Relevance of the Exocyst in Arabidopsis exo70e2 Mutant for Cellular Homeostasis under Stress
Abstract
:1. Introduction
2. Results
2.1. exo70e2 T-DNA Plants Exhibit Developmental Defects under Stress
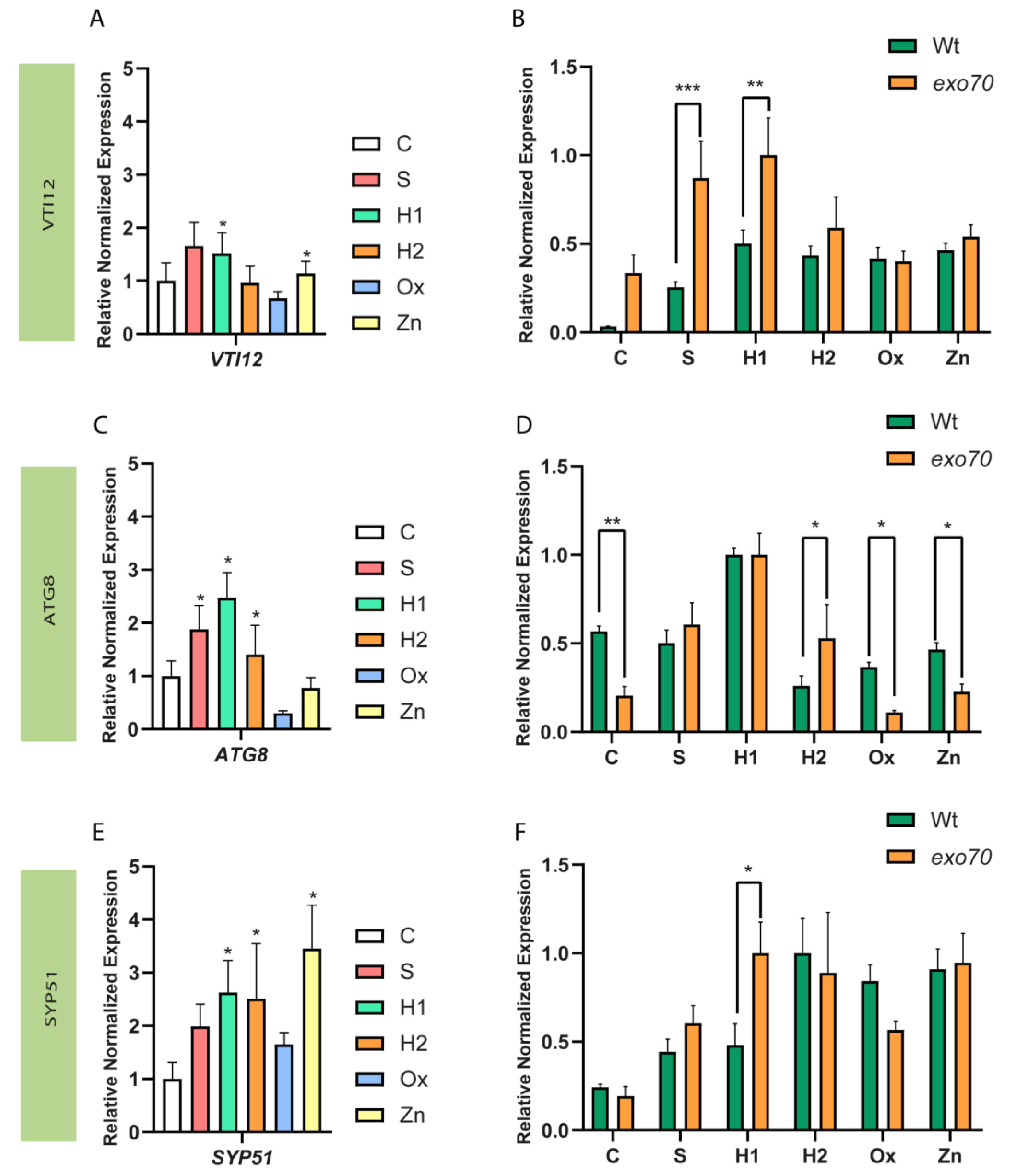
2.2. Expression of EXPO-Related Genes Is Altered in the Mutant Background
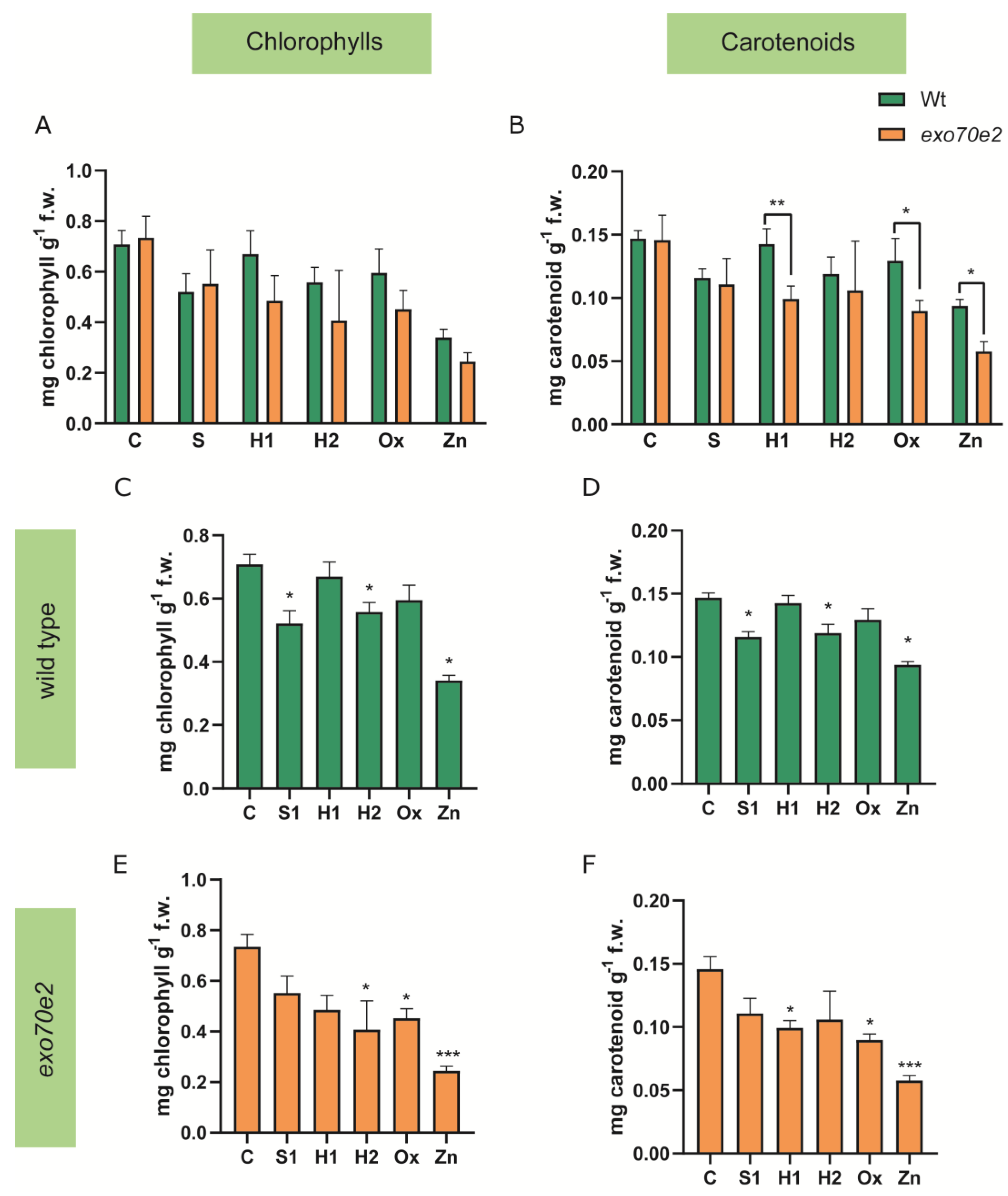
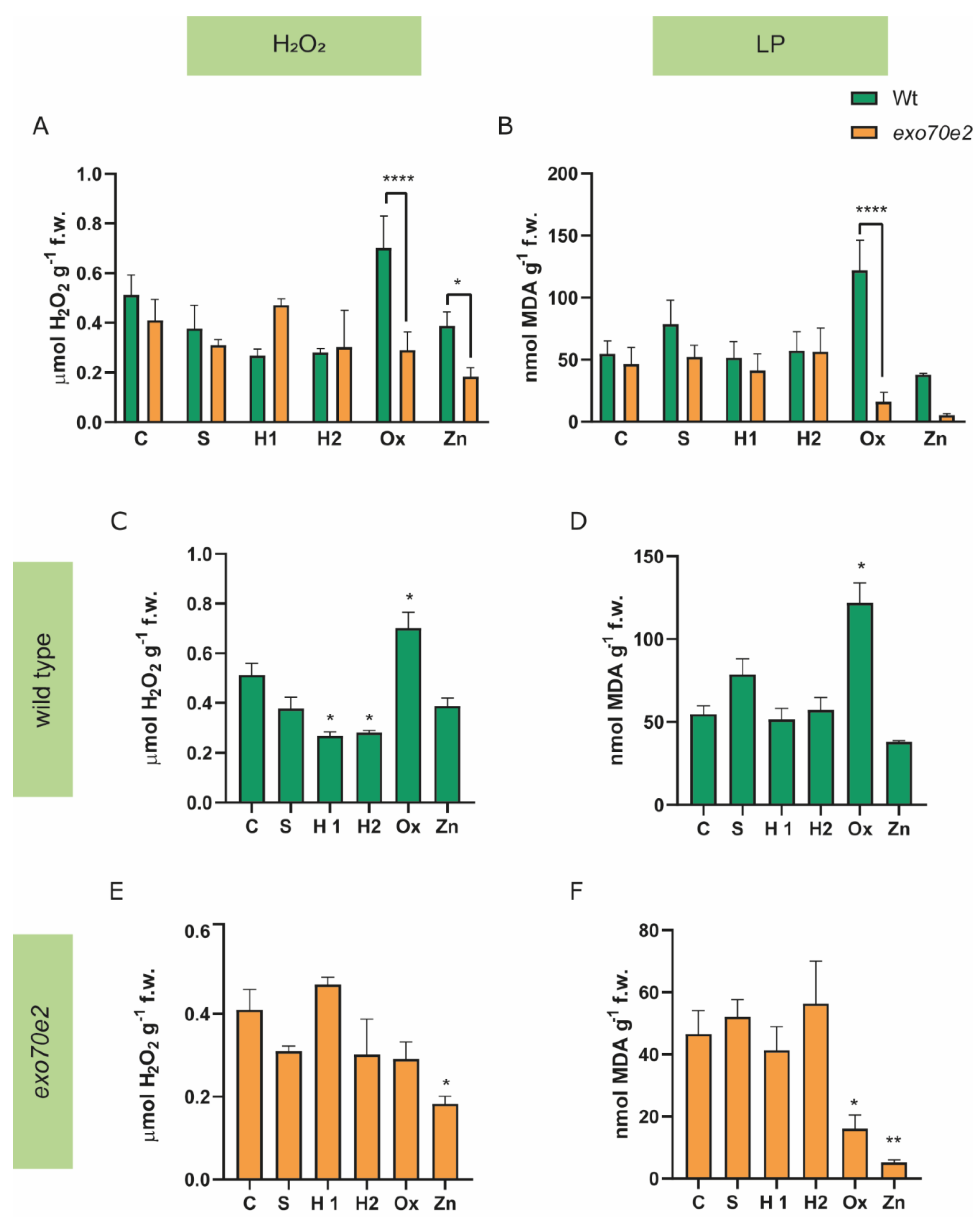
2.3. Analysis of Biochemical Endpoints
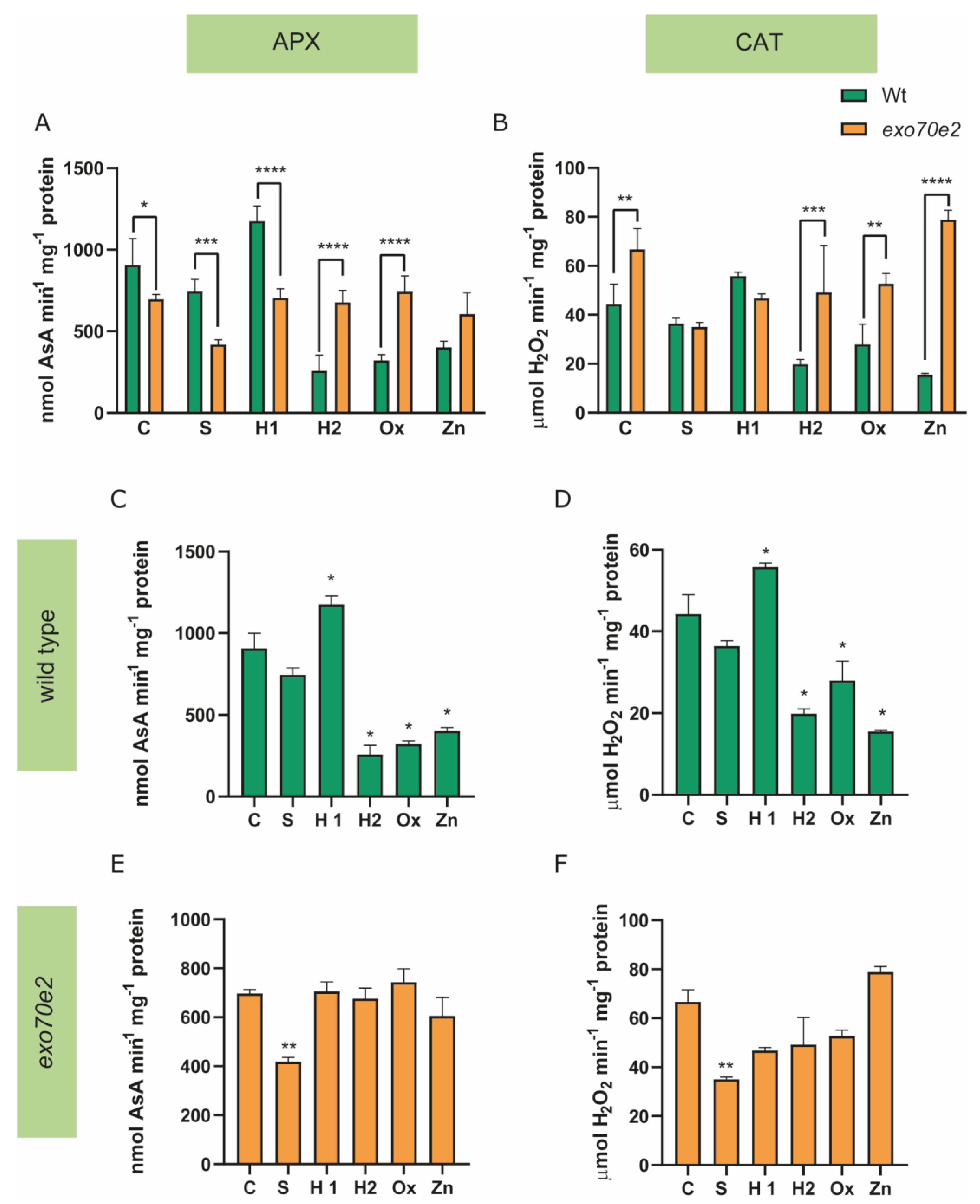
2.4. Evaluation of the Antioxidant System in exo70e2 Mutant Plants
3. Discussion
3.1. Characterisation of exo70e2 Mutant Line under Abiotic Stress
3.2. Relative Expression of EXPO-Related Genes
3.3. Characterisation of the AOX Response in the exo70e2 Background
4. Materials and Methods
4.1. Germination and Stress Experiments
4.2. Biochemical Assays
4.2.1. Photosynthetic Pigments Quantification
4.2.2. Determination of Lipid Peroxidation (LP)
4.2.3. Quantification of H2O2
4.2.4. Determination of Proline (Pro) Levels
4.2.5. Determination of Reduced Glutathione (GSH)
4.2.6. Determination of Catalase (CAT) and Ascorbate Peroxidase (APX) Activity
Extraction and Quantification of Soluble Proteins
Determination of CAT and APX Activity
4.3. Gene Expression Analysis by qPCR
4.3.1. RNA Extraction
4.3.2. cDNA Production
4.3.3. Real-Time Assays
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abberton, M.; Batley, J.; Bentley, A.; Bryant, J.; Cai, H.; Cockram, J.; Costa de Oliveira, A.; Cseke, L.J.; Dempewolf, H.; De Pace, C.; et al. Global agricultural intensification during climate change: A role for genomics. Plant Biotechnol. J. 2016, 14, 1095–1098. [Google Scholar] [CrossRef] [Green Version]
- Mousavi-Derazmahalleh, M.; Bayer, P.E.; Hane, J.K.; Valliyodan, B.; Nguyen, H.T.; Nelson, M.N.; Erskine, W.; Varshney, R.K.; Papa, R.; Edwards, D. Adapting legume crops to climate change using genomic approaches. Plant Cell Environ. 2019, 42, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Kalinowska, K.; Isono, E. All roads lead to the vacuole—Autophagic transport as part of the endomembrane trafficking network in plants. J. Exp. Bot. 2018, 69, 1313–1324. [Google Scholar] [CrossRef]
- Soares, C.; Carvalho, M.E.A.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges—A little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Park, C.J.; Park, J.M. Endoplasmic reticulum plays a critical role in integrating signals generated by both biotic and abiotic stress in plants. Front. Plant Sci. 2019, 10, 399. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, N.; Martinez, I.M.; Kimata, Y.; Kohno, K.; Sano, H.; Chrispeels, M.J. Molecular characterization of two Arabidopsis Ire1 homologs, endoplasmic reticulum-located transmembrane protein kinases. Plant Physiol. 2001, 127, 949–962. [Google Scholar] [CrossRef]
- Liu, Y.; Bassham, D.C. Autophagy: Pathways for self-eating in plant cells. Annu. Rev. Plant Biol. 2012, 63, 215–237. [Google Scholar] [CrossRef] [Green Version]
- Neves, J.; Sampaio, M.; Séneca, A.; Pereira, S.; Pissarra, J.; Pereira, C. Abiotic Stress Triggers the Expression of Genes Involved in Protein Storage Vacuole and Exocyst-Mediated Routes. Int. J. Mol. Sci. 2021, 22, 10644. [Google Scholar] [CrossRef]
- Tang, R.J.; Zhao, F.G.; Garcia, V.J.; Kleist, T.J.; Yang, L.; Zhang, H.X.; Luan, S. Tonoplast CBL-CIPK calcium signaling network regulates magnesium homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 3134–3139. [Google Scholar] [CrossRef] [Green Version]
- Queval, G.; Jaillard, D.; Zechmann, B.; Noctor, G. Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant Cell Environ. 2011, 34, 21–32. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yamada, K.; Shimada, T.; Matsushima, R.; Nishizawa, N.K.; Nishimura, M.; Hara-Nishimura, I. A proteinase-storing body that prepares for cell death or stresses in the epidermal cells of arabidopsis. Plant Cell Physiol. 2001, 42, 894–899. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, M.; Neves, J.; Cardoso, T.; Pissarra, J.; Pereira, S.; Pereira, C. Coping with Abiotic Stress in Plants—An Endomembrane Trafficking Perspective. Plants 2022, 11, 338. [Google Scholar] [CrossRef]
- Žárský, V.; Kulich, I.; Fendrych, M.; Pečenková, T. Exocyst complexes multiple functions in plant cells secretory pathways. Curr. Opin. Plant Biol. 2013, 16, 726–733. [Google Scholar] [CrossRef]
- Wang, J.J.; Ding, Y.; Wang, J.J.; Hillmer, S.; Miao, Y.; Lo, S.W.; Wang, X.; Robinson, D.G.; Jiang, L. EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in arabidopsis and tobacco cells. Plant Cell 2010, 22, 4009–4030. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Robinson, D.G.; Jiang, L. Unconventional protein secretion (UPS) pathways in plants. Curr. Opin. Cell Biol. 2014, 29, 107–115. [Google Scholar] [CrossRef]
- Robinson, D.G.; Ding, Y.; Jiang, L. Unconventional protein secretion in plants: A critical assessment. Protoplasma 2016, 253, 31–43. [Google Scholar] [CrossRef]
- Wang, X.; Chung, K.P.; Lin, W.; Jiang, L. Protein secretion in plants: Conventional and unconventional pathways and new techniques. J. Exp. Bot. 2018, 69, 21–37. [Google Scholar] [CrossRef] [Green Version]
- Synek, L.; Schlager, N.; Eliás, M.; Quentin, M.; Hauser, M.-T.; Zárský, V. AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. Plant J. 2006, 48, 54–72. [Google Scholar] [CrossRef] [Green Version]
- Cvrčková, F.; Grunt, M.; Bezvoda, R.; Hála, M.; Kulich, I.; Rawat, A.; Zárský, V. Evolution of the land plant exocyst complexes. Front. Plant Sci. 2012, 3, 159. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; van Os, G.M.A.; Ren, S.; Yu, D.; Ketelaar, T.; Emons, A.M.C.; Liu, C.M. Expression and functional analyses of EXO70 genes in arabidopsis implicate their roles in regulating cell type-specific exocytosis. Plant Physiol. 2010, 154, 1819–1830. [Google Scholar] [CrossRef] [Green Version]
- Sharp, R.E.; Poroyko, V.; Hejlek, L.G.; Spollen, W.G.; Springer, G.K.; Bohnert, H.J.; Nguyen, H.T. Root growth maintenance during water deficits: Physiology to functional genomics*. J. Exp. Bot. 2004, 55, 2343–2351. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Peng, Y.; Xu, W. Crop Root Responses to Drought Stress: Molecular Mechanisms, Nutrient Regulations, and Interactions with Microorganisms in the Rhizosphere. Int. J. Mol. Sci. 2022, 23, 9310. [Google Scholar] [CrossRef]
- Sousa, B.; Soares, C.; Oliveira, F.; Martins, M.; Branco-Neves, S.; Barbosa, B.; Ataíde, I.; Teixeira, J.; Azenha, M.; Azevedo, R.A.; et al. Foliar application of 24-epibrassinolide improves Solanum nigrum L. tolerance to high levels of Zn without affecting its remediation potential. Chemosphere 2020, 244, 125579. [Google Scholar] [CrossRef]
- Lin, Y.F.; Aarts, M.G.M. The molecular mechanism of zinc and cadmium stress response in plants. Cell. Mol. Life Sci. 2012, 69, 3187–3206. [Google Scholar] [CrossRef]
- Zhang, Y.; Kaiser, E.; Li, T.; Marcelis, L.F.M. NaCl affects photosynthetic and stomatal dynamics by osmotic effects and reduces photosynthetic capacity by ionic effects in tomato. J. Exp. Bot. 2022, 73, 3637–3650. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Shpilka, T.; Weidberg, H.; Pietrokovski, S.; Elazar, Z. Atg8: An autophagy-related ubiquitin-like protein family. Genome Biol. 2011, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, M.; Ordóñez, A.; Sohn, E.J.; Robert, S.; Sánchez-Serrano, J.J.; Surpin, M.A.; Raikhel, N.V.; Rojo, E. Divergent functions of VTI12 and VTI11 in trafficking to storage and lytic vacuoles in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 3645–3650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zouhar, J. AtVPS45 is a positive regulator of the SYP41/SYP61/VTI12 SNARE complex involved in trafficking of vacuolar cargo. Plant Physiol. 2009, 149, 1668–1678. [Google Scholar] [CrossRef] [Green Version]
- Surpin, M.; Zheng, H.; Morita, M.T.; Saito, C.; Avila, E.; Blakeslee, J.J.; Bandyopadhyay, A.; Kovaleva, V.; Carter, D.; Murphy, A.; et al. The VTI Family of SNARE Proteins Is Necessary for Plant Viability and Mediates Different Protein Transport Pathways. Plant Cell 2003, 15, 2885–2899. [Google Scholar] [CrossRef] [Green Version]
- Bassham, D.C.; Laporte, M.; Marty, F.; Moriyasu, Y.; Ohsumi, Y.; Olsen, L.J.; Yoshimoto, K. Autophagy in development and stress responses of plants. Autophagy 2006, 2, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Hochstrasser, M. Microautophagy regulates proteasome homeostasis. Curr. Genet. 2020, 66, 683–687. [Google Scholar] [CrossRef]
- Han, S.; Yu, B.; Wang, Y.; Liu, Y. Role of plant autophagy in stress response. Protein Cell 2011, 2, 784–791. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xiong, Y.; Bassham, D.C. Autophagy is required for tolerance of drought and salt stress in plants. Autophagy 2009, 5, 954–963. [Google Scholar] [CrossRef] [Green Version]
- Pečenková, T.; Marković, V.; Sabol, P.; Kulich, I.; Zárský, V. Exocyst and autophagy-related membrane trafficking in plants. J. Exp. Bot. 2017, 69, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Su, T.; Li, X.; Yang, M.; Shao, Q.; Zhao, Y.; Ma, C.; Wang, P. Autophagy: An Intracellular Degradation Pathway Regulating Plant Survival and Stress Response. Front. Plant Sci. 2020, 11, 164. [Google Scholar] [CrossRef]
- Su, T.; Wang, P.; Li, H.; Zhao, Y.; Lu, Y.; Dai, P.; Ren, T.; Wang, X.; Li, X.; Shao, Q.; et al. The Arabidopsis catalase triple mutant reveals important roles of catalases and peroxisome-derived signaling in plant development. J. Integr. Plant Biol. 2018, 60, 591–607. [Google Scholar] [CrossRef]
- Barozzi, F.; Papadia, P.; Stefano, G.; Renna, L.; Brandizzi, F.; Migoni, D.; Fanizzi, F.P.; Piro, G.; Di Sansebastiano, G. Pietro Variation in membrane trafficking linked to SNARE AtSYP51 interaction with aquaporin NIP1;1. Front. Plant Sci. 2019, 9, 1949. [Google Scholar] [CrossRef] [Green Version]
- De Benedictis, M.; Bleve, G.; Faraco, M.; Stigliano, E.; Grieco, F.; Piro, G.; Dalessandro, G.; Di Sansebastiano, G. Pietro AtSYP51/52 functions diverge in the post-golgi traffic and differently affect vacuolar sorting. Mol. Plant 2013, 6, 916–930. [Google Scholar] [CrossRef] [Green Version]
- Madeira, A.; Moura, T.F.; Soveral, G. Detecting aquaporin function and regulation. Front. Chem. 2016, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanoparticle Res. 2012, 14, 1125. [Google Scholar] [CrossRef]
- Sousa, B.; Rodrigues, F.; Soares, C.; Martins, M.; Azenha, M.; Lino-Neto, T.; Santos, C.; Cunha, A.; Fidalgo, F. Impact of Combined Heat and Salt Stresses on Tomato Plants—Insights into Nutrient Uptake and Redox Homeostasis. Antioxidants 2022, 11, 478. [Google Scholar] [CrossRef]
- Branco-Neves, S.; Soares, C.; De Sousa, A.; Martins, V.; Azenha, M.; Gerós, H.; Fidalgo, F. An efficient antioxidant system and heavy metal exclusion from leaves make Solanum cheesmaniae more tolerant to Cu than its cultivated counterpart. Food Energy Secur. 2017, 6, 123–133. [Google Scholar] [CrossRef]
- Fujita, M.; Hasanuzzaman, M. Approaches to Enhancing Antioxidant Defense in Plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef]
- Spormann, S.; Soares, C.; Martins, V.; Azenha, M.; Gerós, H.; Fidalgo, F. Early Activation of Antioxidant Responses in Ni-Stressed Tomato Cultivars Determines Their Resilience Under Co-exposure to Drought. J. Plant Growth Regul. 2022, 1–15. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.-J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [Green Version]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Cummins, I.; Dixon, D.P.; Freitag-Pohl, S.; Skipsey, M.; Edwards, R. Multiple roles for plant glutathione transferases in xenobiotic detoxification. Drug Metab. Rev. 2011, 43, 266–280. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mullen, R.T.; Lisenbee, C.S.; Miernyk, J.A.; Trelease, R.N. Peroxisomal membrane ascorbate peroxidase is sorted to a membranous network that resembles a subdomain of the endoplasmic reticulum. Plant Cell 1999, 11, 2167–2185. [Google Scholar] [CrossRef]


| Stress Type | Nomenclature | Stress Inducer | Concentration |
|---|---|---|---|
| Control | C | - | - |
| Saline | S | Sodium Chloride | 50 mM |
| Hydric | H1 | Mannitol | 50 mM |
| H2 | Mannitol | 100 Mm | |
| Oxidative | Ox | Hydrogen Peroxide | 0.5 mM |
| Zinc | Zn | Zinc Sulphate | 150 µM |
| Genes | Primer Forward | Primer Reverse |
|---|---|---|
| SAND1 | AACTCTATGCAGCATTTGATCCACT | TGATTGCATATCTTTATCGCCATC |
| GAPDH1 | TTGGTGACAACAGGTCCAAGCA | AAACTTGTCGCTCAATGCAATC |
| EXO70 | TCCCCGATGAAACAGGCTCGTC | GCCTCCATGAAAGGGGCGTGT |
| ATG8 | TTGCTTGCTTGAAATTCGCA | TTCACTCATCCTTGCCTCGA |
| VTI12 | GCAATGTCCGTGGAGAGGCTTGA | TGCGCATGAAGGAGGGTTTGG |
| SYP51 | TGGCGTCTTCATCGGATTCATGG | AGCTGAAGCACGACGCTGAGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, J.; Monteiro, J.; Sousa, B.; Soares, C.; Pereira, S.; Fidalgo, F.; Pissarra, J.; Pereira, C. Relevance of the Exocyst in Arabidopsis exo70e2 Mutant for Cellular Homeostasis under Stress. Int. J. Mol. Sci. 2023, 24, 424. https://doi.org/10.3390/ijms24010424
Neves J, Monteiro J, Sousa B, Soares C, Pereira S, Fidalgo F, Pissarra J, Pereira C. Relevance of the Exocyst in Arabidopsis exo70e2 Mutant for Cellular Homeostasis under Stress. International Journal of Molecular Sciences. 2023; 24(1):424. https://doi.org/10.3390/ijms24010424
Chicago/Turabian StyleNeves, João, João Monteiro, Bruno Sousa, Cristiano Soares, Susana Pereira, Fernanda Fidalgo, José Pissarra, and Cláudia Pereira. 2023. "Relevance of the Exocyst in Arabidopsis exo70e2 Mutant for Cellular Homeostasis under Stress" International Journal of Molecular Sciences 24, no. 1: 424. https://doi.org/10.3390/ijms24010424





