase. | [88,93] |
| Osteosarcoma | Downregulating the caspase-1/IL-1β. | ||
| Caffeic acid phenethyl ester (CAPE) | Fibrosarcoma | Enhances the ROS levels and lipid peroxidation alters the mitochondrial membrane potential increased oxidative DNA damage, apoptosis. | [87] |
| Cucurbitacin E (CurE) | Osteosarcoma | Inhibited the PI3K/Akt/mTOR pathway and epithelial-mesenchymal transition (EMT). | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzka, J.; Łapińska, Z.; Szwedowicz, U.; Gajewska-Naryniecka, A.; Gizak, A.; Kulbacka, J. Alternations of NF-κB Signaling by Natural Compounds in Muscle-Derived Cancers. Int. J. Mol. Sci. 2023, 24, 11900. https://doi.org/10.3390/ijms241511900
Radzka J, Łapińska Z, Szwedowicz U, Gajewska-Naryniecka A, Gizak A, Kulbacka J. Alternations of NF-κB Signaling by Natural Compounds in Muscle-Derived Cancers. International Journal of Molecular Sciences. 2023; 24(15):11900. https://doi.org/10.3390/ijms241511900
Chicago/Turabian StyleRadzka, Justyna, Zofia Łapińska, Urszula Szwedowicz, Agnieszka Gajewska-Naryniecka, Agnieszka Gizak, and Julita Kulbacka. 2023. "Alternations of NF-κB Signaling by Natural Compounds in Muscle-Derived Cancers" International Journal of Molecular Sciences 24, no. 15: 11900. https://doi.org/10.3390/ijms241511900