Determination of BRCAness Phenotype in Breast Tumors for the Appointment of Neoadjuvant Chemotherapy Based on Platinum and Taxanes
Abstract
1. Introduction
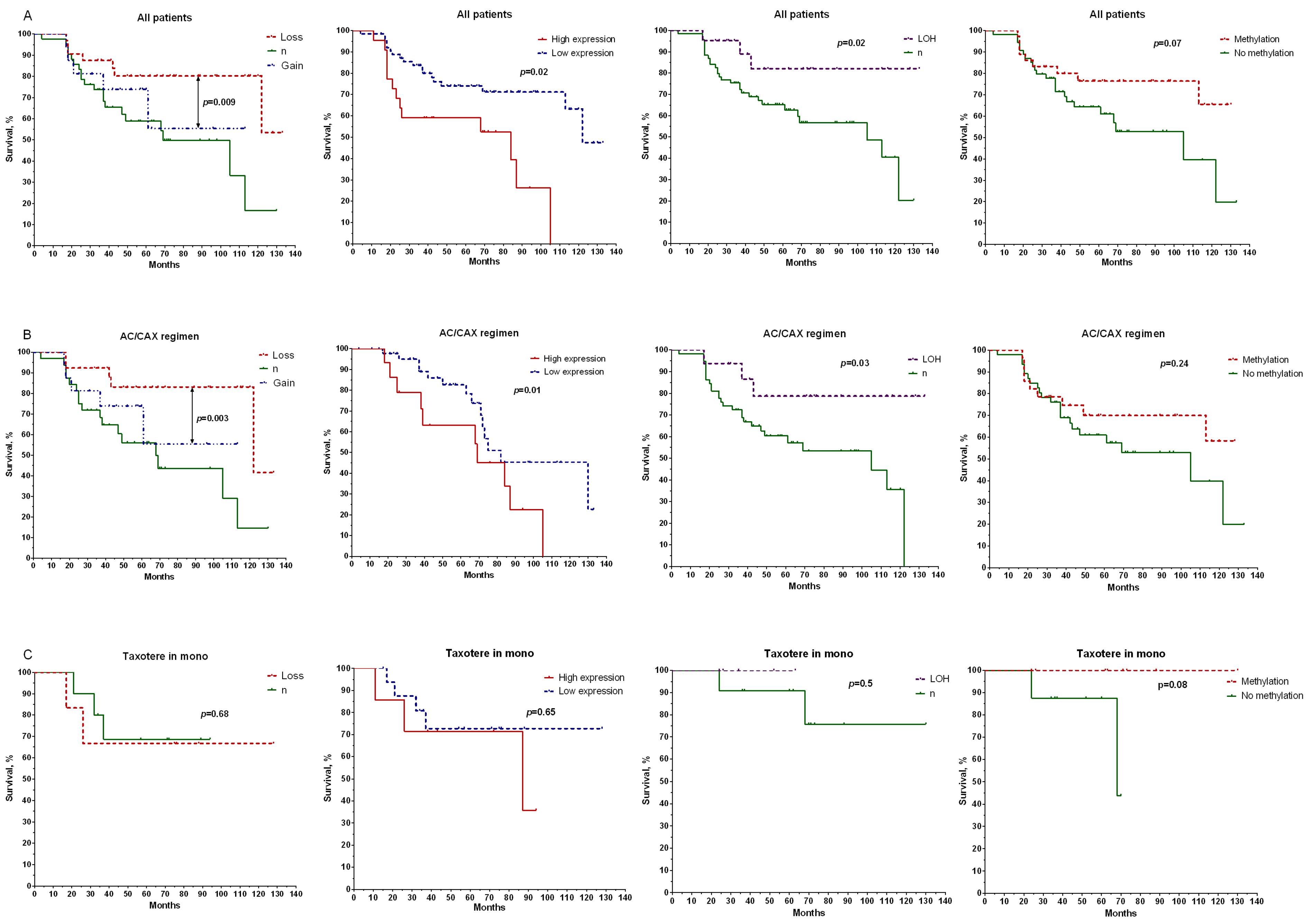
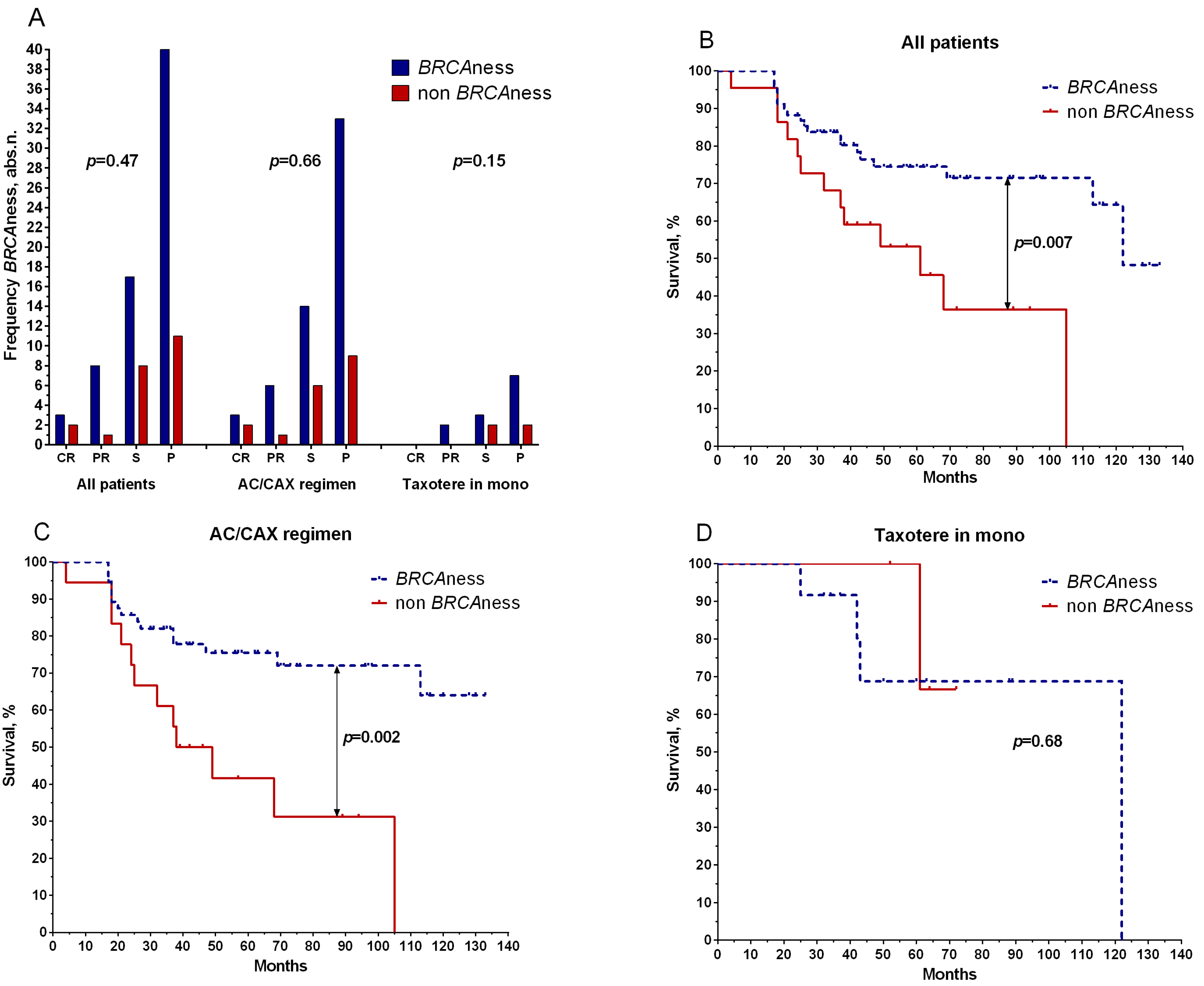
2. Results
2.1. Retrospective Study
2.2. Prospective Study
3. Discussion
4. Materials and Methods
4.1. RNA Isolation
4.2. Quantitative PCR
4.3. DNA Extraction
4.4. Methyl-Sensitive PCR
4.5. Microarray Analysis
4.6. Statistical Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turner, N.; Tutt, A.; Ashworth, A. Hallmarks of'BRCAness’ in sporadic cancers. Nat. Rev. Cancer 2004, 4, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Venkitaraman, A.R. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 2002, 108, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Sueta, A.; Yamamoto–Ibusuki, M.; Tomiguchi, M.; Fujiki, Y.; Goto-Yamaguchi, L.; Iwase, H.; Yamamoto, Y. Predictive and prognostic significance of BRCAness in HER2-negative breast cancer. Breast Cancer 2022, 29, 368–376. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Wang, Y.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; Lin, B.; Xie, Y. Prevalence of BRCA1 mutations and responses to neoadjuvant chemotherapy among BRCA1 carriers and non–carriers with triple-negative breast cancer. Ann. Oncol. 2015, 26, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Gluz, O.; Nitz, U.; Christgen, M.; Grischke, E.-M.; Forstbauer, H.; Braun, M.W.; Warm, M.; Uleer, C.; Aktas, B.; Schumacher, C. Efficacy of 12 weeks neoadjuvant nab–paclitaxel combined with carboplatinum vs. gemcitabine in triple-negative breast cancer: WSG-ADAPT TN randomized phase II trial. J. Clin. Oncol. 2015, 33, 1032. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. BRCAness revisited. Nat. Rev. Cancer 2016, 16, 110. [Google Scholar] [CrossRef]
- Byrum, A.K.; Vindigni, A.; Mosammaparast, N. Defining and modulating ‘BRCAness’. Trends Cell Biol. 2019, 29, 740–751. [Google Scholar] [CrossRef]
- Turner, N.C.; Reis-Filho, J.S. Basal-like breast cancer and the BRCA1 phenotype. Oncogene 2006, 25, 5846–5853. [Google Scholar] [CrossRef]
- Tian, T.; Shan, L.; Yang, W.; Zhou, X.; Shui, R. Evaluation of the BRCAness phenotype and its correlations with clinicopathological features in triple-negative breast cancers. Hum. Pathol. 2019, 84, 231–238. [Google Scholar] [CrossRef]
- Carser, J.E.; Quinn, J.E.; Michie, C.O.; O’Brien, E.J.; McCluggage, W.G.; Maxwell, P.; Lamers, E.; Lioe, T.F.; Williams, A.R.; Kennedy, R.D. BRCA1 is both a prognostic and predictive biomarker of response to chemotherapy in sporadic epithelial ovarian cancer. Gynecol. Oncol. 2011, 123, 492–498. [Google Scholar] [CrossRef]
- Kosaka, Y.; Yamamoto, Y.; Tanino, H.; Nishimiya, H.; Yamamoto-Ibusuki, M.; Hirota, Y.; Iwase, H.; Nakamura, S.; Akashi–Tanaka, S. BRCAness as an Important Prognostic Marker in Patients with Triple-negative breast cancer Treated with Neoadjuvant Chemotherapy: A Multicenter Retrospective Study. Diagnostics 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.; Tovey, H.; Cheang, M.C.U.; Kernaghan, S.; Kilburn, L.; Gazinska, P.; Owen, J.; Abraham, J.; Barrett, S.; Barrett-Lee, P. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: The TNT Trial. Nature medicine. Nat. Med. 2018, 24, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Evers, B.; Schut, E.; van der Burg, E.; Braumuller, T.M.; Egan, D.A.; Holstege, H.; Edser, P.; Adams, D.J.; Wade-Martins, R.; Bouwman, P. A High-Throughput Pharmaceutical Screen Identifies Compounds with Specific Toxicity against BRCA2-Deficient TumorsA Screen for Compounds Toxic to BRCA2-Deficient Tumors. Clin. Cancer Res. 2010, 16, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, V.; Scarpi, E.; Farolfi, A.; Brighi, N.; Rossi, L.; Gurioli, G.; Lolli, C.; Schepisi, G.; Bleve, S.; Gianni, C. Melphalan as a Promising Treatment for BRCA-Related Ovarian Carcinoma. Front. Oncol. 2021, 11, 716467. [Google Scholar] [CrossRef] [PubMed]
- Kaklamani, V.G.; Jeruss, J.S.; Hughes, E.; Siziopikou, K.; Timms, K.M.; Gutin, A.; Abkevich, V.; Sangale, Z.; Solimeno, C.; Brown, K.L. Phase II neoadjuvant clinical trial of carboplatin and eribulin in women with triple negative early-stage breast cancer (NCT01372579). Breast Cancer Res. Treat. 2015, 151, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Timms, K.; Untch, M.; Elkin, E.P.; Fasching, P.A.; Schneeweiss, A.; Salat, C.; Rezai, M.; Blohmer, J.U.; Zahm, D.M. Prediction of pathological complete response (pCR) by Homologous Recombination Deficiency (HRD) after carboplatin-containing neoadjuvant chemotherapy in patients with TNBC: Results from GeparSixto. J. Clin. Oncol. 2015, 33, 1004. [Google Scholar] [CrossRef]
- Quereda, V.; Bayle, S.; Vena, F.; Frydman, S.M.; Monastyrskyi, A.; Roush, W.R.; Duckett, D.R. Therapeutic targeting of CDK12/CDK13 in triple-negative breast cancer. Cancer Cell 2019, 36, 545–558. [Google Scholar] [CrossRef]
- Palleschi, M.; Tedaldi, G.; Sirico, M.; Virga, A.; Ulivi, P.; De Giorgi, U. Moving beyond PARP Inhibition: Current State and Future Perspectives in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 7884. [Google Scholar] [CrossRef]
- Tutt, A.; Robson, M.; Garber, J.E.; Domchek, S.M.; Audeh, M.W.; Weitzel, J.N.; Friedlander, M.; Arun, B.; Loman, N.; Schmutzler, R.K. Oral poly (ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet 2010, 376, 235–244. [Google Scholar] [CrossRef]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.-H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Yin, C.; Kulasekaran, M.; Roy, T.; Decker, B.; Alexander, S.; Margolis, M.; Jha, R.C.; Kupfer, G.M.; He, A.R. Homologous Recombination Repair in Biliary Tract Cancers: A Prime Target for PARP Inhibition? Cancers 2022, 14, 2561. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.-Y.; Lee, H. Inhibition of poly (ADP-Ribose) polymerase: A promising strategy targeting pancreatic cancer with BRCAness phenotype. World J. Gastrointest. Oncol. 2021, 13, 1544. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Matsunaga, Y.; Tsurutani, J.; Akashi-Tanaka, S.; Masuda, H.; Ide, Y.; Hashimoto, R.; Inuzuka, M.; Watanabe, C.; Taruno, K. BRCAness as a prognostic indicator in patients with early breast cancer. Sci. Rep. 2020, 10, 21173. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, S.; Sato, E.; Narui, K.; Yamada, A.; Fujita, T.; Yamada, K.; Oba, M.; Ishikawa, T. Neoadjuvant chemotherapy with anthracycline-based regimen for BRCAness tumors in triple-negative breast cancer. J. Surg. Res. 2020, 250, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Jacot, W.; Lopez-Crapez, E.; Mollevi, C.; Boissière-Michot, F.; Simony-Lafontaine, J.; Ho-Pun-Cheung, A.; Chartron, E.; Theillet, C.; Lemoine, A.; Saffroy, R. BRCA1 promoter hypermethylation is associated with good prognosis and chemosensitivity in triple-negative breast cancer. Cancers 2020, 12, 828. [Google Scholar] [CrossRef] [PubMed]
- Telli, M.L.; Jensen, K.C.; Vinayak, S.; Kurian, A.W.; Lipson, J.A.; Flaherty, P.J.; Timms, K.; Abkevich, V.; Schackmann, E.A.; Wapnir, I.L. Phase II study of gemcitabine, carboplatin, and iniparib as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancer with assessment of a tumor–based measure of genomic instability: PrECOG 0105. J. Clin. Oncol. 2015, 33, 1895. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Spentzos, D.; Karlan, B.Y.; Taniguchi, T.; Fountzilas, E.; Francoeur, N.; Levine, D.A.; Cannistra, S.A. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J. Clin. Oncol. 2010, 28, 3555. [Google Scholar] [CrossRef]
- Ruscito, I.; Dimitrova, D.; Vasconcelos, I.; Gellhaus, K.; Schwachula, T.; Bellati, F.; Zeillinger, R.; Benedetti-Panici, P.; Vergote, I.; Mahner, S. BRCA1 gene promoter methylation status in high-grade serous ovarian cancer patients—A study of the tumour Bank ovarian cancer (TOC) and ovarian cancer diagnosis consortium (OVCAD). Eur. J. Cancer 2014, 50, 2090–2098. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Schwartz, G.F.; Hortobagyi, G.N. Proceedings of the consensus conference on neoadjuvant chemotherapy in carcinoma of the breast, April 26–28, 2003, Philadelphia, Pennsylvania. Breast J. 2004, 10, 273–294. [Google Scholar] [CrossRef]
- Schroeder, A.; Mueller, O.; Stocker, S.; Salowsky, R.; Leiber, M.; Gassmann, M.; Lightfoot, S.; Menzel, W.; Granzow, M.; Ragg, T. The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Mol. Biol. 2006, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]


| Clinical and Pathological Parameter | Retrospective Group (n = 90); Number of Patients (abs. p. (%)) | Prospective Group (n = 40); Number of Patients (abs. p. (%)) | p-Value | |
|---|---|---|---|---|
| Age | ≤45 | 35 (38.9) | 22 (55.0) | 0.12 |
| >45 | 55 (61.1) | 18 (45.0) | ||
| Menstrual status | Premenopause | 51 (56.7) | 33 (82.5) | 0.005 * |
| Postmenopause | 39 (43.3) | 7 (17.5) | ||
| Tumor size | T1 | 12 (13.3) | 5 (12.5) | 0.38 |
| T2 | 66 (73.3) | 30 (75.0) | ||
| T3 | 4 (4.4) | 4 (10.0) | ||
| T4 | 8 (8.9) | 1 (2.5) | ||
| Lymphogenous metastasis | N0 | 38 (42.2) | 16 (40.0) | 0.46 |
| N1 | 37 (41.1) | 20 (50.0) | ||
| N2 | 6 (6.7) | 3 (7.5) | ||
| N3 | 9 (10.0) | 1 (2.5) | ||
| Molecular subtype | Luminal B | 60 (66.7) | 37 (92.5) | 0.007 * |
| Triple-negative | 19 (21.1) | 2 (5.0) | ||
| HER2-positive | 11 (12.2) | 1 (2.5) | ||
| Histological form | Unicentric | 58 (64.4) | 21 (52.5) | 0.24 |
| Multicentric | 32 (35.6) | 19 (47.5) | ||
| NAC regimen | CAX | 28 (31.1) | - | - |
| AC | 46 (51.1) | - | ||
| Taxotere | 16 (17.8) | 26 (65.0) | ||
| CP | - | 14 (35.0) | ||
| NAC response | Progression | 9 (10.0) | 0 (0.0) | 0.007 * |
| Stabilization | 25 (27.8) | 6 (15.0) | ||
| Partial regression | 51 (56.7) | 26 (65.0) | ||
| Complete regression | 5 (5.6) | 8 (20.0) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsyganov, M.M.; Ibragimova, M.K.; Garbukov, E.Y.; Bragina, O.D.; Karchevskaya, A.A.; Usynin, E.A.; Litvyakov, N.V. Determination of BRCAness Phenotype in Breast Tumors for the Appointment of Neoadjuvant Chemotherapy Based on Platinum and Taxanes. Int. J. Mol. Sci. 2023, 24, 207. https://doi.org/10.3390/ijms24010207
Tsyganov MM, Ibragimova MK, Garbukov EY, Bragina OD, Karchevskaya AA, Usynin EA, Litvyakov NV. Determination of BRCAness Phenotype in Breast Tumors for the Appointment of Neoadjuvant Chemotherapy Based on Platinum and Taxanes. International Journal of Molecular Sciences. 2023; 24(1):207. https://doi.org/10.3390/ijms24010207
Chicago/Turabian StyleTsyganov, Matvey Mihajlovich, Marina K. Ibragimova, Evgeniy Y. Garbukov, Olga D. Bragina, Ariana A. Karchevskaya, Evgeny A. Usynin, and Nikolai V. Litvyakov. 2023. "Determination of BRCAness Phenotype in Breast Tumors for the Appointment of Neoadjuvant Chemotherapy Based on Platinum and Taxanes" International Journal of Molecular Sciences 24, no. 1: 207. https://doi.org/10.3390/ijms24010207
APA StyleTsyganov, M. M., Ibragimova, M. K., Garbukov, E. Y., Bragina, O. D., Karchevskaya, A. A., Usynin, E. A., & Litvyakov, N. V. (2023). Determination of BRCAness Phenotype in Breast Tumors for the Appointment of Neoadjuvant Chemotherapy Based on Platinum and Taxanes. International Journal of Molecular Sciences, 24(1), 207. https://doi.org/10.3390/ijms24010207







