Sodium Alginate-g-acrylamide/acrylic Acid Hydrogels Obtained by Electron Beam Irradiation for Soil Conditioning
Abstract
:1. Introduction
2. Results and Discussion
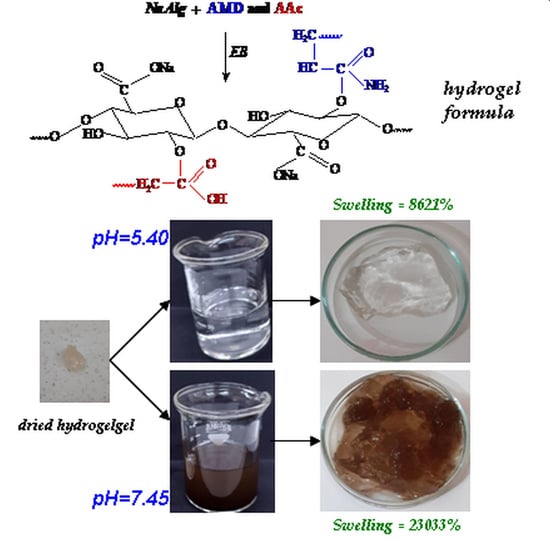
2.1. Percentages of Gel Fraction, Swelling and Equilibrium Water Content
2.2. Cross-Link Density, Porosity and Mesh Size
2.3. Swelling Kinetics, Swelling Power and Diffusion Coefficients Determination
2.4. Absorption and Release Capacities
2.5. Spectral Characterization
2.6. Morphological Characterization
3. Materials and Methods
3.1. Materials
3.2. Experimental Installation and Samples Preparation
3.3. Sodium Alginate-g-acrylamide/acrylic Acid Hydrogels Characterization
3.3.1. Gel Fraction, Swelling and Equilibrium Water Content Measurements
3.3.2. Network Studies
3.3.3. Swelling Kinetics and Swelling Power
3.3.4. Evaluation of Hydrogels Capacity to Absorb and Release Aqueous Nutrients Solutions by Multiple Usage Experiments
3.3.5. Spectral Characterization by Fourier Transform Infrared Spectroscopy (FTIR)
3.3.6. Morphological Investigations by Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Water Use in Agriculture. Available online: https://energypedia.info/wiki/Water_Use_in_Agriculture (accessed on 3 December 2022).
- Available online: http://www.fao.org/assets/infographics/FAO-Infographic-water-thirsty-en.pdf (accessed on 3 December 2022).
- Durpekova, S.; Di Martino, A.; Dusankova, M.; Drohsler, P.; Sedlarik, V. Biopolymer Hydrogel Based on Acid Whey and Cellulose Derivatives for Enhancement Water Retention Capacity of Soil and Slow Release of Fertilizers. Polymers 2021, 13, 3274. [Google Scholar] [CrossRef] [PubMed]
- Oladosu, Y.; Rafii, M.Y.; Arolu, F.; Chukwu, S.C.; Salisu, M.A.; Fagbohun, I.K.; Muftaudeen, T.K.; Swaray, S.; Haliru, B.S. Superabsorbent Polymer Hydrogels for Sustainable Agriculture: A Review. Horticulturae 2022, 8, 605. [Google Scholar] [CrossRef]
- Patra, S.K.; Poddar, R.; Brestic, M.; Acharjee, P.U.; Bhattacharya, P.; Sengupta, S.; Pal, P.; Bam, N.; Biswas, B.; Barek, V.; et al. Prospects of Hydrogels in Agriculture for Enhancing Crop and Water Productivity under Water Deficit Condition. Int. J. Polym. Sci. 2022, 49, 14836. [Google Scholar] [CrossRef]
- Mikkelsen, R.L. Using hydrophilic polymers to control nutrient release. Fert. Res. 1994, 38, 53–59. Available online: https://link.springer.com/content/pdf/10.1007/BF00750062.pdf?pdf=button (accessed on 3 December 2022). [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Palanivelu, S.D.; Armir, N.A.Z.; Zulkifli, A.; Hair, A.H.A.; Salleh, K.M.; Lindsey, K.; Che-Othman, M.H.; Zakaria, S. Hydrogel Application in Urban Farming: Potentials and Limitations—A Review. Polymers 2022, 14, 2590. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A Review on the Design and Hydration Properties of Natural Polymer-Based Hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Biswas, G.; Majee, S.; Roy, A. Combination of synthetic and natural polymers in hydrogel: An impact on drug permeation. J. Appl. Pharm. Sci. 2016, 6, 158–164. Available online: https://japsonline.com/admin/php/uploads/2065_pdf.pdf (accessed on 3 December 2022). [CrossRef] [Green Version]
- Fan, L.; Du, Y.; Zhang, B.; Yang, J.; Cai, J.; Zhang, L.; Zhou, J. Preparation and Properties of Alginate/Water-Soluble Chitin Blend Fibers. J. Macromol. Sci. A 2005, 42, 723–732. [Google Scholar] [CrossRef]
- Kushibiki, T.; Tomoshige, R.; Iwanga, K.; Kakemi, M.; Tabata, Y. Controlled release of plasmid DNA from hydrogels prepared from gelatin cationized by different amine compounds. J. Control. Release 2006, 112, 249–256. [Google Scholar] [CrossRef]
- Aroguz, A.Z.; Baysal, K.; Adiguzel, Z.; Baysal, B.M. Alginate/Polyoxyethylene and Alginate/Gelatin Hydrogels: Preparation, Characterization, and Application in Tissue Engineering. Appl. Biochem. Biotechnol. 2014, 173, 433–448. Available online: https://link.springer.com/article/10.1007/s12010-014-0851-0 (accessed on 3 December 2022). [CrossRef]
- Boanini, E.; Rubini, K.; Panzavolta, S.; Bigi, A. Chemico-physical characterization of gelatin films modified with oxidized alginate. Acta Biomater. 2010, 6, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Man, E.; Lamprou, D.; Easdon, C.; McLellan, I.; Yiu, H.H.P.; Hoskins, C. Exploration of Dual Ionic Cross-Linked Alginate Hydrogels Via Cations of Varying Valences towards Wound Healing. Polymers 2022, 14, 5192. [Google Scholar] [CrossRef]
- Singh, R.P.; Tripathy, T.; Karmakar, G.P.; Rath, S.K.; Karmakar, N.C.; Pandey, S.R.; Kannan, K.; Jain, S.K.; Lan, N.T. Novel biodegradable flocculants based on polysaccharides. Curr. Sci. India 2000, 78, 798–803. [Google Scholar]
- Swanson, C.L.; Shogren, R.L.; Fanta, G.F.; Imam, S.H. Starch-plastic materials—Preparation, physical properties, and biodegradability (a review of recent USDA research). J. Environ. Polym. Degrad. 1993, 1, 155–166. [Google Scholar] [CrossRef]
- Singh, R.P. Advanced Turbulent Drag Reducing and Flocculating Materials Based on Polysaccharides. In Polymers and Other Advanced Materials: Emerging Technologies and Business Opportunities, 1st ed.; Prasad, P.N., Mark, E., Fai, T.J., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 227–249. [Google Scholar]
- Manaila, E.; Craciun, G.; Ighigeanu, D.; Campeanu, C.; Barna, C.; Fugaru, V. Hydrogels synthesized by electron beam irradiation for heavy metal adsorption. Materials 2017, 10, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craciun, G.; Manaila, E.; Stelescu, M.D. Electron Beam Synthesis and Characterization of Acrylamide/Acrylic Acid Hydrogels Using Trimethylolpropane Trimethacrylate as Cross-Linker. J. Chem. 2016, 2016, 1470965. [Google Scholar] [CrossRef]
- El-Naggar, A.A. Radiation synthesis of superabsorbent hydrogels based on carboxymethyl cellulose/sodium alginate for absorbent of heavy metal ions from waste water. J. Thermoplast. Compos. 2016, 29, 16–27. [Google Scholar] [CrossRef]
- Erizal; Sudirman, S.; Budianto, E.; Mahendra, A.; Yudianti, R. Radiation Synthesis of Superabsorbent Poly(acrylamide-co-acrylic acid)-Sodium Alginate Hydrogels. Adv. Mat. Res. 2013, 746, 88–96. Available online: http://repo.unand.ac.id/27461/1/PR_DDH_06%20artikel.pdf (accessed on 4 December 2022). [CrossRef]
- Nagasawa, N.; Mitomo, H.; Yoshii, F.; Kume, T. Radiation-induced degradation of sodium alginate. Polym. Degrad. Stabil. 2000, 69, 279–285. [Google Scholar] [CrossRef]
- Abd El-Mohdy, H.L. Radiation-induced degradation of sodium alginate and its plant growth promotion effect. Arab. J. Chem. 2017, 10, S431–S438. [Google Scholar] [CrossRef]
- Şen, M. Effects of molecular weight and ratio of guluronic acid to mannuronic acid on the antioxidant properties of sodium alginate fractions prepared by radiation-induced degradation. Appl. Radiat. Isotopes 2011, 69, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Jing, R.; Yancun, Z.; Jiuqiang, L.; Hongfei, H. Radiation synthesis and characteristic of IPN hydrogels composed of poly(diallyldimethylammonium chloride) and Kappa-Carrageenan. Radiat. Phys. Chem. 2001, 62, 277–281. [Google Scholar] [CrossRef]
- Thakur, A.; Wanchoo, R.K.; Singh, P. Structural Parameters and Swelling Behavior of pHSensitive Poly(acrylamide-co-acrylic acid) Hydrogels. Chem. Biochem. Eng. Q. 2011, 25, 181–194. Available online: https://www.researchgate.net/publication/266502664 (accessed on 3 December 2022).
- Erceg, T.; Brakus, G.; Stupar, A.; Cvetinov, M.; Hadnadev, M.; Ristic, I. Synthesis and Characterization of Chitosan-Acrylic Acid Based Hydrogels and Investigation the Properties of Bilayered Design with Incorporated Alginate Beads. J. Polym. Environ. 2022, 30, 3737–3760. [Google Scholar] [CrossRef]
- Khan, M.A.; Azad, A.K.; Safdar, M.; Nawaz, A.; Akhlaq, M.; Paul, P.; Hossain, M.K.; Rahman, M.H.; Baty, R.S.; El-Kott, A.F.; et al. Synthesis and Characterization of Acrylamide/Acrylic Acid Co-Polymers and Glutaraldehyde Crosslinked pH-Sensitive Hydrogels. Gels 2022, 8, 47. [Google Scholar] [CrossRef]
- Dergunov, S.A.; Nam, I.K.; Mun, G.A.; Nurkeeva, Z.S.; Shaikhutdinov, E.M. Radiation synthesis and characterization of stimulisensitive chitosan–polyvinyl pyrrolidone hydrogels. Radiat. Phys. Chem. 2005, 72, 619–623. [Google Scholar] [CrossRef]
- Karadag, E.; Saraydin, D. Swelling studies of super water retainer acrylamide/crotonic acid hydrogels crosslinked by trimethylolpropane triacrylate and 1,4-butanediol dimethacrylate. Polym. Bull. 2002, 48, 299–307. [Google Scholar] [CrossRef]
- Ostrowska-Czubenko, J.; Gierszewska-Drużyńska, M. Mechanism of water diffusion into noncrosslinked and ionically crosslinked chitosan membranes. In Progress on Chemistry and Application of Chitin and Its Derivatives; Jaworska, M.M., Ed.; Polish Chitin Society: Łódź, Poland, 2012; Volume XVII, pp. 59–66. Available online: https://www.researchgate.net/publication/287905244_Mechanism_of_water_diffusion_into_noncrosslinked_and_ionically_crosslinked_chitosan_membranes (accessed on 8 December 2022).
- Khare, A.R.; Peppas, N.A. Swelling/deswelling of anionic copolymer gels. Biomaterials 1995, 16, 559–567. [Google Scholar] [CrossRef]
- Lin, W.C.; Yu, D.G.; Yang, M.C. pH-sensitive polyelectrolyte complex gel microspheres composed of chitosan/sodium tripolyphosphate/dextran sulfate: Swelling kinetics and drug delivery properties. Colloid Surface B 2005, 44, 143–151. [Google Scholar] [CrossRef]
- Rao, K.V.R.; Devi, K.P.; Buri, P. Cellulose matrices for zero-order release of soluble drugs. Drug Dev. Ind. Pharm. 1988, 14, 2299–2320. [Google Scholar] [CrossRef]
- Munday, D.L.; Cox, P.J. Compressed xanthan and karaya gum matrices: Hydration, erosion and drug release mechanisms. Int. J. Pharm. 2000, 203, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.T.; Lim, M.C.H.; Whittaker, A.K. Water diffusion in hydroxyethyl methacrylate (HEMA)-based hydrogels formed by γ-radiolysis. Polym. Int. 1999, 48, 1046–1052. [Google Scholar] [CrossRef]
- Krongauz, V.V. Diffusion in polymers dependence on crosslink density. Eyring approach to mechanism. J. Therm. Anal. Calor. 2010, 102, 435–445. [Google Scholar] [CrossRef]
- Demeter, M.; Virgolici, M.; Vancea, C.; Scarisoreanu, A.; Kaya, M.G.A.; Meltzer, V. Network structure studies on gamma-irradiated collagen-PVP superabsorbent hydrogels. Radiat. Phys. Chem. 2017, 131, 51–59. [Google Scholar] [CrossRef]
- Karadag, E.; Uzum, O.B.; Saraydin, D. Water uptake in chemically crosslinked poly(acrylamide-co-crotonic acid) hydrogels. Mater. Design. 2005, 26, 265–270. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Johnson, S. Superabsorbent hydrogels for removal of divalent toxic ions. Part I: Synthesis and swelling characterization. React. Funct. Polym. 2005, 62, 271–283. [Google Scholar] [CrossRef]
- Jastram, A.; Lindner, T.; Luebbert, C.; Sadowski, G.; Kragl, U. Swelling and Diffusion in PolymerizedIonic Liquids-Based Hydrogels. Polymers 2021, 13, 1834. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Xia, Q.; Bajalis, E.; Xi, H.; Lin, Y. Swelling/Deswelling Kinetics of PNIPAAm Hydrogels Synthesized by Microwave Irradiation. Chem. Eng. J. 2008, 142, 263–270. [Google Scholar] [CrossRef]
- ALSamman, M.T.; Sanchez, J. Chitosan- and Alginate-Based Hydrogels for the Adsorption of Anionic and Cationic Dyes from Water. Polymers 2022, 14, 1498. [Google Scholar] [CrossRef]
- Shamsul Alam, M.D.; Arifuzzaman Khan, G.M.; Abdur Razzaque, S.M.; Alam, M.N.; Mondal, M.I.H. Swelling Property of the Polyacrylamide Hydrogel Prepared by ϒ-ray Irradiation. J. Polym. Mater. 2008, 25, 645–651. Available online: https://www.researchgate.net/publication/257139633 (accessed on 8 December 2022).
- Sadeghi, M.; Godarzi, A.; Khani, F.; Mirdarikvande, S.; Sadeghi, H.; Shasavari, H. Synthesis of a Novel Biopolymer-based alginate Superabsorbent Hydrogel. Bull. Env. Pharmacol. Life Sci. 2014, 3, 169–174. [Google Scholar]
- Couto da Feira, J.M.; Klein, J.M.; Camargo Forte, M.M. Ultrasound-assisted synthesis of polyacrylamide-grafted sodium alginate and its application in dye removal. Polímeros 2018, 28, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Patel, G.M.; Patel, C.P.; Trivedi, H.C. Ceric-induced grafting of methyl acrylate onto sodium salt of partially carboxymethylated sodium alginate. Eur. Polym. J. 1999, 35, 201–208. [Google Scholar] [CrossRef]
- Sand, A.; Vyas, A.; Gupta, A.K. Graft copolymer based on (sodium alginate-g-acrylamide): Characterization and study of Water swelling capacity, metal ion sorption, flocculation and resistance to biodegradability. Int. J. Biol. Macromol. 2016, 90, 37–43. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. Available online: https://www.nature.com/articles/nature11409 (accessed on 3 December 2022). [CrossRef] [Green Version]
- Alberti, A.; Bertini, S.; Gastaldi, G.; Iannaccone, N.; Macciantelli, D.; Torri, G.; Vismara, E. Electron beam irradiated textile cellulose fibres: ESR studies and derivatisation with glycidyl methacrylate (GMA). Eur. Polym. J. 2005, 41, 1787–1797. [Google Scholar] [CrossRef]
- Craciun, G.; Manaila, E.; Ighigeanu, D. New Type of Sodium Alginate-g-acrylamide Polyelectrolyte Obtained by Electron Beam Irradiation: Characterization and Study of Flocculation Efficacy and Heavy Metal Removal Capacity. Polymers 2019, 11, 234. [Google Scholar] [CrossRef] [Green Version]
- Isiklan, N.; Kursun, F. Synthesis and characterization of graft copolymer of sodium alginate and poly(itaconic acid) by the redox system. Polym. Bull. 2013, 70, 1065–1084. Available online: https://www.researchgate.net/publication/257394652 (accessed on 3 December 2022). [CrossRef]
- Singh, T.; Singhal, R. Poly(acrylic acid/acrylamide/sodium humate) Superabsorbent Hydrogels for Metal Ion/Dye Adsorption: Effect of Sodium Humate Concentration. J. Appl. Polym. Sci. 2012, 125, 1267–1283. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Wang, F.; He, T.; Tang, Y.; Yang, J. Preparation and the swelling properties of sodium alginate graft poly (acrylic acid-co-2-acrylamide-2-methyl propane sulfonic acid)/graphene oxide hydrogel composite. Adv. Polym. Technol. 2018, 37, 2885–2893. [Google Scholar] [CrossRef]
- Manaila, E.; Craciun, G.; Ighigeanu, D.; Lungu, I.B.; Dumitru, M.; Stelescu, M.D. Electron Beam Irradiation: A Method for Degradation of Composites Based on Natural Rubber and Plasticized Starch. Polymers 2021, 13, 1950. [Google Scholar] [CrossRef] [PubMed]
- Craciun, G.; Manaila, E.; Ighigeanu, D.; Stelescu, M.D. A method to improve the characteristics of EPDM rubber based eco-composites with electron beam. Polymers 2020, 12, 215. [Google Scholar] [CrossRef] [Green Version]
- Sultana, S.; Islam, M.R.; Dafader, N.C.; Haque, M.E. Preparation Of Carboxymethyl Cellulose/Acrylamide Copoly-Mer Hydrogel Using Gamma Radiation And Investigation of Its Swelling Behavior. J. Bangladesh Chem. Soc. 2012, 25, 132–138. [Google Scholar] [CrossRef]
- Karadag, E.; Saraydin, D.; Sahiner, N.; Güven, O. Radiation induced acrylamide/citric acid hydrogelas and their swelling behaviors. J. Macromol. Sci. Pure 2001, 38, 1105–1121. Available online: https://www.researchgate.net/publication/233016775 (accessed on 3 December 2022). [CrossRef]
- Pourjavadi, A.; Kurdtabar, M. Collagen-based highly porous hydrogel without any porogen: Synthesis and characteristics. Eur. Polym. J. 2007, 43, 877–889. [Google Scholar] [CrossRef]
- Yiamsawas, D.; Kangwansupamonkon, W.; Chailapakul, O.; Kiatkamjornwong, S. Synthesis and swelling properties of poly[acrylamide-co-(crotonic acid)] superabsorbents. Reac. Funct. Polym. 2007, 67, 865–882. [Google Scholar] [CrossRef]
- Ding, Z.Y.; Aklonis, J.J.; Salovey, R. Model filled polymers. VI. Determination of the crosslink density of polymeric beads by swelling. J. Polym. Sci. B 1991, 29, 1035–1038. [Google Scholar] [CrossRef]
- Karadag, E.; Saraydin, D.; Güven, O. Influence of some crosslinkers on the swelling of acrylamide-crotonic acid hydrogels. Turk. J. Chem. 1997, 21, 151–161. Available online: https://www.researchgate.net/publication/282285864 (accessed on 3 December 2022).
- Lee, B.H.; Li, B.; Guelcher, S.A. Gel Microstructure Regulates Proliferation and Differentiation of MC3T3-E1 Cells Encapsulated in Alginate Beads. Acta Biomater. 2012, 8, 1693–1702. [Google Scholar] [CrossRef] [Green Version]
- Karadag, E.; Uzum, O.B.; Saraydin, D. Swelling equilibria and dye adsorption studies of chemically crosslinked superabsorbent acrylamide/maleic acid hydrogels. Eur. Polym. J. 2002, 38, 2133–2141. [Google Scholar] [CrossRef]
- Jabbari, E.; Nozari, S. Swelling behaviour of acrylic acid hydrogels prepared by gamma-radiation crosslinking of polyacrylic acid in aqueous solution. Eur. Polym. J. 2000, 36, 2685–2692. [Google Scholar] [CrossRef]
- Manaila, E.; Craciun, G.; Ighigeanu, D.; Lungu, I.B.; Dumitru Grivei, M.D.; Stelescu, D.M. Degradation by Electron Beam Irradiation of Some Composites Based on Natural Rubber Reinforced with Mineral and Organic Fillers. Int. J. Mol. Sci. 2022, 23, 6925. [Google Scholar] [CrossRef] [PubMed]

















| Samples Codes | Gel Fraction (%) | Swelling (%) | EWC (%) |
|---|---|---|---|
| H1R1-D1 | 81.39 | 24,347 | 99.59 |
| H1R2-D1 | 89.46 | 20,276 | 99.51 |
| H2R1-D1 | 79.55 | 15,460 | 99.36 |
| H2R2-D1 | 80.76 | 35,194 | 99.72 |
| H1R1-D2 | 82.64 | 28,826 | 99.65 |
| H1R2-D2 | 92.21 | 22,555 | 99.56 |
| H2R1-D2 | 81.75 | 52,882 | 99.81 |
| H2R2-D2 | 87.35 | 21,029 | 99.53 |
| Samples Codes | Cross-Link Density (q × 105) | Porosity (%) | Mesh Size, ξ (nm) |
|---|---|---|---|
| H1R1-D1 | 1.09 | 99.74 | 486 |
| H1R2-D1 | 1.66 | 99.65 | 371 |
| H2R1-D1 | 1.42 | 99.73 | 432 |
| H2R2-D1 | 5.30 | 99.79 | 844 |
| H1R1-D2 | 0.77 | 99.77 | 611 |
| H1R2-D2 | 1.50 | 99.64 | 396 |
| H2R1-D2 | 0.72 | 99.80 | 688 |
| H2R2-D2 | 1.42 | 99.67 | 431 |
| Samples Codes | First Order Swelling Rate Constants | Diffusion | |||||
|---|---|---|---|---|---|---|---|
| Mechanisms of Water Diffusion | Diffusion Coefficient | ||||||
| k1,S × 103 | R2 | n | k | R2 | D × 103 | R2 | |
| H1R1-D1 | 0.640 | 0.998 | 0.881 | 0.281 | 0.995 | 4.321 | 0.979 |
| H1R1-D2 | 0.622 | 0.994 | 0.923 | 0.250 | 0.990 | 5.070 | 0.974 |
| H1R2-D1 | 0.555 | 0.999 | 0.905 | 0.178 | 0.998 | 2.435 | 0.975 |
| H1R2-D2 | 0.559 | 0.995 | 0.891 | 0.216 | 0.996 | 2.843 | 0.987 |
| H2R1-D1 | 0.639 | 0.998 | 0.919 | 0.146 | 0.998 | 0.501 | 0.981 |
| H2R1-D2 | 0.505 | 0.996 | 1.093 | 0.152 | 0.997 | 3.517 | 0.953 |
| H2R2-D1 | 0.396 | 0.998 | 0.968 | 0.159 | 0.997 | 0.939 | 0.976 |
| H2R2-D2 | 0.482 | 0.997 | 0.921 | 0.151 | 0.998 | 0.571 | 0.976 |
| Wavenumber cm−1 | Band Assignments | ||
|---|---|---|---|
| Na-Alg | AMD | Hydrogel | |
| – | 3360 | 3330–3345 | -H stretching vibration |
| 3280 | 3190 | 3190–3200 | -OH stretching vibration |
| 2950 | 2930/2860 | 2930–2940 | C-H, CH2 stretching vibration |
| – | 1654 | 1649–1655 | C=O stretching vibration |
| 1620 | 1613 | 1600–1620 | -COO- symmetric bending |
| 1417 | 1429 | 1415–1450 | -COO- symmetric bending |
| – | 1353 | – | -CN stretching vibration |
| 1349 | – | 1345–1350 | CH2, C-H bending mode |
| 1120 | 1121 | 1105–1120 | C-O-C stretching |
| 1030 | 1037 | 1032–1045 | C-O stretching vibrations |
| Row Materials | Chemical Characteristics |
|---|---|
| Sodium alginate, Na-Alg (C6H7NaO6)n or C6H9NaO7 |
|
| Acrylic acid, AA C3H4O2 |
|
| Acrylamide, AMD C3H5NO |
|
| Potassium persulfate, PP (used as reaction initiator) K2S2O8 |
|
| Trimethylolpropane trimethacrylate, TMPT (used as cross-linker) C18H26O6 |
|
| Nutrients Solutions | Characteristics |
|---|---|
| Solution no. 1: Liquid fertilizer for balcony flowers (produced by AGRO CS, Lucenec, Slovakia); used according to the manufacturer instructions: 15 mL diluted in 1000 mL water |
|
| Solution no. 2: Biopon natural biohumus for vegetables and greens (produced by Bros Sp. z o.o. sp. k., Poznan, Poland); used according to the manufacturer instructions: 120 mL diluted in 1000 mL water |
|
| Samples Codes | Amount of Chemicals (g/100 mL Solution) | Irradiation Dose (kGy) | ||||
|---|---|---|---|---|---|---|
| Na-Alg | AA | AMD | PP | TMPT | ||
| H1R1-D1 | 1 | 12.5 | 18.75 | 0.025 | 0.02 | 5 |
| H1R1-D2 | 6 | |||||
| H1R2-D1 | 18.75 | 18.75 | 0.025 | 0.02 | 5 | |
| H1R2-D2 | 6 | |||||
| H2R1-D1 | 2 | 12.5 | 18.75 | 0.025 | 0.02 | 5 |
| H2R1-D2 | 6 | |||||
| H2R2-D1 | 18.75 | 18.75 | 0.025 | 0.02 | 5 | |
| H2R2-D2 | 6 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manaila, E.; Craciun, G.; Calina, I.C. Sodium Alginate-g-acrylamide/acrylic Acid Hydrogels Obtained by Electron Beam Irradiation for Soil Conditioning. Int. J. Mol. Sci. 2023, 24, 104. https://doi.org/10.3390/ijms24010104
Manaila E, Craciun G, Calina IC. Sodium Alginate-g-acrylamide/acrylic Acid Hydrogels Obtained by Electron Beam Irradiation for Soil Conditioning. International Journal of Molecular Sciences. 2023; 24(1):104. https://doi.org/10.3390/ijms24010104
Chicago/Turabian StyleManaila, Elena, Gabriela Craciun, and Ion Cosmin Calina. 2023. "Sodium Alginate-g-acrylamide/acrylic Acid Hydrogels Obtained by Electron Beam Irradiation for Soil Conditioning" International Journal of Molecular Sciences 24, no. 1: 104. https://doi.org/10.3390/ijms24010104