A General Use QSAR-ARX Model to Predict the Corrosion Inhibition Efficiency of Drugs in Terms of Quantum Mechanical Descriptors and Experimental Comparison for Lidocaine
Abstract
:1. Introduction
1.1. Corrosion Inhibition and QSAR Fundamentals
1.2. QSAR Paradigm and HSAB Descriptors
2. Theoretical and Experimental Methods
2.1. NARMAX System Identification Approach
2.2. ARX Theoretical Model
2.2.1. Arrangement of Candidate Terms
2.2.2. FROLS and ERR Algorithms for Model Structure Selection
2.2.3. Cross-Validation
2.3. Experimental Details
2.3.1. Solution Preparation
2.3.2. Electrochemical Evaluation
2.3.3. Characterization by Atomic Force Microscopy (AFM)
3. Results and Discussion
3.1. Model Determination
3.1.1. Data Processing into an ARX Linear System
3.1.2. Term Selection through FROLS and ERR
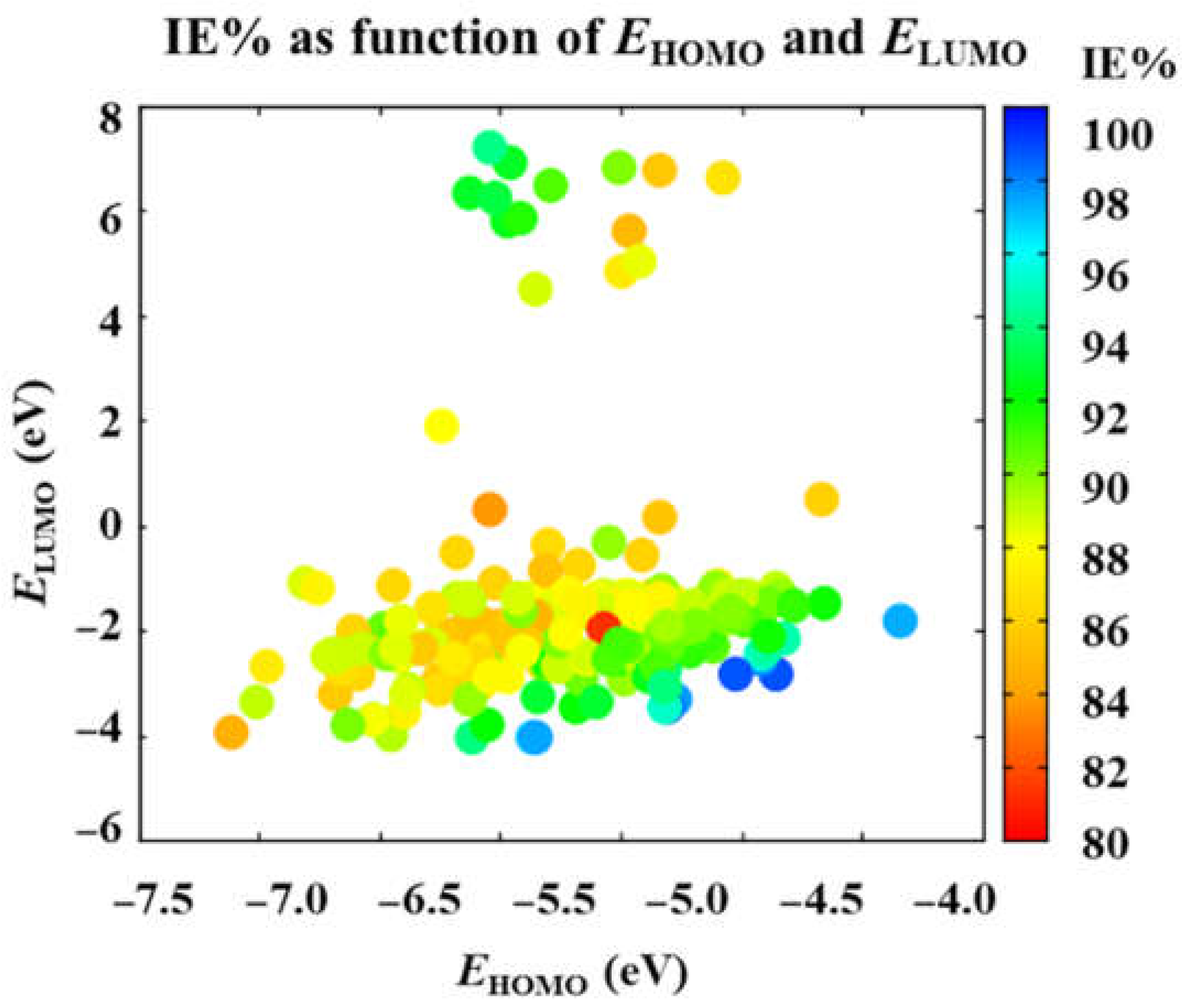
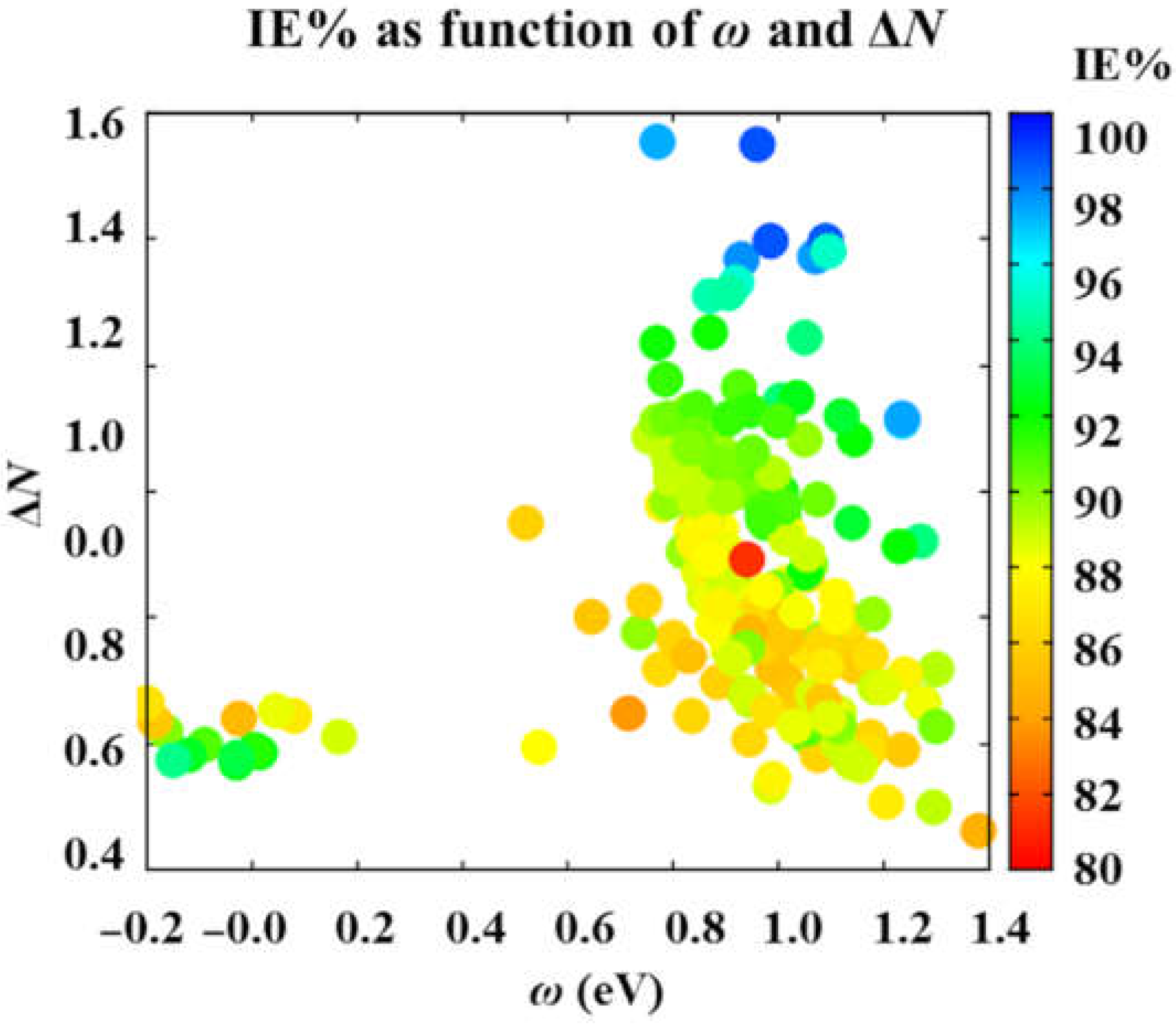
3.2. Main Tendencies
3.3. High-Efficiency Corrosion Inhibitors
3.4. Experimental Verification
- (a)
- Open circuit potential (OCP)
- (b)
- Concentration effect of lidocaine by EIS
- (c)
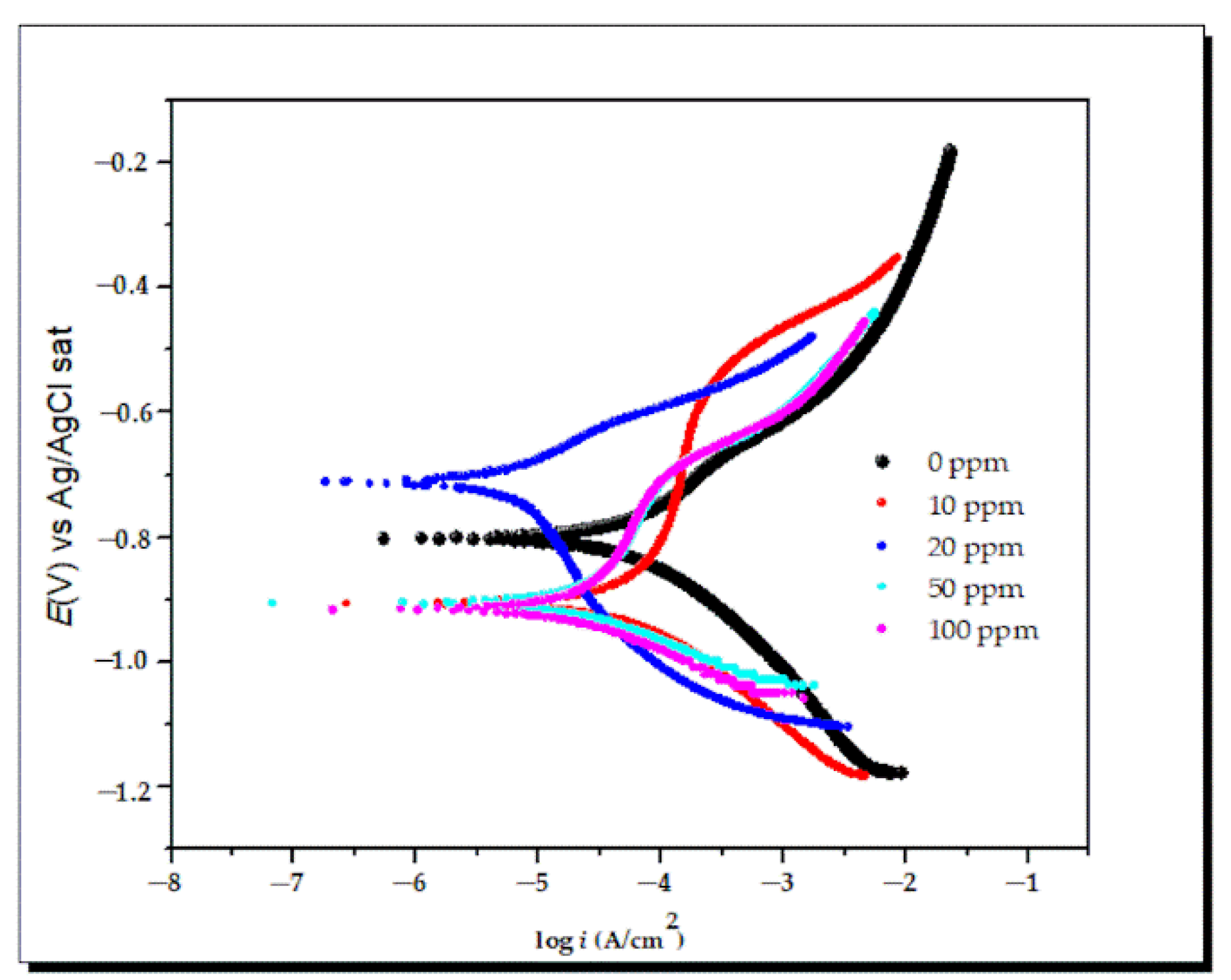
- Polarization curves
- (d)
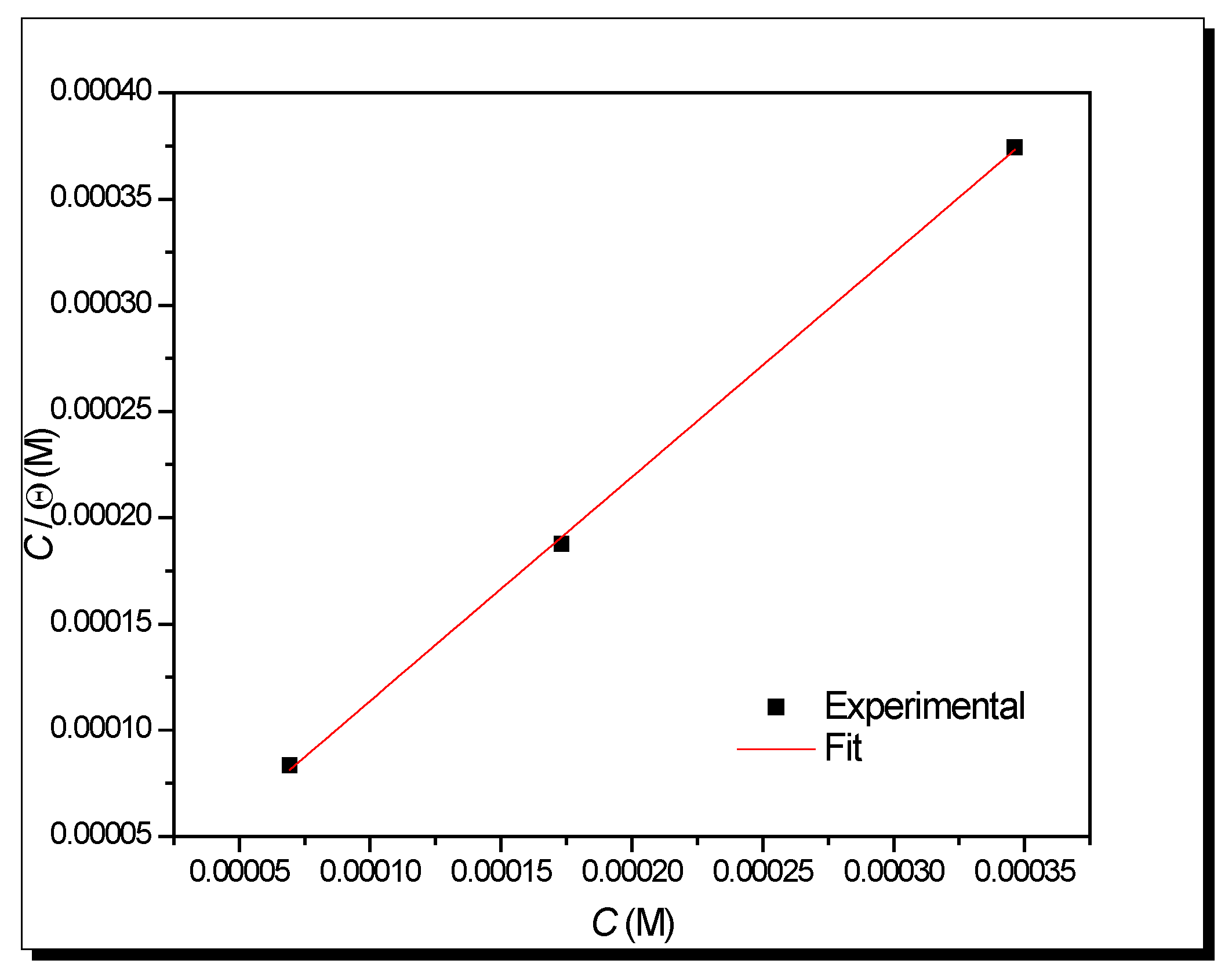
- Adsorption process
- (e)
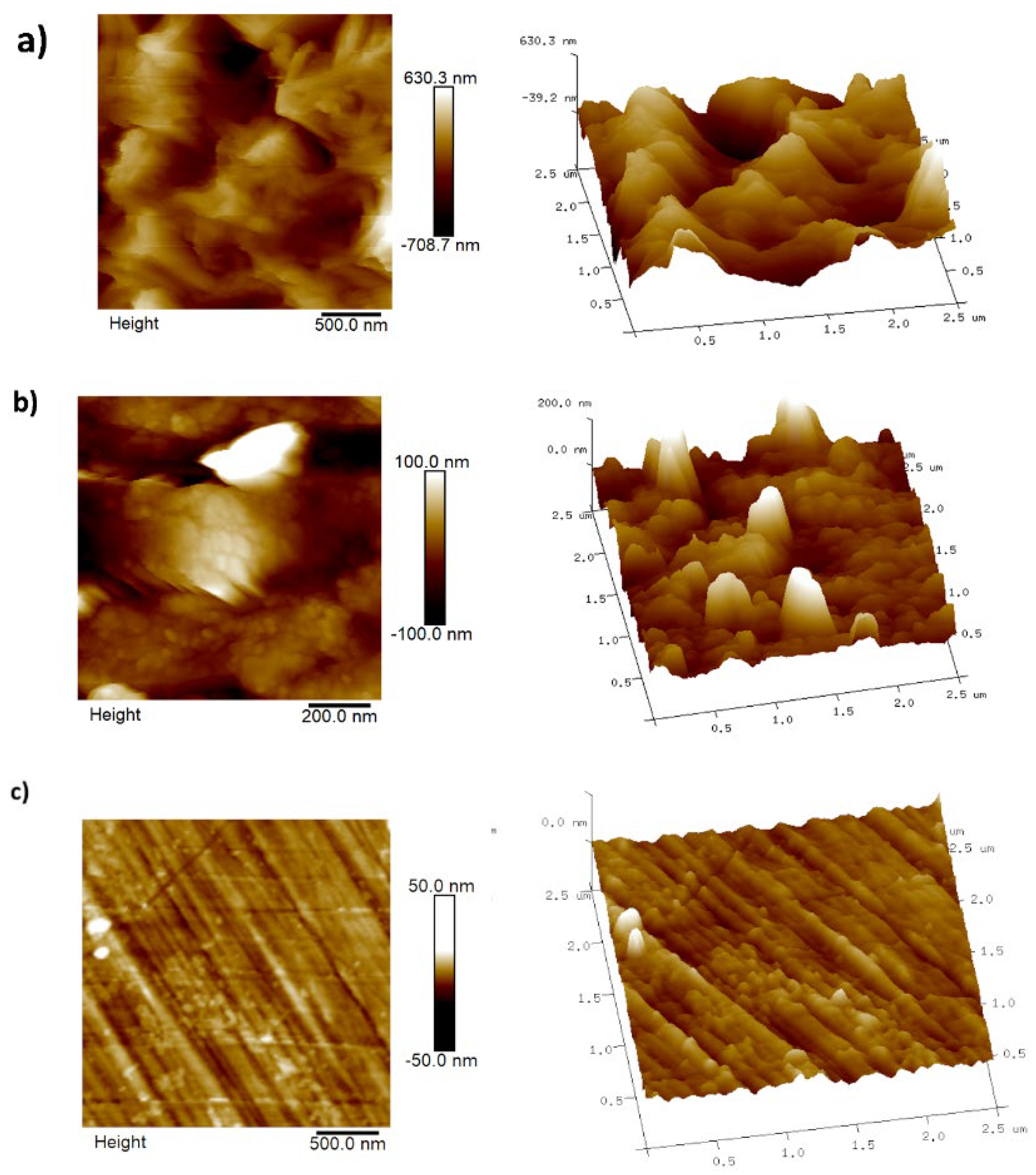
- AFM analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mineral Commodity Summaries 2021; Mineral Commodity Summaries: Reston, VA, USA, 2021; p. 200.
- Sastri, V.S. Green Corrosion Inhibitors: Theory and Practice; John Wiley and Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Shamnamol, G.K.; Sreelakshmi, K.P.; Ajith, G.; Jacob, J.M. Effective Utilization of Drugs as Green Corrosion Inhibitor—A Review. In AIP Conference Proceedings; AIP Publishing LLC.: Puducherry, India, 2020; p. 070006. [Google Scholar]
- Sharma, S.; Kumar, A. Recent Advances in Metallic Corrosion Inhibition: A Review. J. Mol. Liq. 2021, 322, 114862. [Google Scholar] [CrossRef]
- Nduma, R.C.; Fayomi, O.S.I.; Nkiko, M.O.; Inegbenebor, A.O.; Udoye, N.E.; Onyisi, O.; Sanni, O.; Fayomi, J. Review of Metal Protection Techniques and Application of Drugs as Corrosion Inhibitors on Metals. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1107, 012023. [Google Scholar] [CrossRef]
- Elsaoud, A.A.; Mabrouk, E.M.; Seyam, D.F.; El-Etre, A. Recyclization of Expired Megavit Zinc (MZ) Drug as Metallic Corrosion Inhibitor for Copper Alloy C10100 in Nitric Acid Solution. J. Bio-Tribo-Corros. 2021, 7, 64. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Tan, B.; Qiang, Y.; Li, W.; Chen, S.; Guo, L. Investigation of Losartan Potassium as an Eco-Friendly Corrosion Inhibitor for Copper in 0.5 M H2SO4. J. Mol. Liq. 2020, 305, 112789. [Google Scholar] [CrossRef]
- Tasić, Ž.Z.; Petrović Mihajlović, M.B.; Radovanović, M.B.; Simonović, A.T.; Antonijević, M.M. Experimental and Theoretical Studies of Paracetamol as a Copper Corrosion Inhibitor. J. Mol. Liq. 2021, 327, 114817. [Google Scholar] [CrossRef]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A.R. Review of Corrosive Environments for Copper and Its Corrosion Inhibitors. Arab. J. Chem. 2017, 13, 481–544. [Google Scholar] [CrossRef]
- Su, P. Expired Drug Theophylline as Potential Corrosion Inhibitor for 7075 Aluminium Alloy in 1M NaOH Solution. Int. J. Electrochem. Sci. 2020, 15, 1412–1425. [Google Scholar] [CrossRef]
- Chaubey, N.; Singh, V.K.; Quraishi, M.A. Papaya Peel Extract as Potential Corrosion Inhibitor for Aluminium Alloy in 1 M HCl: Electrochemical and Quantum Chemical Study. Ain. Shams. Eng. J. 2018, 9, 1131–1140. [Google Scholar] [CrossRef] [Green Version]
- Nathiya, R.S.; Perumal, S.; Murugesan, V.; Raj, V. Evaluation of Extracts of Borassus Flabellifer Dust as Green Inhibitors for Aluminium Corrosion in Acidic Media. Mater. Sci. Semicond. Process. 2019, 104, 104674. [Google Scholar] [CrossRef]
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study. NACE Int. 2016, 216, 2–3. [Google Scholar]
- Miralrio, A.; Espinoza Vázquez, A. Plant Extracts as Green Corrosion Inhibitors for Different Metal Surfaces and Corrosive Media: A Review. Processes 2020, 8, 942. [Google Scholar] [CrossRef]
- Kadhim, A.; Al-Amiery, A.A. Corrosion Inhibitors. A Review. Int. J. Corros. Scale Inhib. 2021, 10, 54–67. [Google Scholar] [CrossRef]
- Papavinasam, S. Corrosion Inhibitors. In Uhlig’s Corrosion Handbook; Revie, R.W., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 1021–1032. [Google Scholar]
- Salleh, S.Z.; Yusoff, A.H.; Zakaria, S.K.; Taib, M.A.A.; Abu Seman, A.; Masri, M.N.; Mohamad, M.; Mamat, S.; Ahmad Sobri, S.; Ali, A.; et al. Plant Extracts as Green Corrosion Inhibitor for Ferrous Metal Alloys: A Review. J. Clean. Prod. 2021, 304, 127030. [Google Scholar] [CrossRef]
- Majd, M.T.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Probing Molecular Adsorption/Interactions and Anti-Corrosion Performance of Poppy Extract in Acidic Environments. J. Mol. Liq. 2020, 304, 112750. [Google Scholar] [CrossRef]
- Kalaiselvi, K.; Chung, I.-M.; Kim, S.-H.; Prabakaran, M. Corrosion Resistance of Mild Steel in Sulphuric Acid Solution by Coreopsis Tinctoria Extract: Electrochemical and Surface Studies. Anti-Corros. Methods Mater. 2018, 65, 408–416. [Google Scholar] [CrossRef]
- Saxena, A.; Prasad, D.; Haldhar, R.; Singh, G.; Kumar, A. Use of Saraca Ashoka Extract as Green Corrosion Inhibitor for Mild Steel in 0.5 M H2SO4. J. Mol. Liq. 2018, 258, 89–97. [Google Scholar] [CrossRef]
- Aysel, B.; Meltem, D. The Use of Papaver Somniferum L. Plant Extract as Corrosion Inhibitor. Prot. Met. Phys. Chem. Surf. 2019, 55, 1182–1194. [Google Scholar] [CrossRef]
- Alrefaee, S.H.; Rhee, K.Y.; Verma, C.; Quraishi, M.A.; Ebenso, E.E. Challenges and Advantages of Using Plant Extract as Inhibitors in Modern Corrosion Inhibition Systems: Recent Advancements. J. Mol. Liq. 2021, 321, 114666. [Google Scholar] [CrossRef]
- Baitule, P.K.; Victoria, S.N.; Manivannan, R. Review on Assessment of Corrosion of Mild Steel in Alkaline Environment by Using Plant Extract. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1057, 012012. [Google Scholar] [CrossRef]
- Espinoza-Vázquez, A.; Rodríguez-Gómez, F.J.; Negrón-Silva, G.E.; González-Olvera, R.; Ángeles-Beltrán, D.; Palomar-Pardavé, M.; Miralrio, A.; Castro, M. Fluconazole and Fragments as Corrosion Inhibitors of API 5L X52 Steel Immersed in 1M HCl. Corros. Sci. 2020, 174, 108853. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.J.; Valdelamar, M.P.; Vazquez, A.E.; Del Valle Perez, P.; Mata, R.; Miralrio, A.; Castro, M. Mycophenolic Acid as a Corrosion Inhibitor of Carbon Steel in 3% Wt. NaCl Solution. An Experimental and Theoretical Study. J. Mol. Struct. 2019, 1183, 168–181. [Google Scholar] [CrossRef]
- Haruna, K.; Saleh, T.A.; Quraishi, M.A. Expired Metformin Drug as Green Corrosion Inhibitor for Simulated Oil/Gas Well Acidizing Environment. J. Mol. Liq. 2020, 315, 113716. [Google Scholar] [CrossRef]
- Ma, X. Electrochemical Studies of Expired Drug (Formoterol) as Oilfield Corrosion Inhibitor for Mild Steel in H2SO4 Media. Int. J. Electrochem. Sci. 2020, 15, 1964–1981. [Google Scholar] [CrossRef]
- Farahati, R.; Mousavi-Khoshdel, S.M.; Ghaffarinejad, A.; Behzadi, H. Experimental and Computational Study of Penicillamine Drug and Cysteine as Water-Soluble Green Corrosion Inhibitors of Mild Steel. Prog. Org. Coat. 2020, 142, 105567. [Google Scholar] [CrossRef]
- Abdollahi, F.; Foroughi, M.M.; Shahidi Zandi, M.; Kazemipour, M. Electrochemical Investigation of Meloxicam Drug as a Corrosion Inhibitor for Mild Steel in Hydrochloric and Sulfuric Acid Solutions. Prog. Color Color. Coat. 2020, 13, 155–165. [Google Scholar] [CrossRef]
- El-Haddad, M.N.; Fouda, A.E.S. Evaluation of Curam Drug as an Ecofriendly Corrosion Inhibitor for Protection of Stainless Steel-304 in Hydrochloric Acid Solution: Chemical, Electrochemical, and Surface Morphology Studies. J. Chin. Chem. Soc. 2021, 68, 826–836. [Google Scholar] [CrossRef]
- Gece, G. Drugs: A Review of Promising Novel Corrosion Inhibitors. Corros. Sci. 2011, 53, 3873–3898. [Google Scholar] [CrossRef]
- Vaszilcsin, N.; Ordodi, V.; Borza, A. Corrosion Inhibitors from Expired Drugs. Int. J. Pharm. 2012, 431, 241–244. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Bahadur, I.; Quraishi, M.A. An Overview on Plant Extracts as Environmental Sustainable and Green Corrosion Inhibitors for Metals and Alloys in Aggressive Corrosive Media. J. Mol. Liq. 2018, 266, 577–590. [Google Scholar] [CrossRef]
- Neves, B.J.; Braga, R.C.; Melo-Filho, C.C.; Moreira-Filho, J.T.; Muratov, E.N.; Andrade, C.H. QSAR-Based Virtual Screening: Advances and Applications in Drug Discovery. Front. Pharmacol. 2018, 9, 1275. [Google Scholar] [CrossRef] [Green Version]
- Selassie, C.; Verma, R.P. History of Quantitative Structure-Activity Relationships. In Burger’s Medicinal Chemistry and Drug Discovery; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; p. bmc001. [Google Scholar]
- Hu, S.; Chen, P.; Gu, P.; Wang, B. A Deep Learning-Based Chemical System for QSAR Prediction. IEEE J. Biomed. Health Inform. 2020, 24, 3020–3028. [Google Scholar] [CrossRef] [PubMed]
- Toropov, A.A.; Toropova, A.P. QSPR/QSAR: State-of-Art, Weirdness, the Future. Molecules 2020, 25, 1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaled, K.F. Modeling Corrosion Inhibition of Iron in Acid Medium by Genetic Function Approximation Method: A QSAR Model. Corros. Sci. 2011, 53, 3457–3465. [Google Scholar] [CrossRef]
- Keshavarz, M.H.; Esmaeilpour, K.; Golikand, A.N.; Shirazi, Z. Simple Approach to Predict Corrosion Inhibition Efficiency of Imidazole and Benzimidazole Derivatives as Well as Linear Organic Compounds Containing Several Polar Functional Groups: Simple Approach to Predict Corrosion Inhibition. Zeitschrift für anorganische und allgemeine Chemie 2016, 642, 906–913. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Wu, W.; Xiong, Y.; Sun, C.; Yuan, L.; Li, M. A Machine Learning-Based QSAR Model for Benzimidazole Derivatives as Corrosion Inhibitors by Incorporating Comprehensive Feature Selection. Interdiscip. Sci. Comput. Life Sci. 2019, 11, 738–747. [Google Scholar] [CrossRef]
- Ser, C.T.; Žuvela, P.; Wong, M.W. Prediction of Corrosion Inhibition Efficiency of Pyridines and Quinolines on an Iron Surface Using Machine Learning-Powered Quantitative Structure-Property Relationships. Appl. Surf. Sci. 2020, 512, 145612. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Giri, S. Electrophilicity Index within a Conceptual DFT Framework. Annu. Rep. Sect. C Phys. Chem. 2009, 105, 13. [Google Scholar] [CrossRef]
- Lukovits, I.; Kálmán, E.; Zucchi, F. Corrosion Inhibitors—Correlation between Electronic Structure and Efficiency. CORROSION 2001, 57, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Bulat, F.A.; Toro-Labbé, A.; Brinck, T.; Murray, J.S.; Politzer, P. Quantitative Analysis of Molecular Surfaces: Areas, Volumes, Electrostatic Potentials and Average Local Ionization Energies. J. Mol. Model. 2010, 16, 1679–1691. [Google Scholar] [CrossRef]
- Aradi, B.; Hourahine, B.; Frauenheim, T. DFTB+, a Sparse Matrix-Based Implementation of the DFTB Method. J. Phys. Chem. A 2007, 111, 5678–5684. [Google Scholar] [CrossRef] [PubMed]
- Hourahine, B.; Aradi, B.; Blum, V.; Bonafé, F.; Buccheri, A.; Camacho, C.; Cevallos, C.; Deshaye, M.Y.; Dumitrică, T.; Dominguez, A.; et al. DFTB+, a Software Package for Efficient Approximate Density Functional Theory Based Atomistic Simulations. J. Chem. Phys. 2020, 152, 124101. [Google Scholar] [CrossRef] [PubMed]
- Billings, S.A. Nonlinear System Identification: NARMAX Methods in the Time, Frequency, and Spatio-Temporal Domains; John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar]
- Yassin, I.M.; Abdul Khalid, M.F.; Herman, S.H.; Pasya, I.; Wahab, N.A.; Awang, Z. Multi-Layer Perceptron (MLP)-Based Nonlinear Auto-Regressive with Exogenous Inputs (NARX) Stock Forecasting Model. Int. J. Adv. Sci. Eng. Inf. Technol. 2017, 7, 1098. [Google Scholar] [CrossRef] [Green Version]
- Chadalawada, J.; Havlicek, V.; Babovic, V. A Genetic Programming Approach to System Identification of Rainfall-Runoff Models. Water Resour. Manag. 2017, 31, 3975–3992. [Google Scholar] [CrossRef]
- Cheah, K.W.; Ahmad, N.A. Fuzzy Recursive Least-Squares Approach in Speech System Identification: A Transformed Domain LPC Model. Int. J. Electr. Comput. Eng. IJECE 2017, 7, 842. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.K.; Wang, P.P. Genetic Algorithms for Pattern Recognition, 1st ed.; Pal, S.K., Wang, P.P., Eds.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Harris, J.; Arthurs, F.; Henrickson, J.V.; Valasek, J. Aircraft System Identification Using Artificial Neural Networks with Flight Test Data. In Proceedings of the 2016 International Conference on Unmanned Aircraft Systems (ICUAS, IEEE), Arlington, VA, USA, 7–10 June 2016; pp. 679–688. [Google Scholar]
- Gao, Y.; Liu, S.; Li, F.; Liu, Z. Fault Detection and Diagnosis Method for Cooling Dehumidifier Based on LS-SVM NARX Model. Int. J. Refrig. 2016, 61, 69–81. [Google Scholar] [CrossRef]
- Chen, S.; Billings, S.A.; Luo, W. Orthogonal Least Squares Methods and Their Application to Non-Linear System Identification. Int. J. Control 1989, 50, 1873–1896. [Google Scholar] [CrossRef]
- Billings, S.A.; Korenberg, M.J.; Chen, S. Identification of Non-Linear Output-Affine Systems Using an Orthogonal Least-Squares Algorithm. Int. J. Syst. Sci. 1988, 19, 1559–1568. [Google Scholar] [CrossRef]
- Korenberg, M.; Billings, S.A.; Liu, Y.P.; McILROY, P.J. Orthogonal Parameter Estimation Algorithm for Non-Linear Stochastic Systems. Int. J. Control 1988, 48, 193–210. [Google Scholar] [CrossRef]
- Yoshida, H.; Kumar, S. Development of ARX Model Based Off-Line FDD Technique for Energy Efficient Buildings. Renew. Energy 2001, 22, 53–59. [Google Scholar] [CrossRef]
- Antonucci, D.; Filippi Oberegger, U.; Pasut, W.; Gasparella, A. Building Performance Evaluation through a Novel Feature Selection Algorithm for Automated Arx Model Identification Procedures. Energy Build. 2017, 150, 432–446. [Google Scholar] [CrossRef]
- Romero-Ugalde, H.M.; Garnotel, M.; Doron, M.; Jallon, P.; Charpentier, G.; Franc, S.; Huneker, E.; Simon, C.; Bonnet, S. ARX Model for Interstitial Glucose Prediction during and after Physical Activities. Control Eng. Pract. 2019, 90, 321–330. [Google Scholar] [CrossRef]
- Ayala Solares, J.R.; Wei, H.-L.; Boynton, R.J.; Walker, S.N.; Billings, S.A. Modeling and Prediction of Global Magnetic Disturbance in Near-Earth Space: A Case Study for K p Index Using NARX Models: MODELING AND PREDICTION OF K p INDEX. Space Weather 2016, 14, 899–916. [Google Scholar] [CrossRef] [Green Version]
- Ayala-Solares, J.R.; Wei, H.-L.; Bigg, G.R. The Variability of the Atlantic Meridional Circulation since 1980, as Hindcast by a Data-Driven Nonlinear Systems Model. Acta Geophys. 2018, 66, 683–695. [Google Scholar] [CrossRef] [Green Version]
- Rashid, M.T.; Frasca, M.; Ali, A.A.; Ali, R.S.; Fortuna, L.; Xibilia, M.G. Nonlinear Model Identification for Artemia Population Motion. Nonlinear Dyn. 2012, 69, 2237–2243. [Google Scholar] [CrossRef]
- Luna, C.; Coca, D. An Empirical Model of Drosophila Photoreceptor-LMC Network. BMC Neurosci. 2015, 16, 47. [Google Scholar] [CrossRef]
- Li, Y.; Cui, W.-G.; Guo, Y.-Z.; Huang, T.; Yang, X.-F.; Wei, H.-L. Time-Varying System Identification Using an Ultra-Orthogonal Forward Regression and Multiwavelet Basis Functions With Applications to EEG. IEEE Trans. Neural Netw. Learn. Syst. 2017, 29, 2960–2972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berk, R.A. Statistical Learning from a Regression Perspective; Springer Texts in Statistics; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer Texts in Statistics; Springer: New York, NY, USA, 2013; Volume 103. [Google Scholar]
- Chen, Y.; Elenee Argentinis, J.; Weber, G. IBM Watson: How Cognitive Computing Can Be Applied to Big Data Challenges in Life Sciences Research. Clin. Ther. 2016, 38, 688–701. [Google Scholar] [CrossRef] [Green Version]
- Ogunyemi, B.T.; Latona, D.F.; Adejoro, I.A. Molecular Modeling and Quantitative Structure–Property Relationships (QSPRs) of Purine Derivatives as Corrosion Inhibitor in Acid Medium. Sci. Afr. 2020, 8, e00336. [Google Scholar] [CrossRef]
- Yesudass, S.; Olasunkanmi, L.O.; Bahadur, I.; Kabanda, M.M.; Obot, I.B.; Ebenso, E.E. Experimental and Theoretical Studies on Some Selected Ionic Liquids with Different Cations/Anions as Corrosion Inhibitors for Mild Steel in Acidic Medium. J. Taiwan Inst. Chem. Eng. 2016, 64, 252–268. [Google Scholar] [CrossRef]
- Jalgham, R.T.T. Theoretical, Monte Carlo Simulations and QSAR Studies on Some Triazole Derivatives as Corrosion Inhibitors for Mild Steel in 1 M HCl. ES Energy Environ. 2021, 13, 37–49. [Google Scholar] [CrossRef]
- Obot, I.B. Experimental, DFT and QSAR Models for the Discovery of New Pyrazines Corrosion Inhibitors for Steel in Oilfield Acidizing Environment. Int. J. Electrochem. Sci. 2020, 15, 9066–9080. [Google Scholar] [CrossRef]
- Shukla, S.K.; Singh, A.K.; Ahamad, I.; Quraishi, M.A. Streptomycin: A Commercially Available Drug as Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Solution. Mater. Lett. 2009, 63, 819–822. [Google Scholar] [CrossRef]
- Ahamad, I.; Prasad, R.; Quraishi, M.A. Experimental and Theoretical Investigations of Adsorption of Fexofenadine at Mild Steel/Hydrochloric Acid Interface as Corrosion Inhibitor. J. Solid State Electrochem. 2010, 14, 2095–2105. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Obot, I.B.; Murulana, L.C. Quinoline and Its Derivatives as Effective Corrosion Inhibitors for Mild Steel in Acidic Medium. Int. J. Electrochem. Sci. 2010, 5, 1574–1586. [Google Scholar]
- Martínez Jiménez, P.; Espinoza Vázquez, A.; Rodríguez Gómez, F.J. Evaluation of N,N-Dimethylformamide as Corrosion Inhibitor on API 5L X70 Using 3% NaCl. MRS Adv. 2019, 4, 2391–2399. [Google Scholar] [CrossRef]
- IBM Watson Studio—AutoML—IBM AutoAI. Available online: https://www.ibm.com/in-en/cloud/watson-studio/autoai (accessed on 9 February 2022).
- Quadri, T.W.; Olasunkanmi, L.O.; Akpan, E.D.; Fayemi, O.E.; Lee, H.-S.; Lgaz, H.; Verma, C.; Guo, L.; Kaya, S.; Ebenso, E.E. Development of QSAR-Based (MLR/ANN) Predictive Models for Effective Design of Pyridazine Corrosion Inhibitors. Mater. Today Commun. 2022, 30, 103163. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Gong, S.; Zhao, H.; Bai, Y.; Li, Q.; Ji, L. The Discussion of Descriptors for the QSAR Model and Molecular Dynamics Simulation of Benzimidazole Derivatives as Corrosion Inhibitors. Corros. Sci. 2015, 99, 76–88. [Google Scholar] [CrossRef]
- Al-Fakih, A.M.; Algamal, Z.Y.; Lee, M.H.; Abdallah, H.H.; Maarof, H.; Aziz, M. Quantitative Structure-Activity Relationship Model for Prediction Study of Corrosion Inhibition Efficiency Using Two-Stage Sparse Multiple Linear Regression: QSAR Study Using Two-Stage SMLR. J. Chemom. 2016, 30, 361–368. [Google Scholar] [CrossRef]
- Gu, Y.; Yang, Y.; Dewald, J.P.A.; van der Helm, F.C.T.; Schouten, A.C.; Wei, H.-L. Nonlinear Modeling of Cortical Responses to Mechanical Wrist Perturbations Using the NARMAX Method. IEEE Trans. Biomed. Eng. 2021, 68, 948–958. [Google Scholar] [CrossRef]
- Boynton, R.J.; Balikhin, M.A.; Billings, S.A.; Sharma, A.S.; Amariutei, O.A. Data Derived NARMAX Dst Model. Ann. Geophys. 2011, 29, 965–971. [Google Scholar] [CrossRef] [Green Version]
- Pisoni, E.; Farina, M.; Carnevale, C.; Piroddi, L. Forecasting Peak Air Pollution Levels Using NARX Models. Eng. Appl. Artif. Intell. 2009, 22, 593–602. [Google Scholar] [CrossRef]
- Boynton, R.; Balikhin, M.; Wei, H.-L.; Lang, Z.-Q. Applications of NARMAX in Space Weather. In Machine Learning Techniques for Space Weather; Elsevier: Amsterdam, the Netherlands, 2018; pp. 203–236. [Google Scholar]
- Kukula-Koch, W.A.; Widelski, J. Alkaloids. In Pharmacognosy; Elsevier: Amsterdam, the Netherlands, 2017; pp. 163–198. [Google Scholar]
- Nunomura, N.; Sunada, S. Density Functional Theory Study of The Interaction Of Hydroxyl Groups With Iron Surface. Arch. Metall. Mater. 2015, 60, 931–933. [Google Scholar] [CrossRef]
- Lepetit, C.; Maraval, V.; Canac, Y.; Chauvin, R. On the Nature of the Dative Bond: Coordination to Metals and beyond. The Carbon Case. Coord. Chem. Rev. 2016, 308, 59–75. [Google Scholar] [CrossRef]
- Qiang, Y.; Guo, L.; Li, H.; Lan, X. Fabrication of Environmentally Friendly Losartan Potassium Film for Corrosion Inhibition of Mild Steel in HCl Medium. Chem. Eng. J. 2021, 406, 126863. [Google Scholar] [CrossRef]
- Anadebe, V.C.; Nnaji, P.C.; Onukwuli, O.D.; Okafor, N.A.; Abeng, F.E.; Chukwuike, V.I.; Okoye, C.C.; Udoh, I.I.; Chidiebere, M.A.; Guo, L.; et al. Multidimensional Insight into the Corrosion Inhibition of Salbutamol Drug Molecule on Mild Steel in Oilfield Acidizing Fluid: Experimental and Computer Aided Modeling Approach. J. Mol. Liq. 2022, 349, 118482. [Google Scholar] [CrossRef]
- Abeng, F.E.; Anadebe, V.C.; Idim, V.D.; Edim, M.M. Anti-Corrosion Behaviour of Expired Tobramycin Drug on Carbon Steel in Acidic Medium. S. Afr. J. Chem. 2020, 73, 125–130. [Google Scholar]
- Onyeachu, I.B.; Abdel-Azeim, S.; Chauhan, D.S.; Quraishi, M.A. Electrochemical and Computational Insights on the Application of Expired Metformin Drug as a Novel Inhibitor for the Sweet Corrosion of C1018 Steel. ACS Omega 2021, 6, 65–76. [Google Scholar] [CrossRef]
- Abeng, F.E.; Ikpi, M.E.; Anadebe, V.C.; Emori, W. Metolazone Compound as Corrosion Inhibitor for API 5L X-52 Steel in Hydrochloric Acid Solution. Bull. Chem. Soc. Ethiop. 2020, 34, 407–418. [Google Scholar] [CrossRef]
- Ikpi, M.E.; Abeng, F.E. Electrochemical and Quantum Chemical Investigation on Adsorption of Nifedipine as Corrosion Inhibitor at API 5L X-52 Streel/HCL ACID Interface. Arch. Metall. Mater. 2020, 65, 125–131. [Google Scholar] [CrossRef]
- Anadebe, V.C.; Onukwuli, O.D.; Abeng, F.E.; Okafor, N.A.; Ezeugo, J.O.; Okoye, C.C. Electrochemical-Kinetics, MD-Simulation and Multi-Input Single-Output (MISO) Modeling Using Adaptive Neuro-Fuzzy Inference System (ANFIS) Prediction for Dexamethasone Drug as Eco-Friendly Corrosion Inhibitor for Mild Steel in 2 M HCl Electrolyte. J. Taiwan Inst. Chem. Eng. 2020, 115, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Gholamhosseinzadeh, M.R.; Aghaie, H.; Zandi, M.S.; Giahi, M. Rosuvastatin Drug as a Green and Effective Inhibitor for Corrosion of Mild Steel in HCl and H2SO4 Solutions. J. Mater. Res. Technol. 2019, 8, 5314–5324. [Google Scholar] [CrossRef]
- Geethamani, P.; Narmatha, M.; Dhanalakshmi, R.; Aejitha, S.; Kasthuri, P.K. Corrosion Inhibition and Adsorption Properties of Mild Steel in 1 M Hydrochloric Acid Medium by Expired Ambroxol Drug. J. Bio-Tribo-Corros. 2019, 5, 16. [Google Scholar] [CrossRef]
- Singh, P.; Chauhan, D.S.; Chauhan, S.S.; Singh, G.; Quraishi, M.A. Chemically Modified Expired Dapsone Drug as Environmentally Benign Corrosion Inhibitor for Mild Steel in Sulphuric Acid Useful for Industrial Pickling Process. J. Mol. Liq. 2019, 286, 110903. [Google Scholar] [CrossRef]
- Abeng, F.E.; Ikpi, M.E.; Ushie, O.A.; Anadebe, V.C.; Nyong, B.E.; Obeten, M.E.; Okafor, N.A.; Chukwuike, V.I.; Nkom, P.Y. Insight into Corrosion Inhibition Mechanism of Carbon Steel in 2 M HCl Electrolyte by Eco-Friendly Based Pharmaceutical Drugs. Chem. Data Collect. 2021, 34, 100722. [Google Scholar] [CrossRef]
- Dahiya, S.; Pahuja, P.; Lgaz, H.; Chung, I.-M.; Lata, S. Advanced Quantum Chemical and Electrochemical Analysis of Ravage Drugs for Corrosion Inhibition of Mild Steel. J. Adhes. Sci. Technol. 2019, 33, 1066–1089. [Google Scholar] [CrossRef]
- Saraswat, V.; Yadav, M. Improved Corrosion Resistant Performance of Mild Steel under Acid Environment by Novel Carbon Dots as Green Corrosion Inhibitor. Colloids Surf. Physicochem. Eng. Asp. 2021, 627, 127172. [Google Scholar] [CrossRef]
- Verma, C.; Abdellattif, M.H.; Alfantazi, A.; Quraishi, M.A. N-Heterocycle Compounds as Aqueous Phase Corrosion Inhibitors: A Robust, Effective and Economic Substitute. J. Mol. Liq. 2021, 340, 117211. [Google Scholar] [CrossRef]
- Rodrigues, F.A.d.S.; Gonçalves, Y.M.H.; Horta, B.A.C.; Santos, I.d.S.; Silva, B.V.; D’Elia, E. Experimental and Theoretical Studies of Isonitrosoacetanilides Derivatives as Corrosion Inhibitors for Mild Steel in 1 Mol L−1 HCl. J. Mol. Struct. 2021, 1245, 131256. [Google Scholar] [CrossRef]
- Berdimurodov, E.; Kholikov, A.; Akbarov, K.; Guo, L. Experimental and Theoretical Assessment of New and Eco–Friendly Thioglycoluril Derivative as an Effective Corrosion Inhibitor of St2 Steel in the Aggressive Hydrochloric Acid with Sulfate Ions. J. Mol. Liq. 2021, 335, 116168. [Google Scholar] [CrossRef]
- Srivastava, V.; Salman, M.; Chauhan, D.S.; Abdel-Azeim, S.; Quraishi, M.A. (E)-2-Styryl-1H-Benzo[d]Imidazole as Novel Green Corrosion Inhibitor for Carbon Steel: Experimental and Computational Approach. J. Mol. Liq. 2021, 324, 115010. [Google Scholar] [CrossRef]
- Espinoza Vázquez, A.; López Reséndiz, L.A.; Figueroa, I.A.; Rodríguez Gómez, F.J.; Figueroa, M.; Ángeles Beltrán, D.; Castro, M.; Miralrio, A. Corrosion Inhibition Assessment on API 5L X70 Steel by Preussomerin G Immersed in Saline and Saline Acetic. J. Adhes. Sci. Technol. 2021, 35, 873–899. [Google Scholar] [CrossRef]
- Toghan, A.; Gadow, H.S.; Dardeer, H.M.; Elabbasy, H.M. New Promising Halogenated Cyclic Imides Derivatives as Potential Corrosion Inhibitors for Carbon Steel in Hydrochloric Acid Solution. J. Mol. Liq. 2021, 325, 115136. [Google Scholar] [CrossRef]
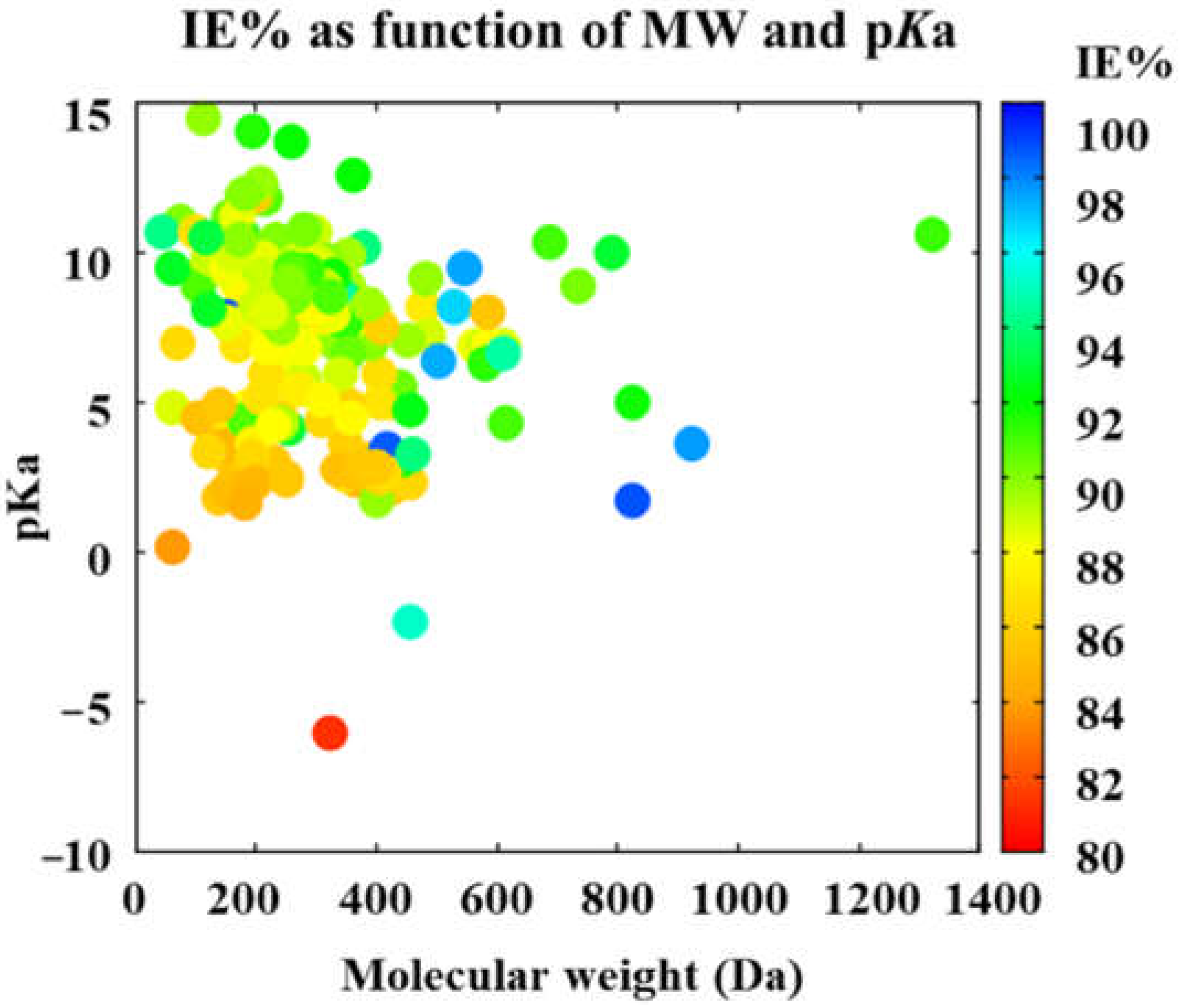
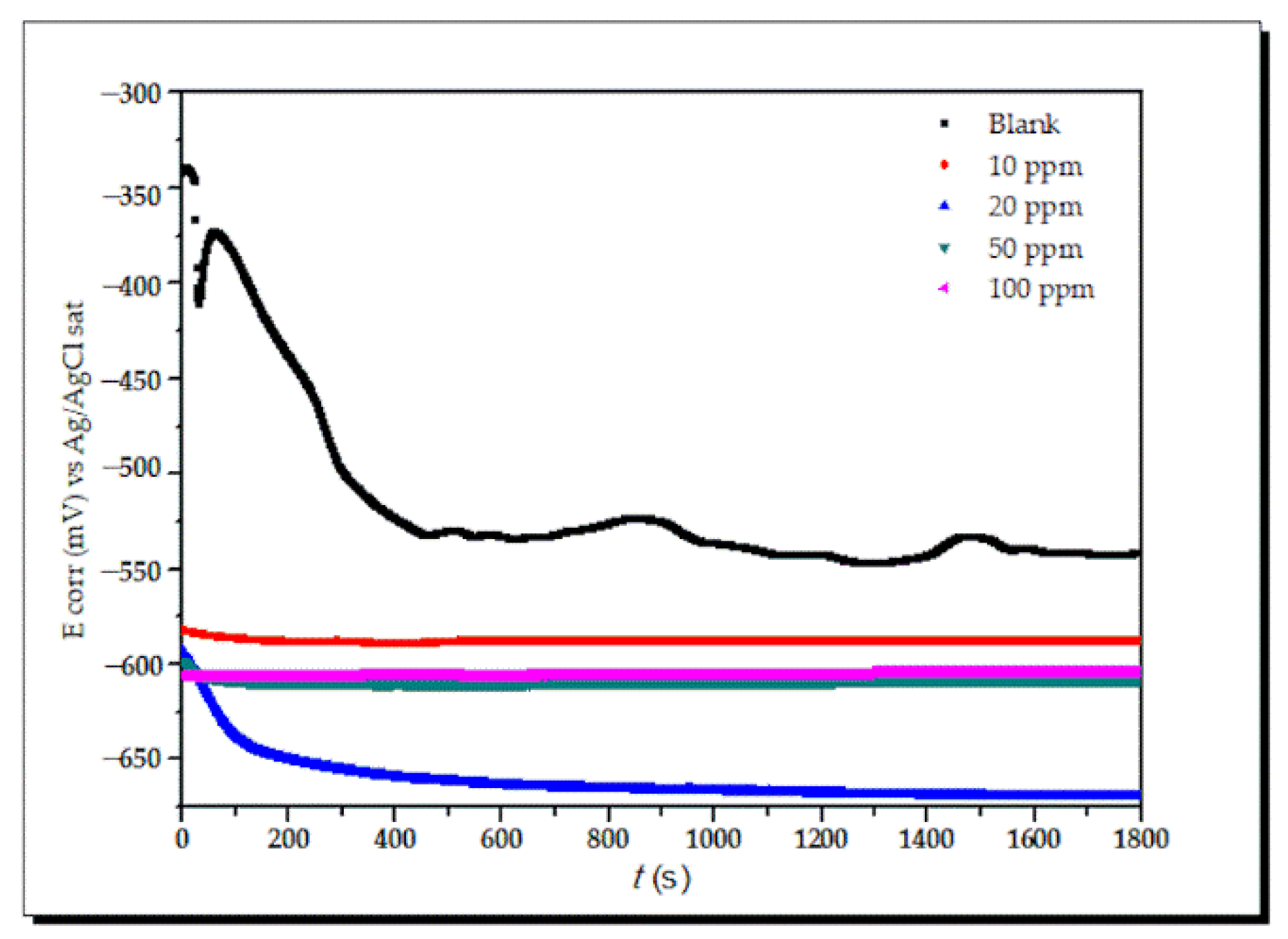
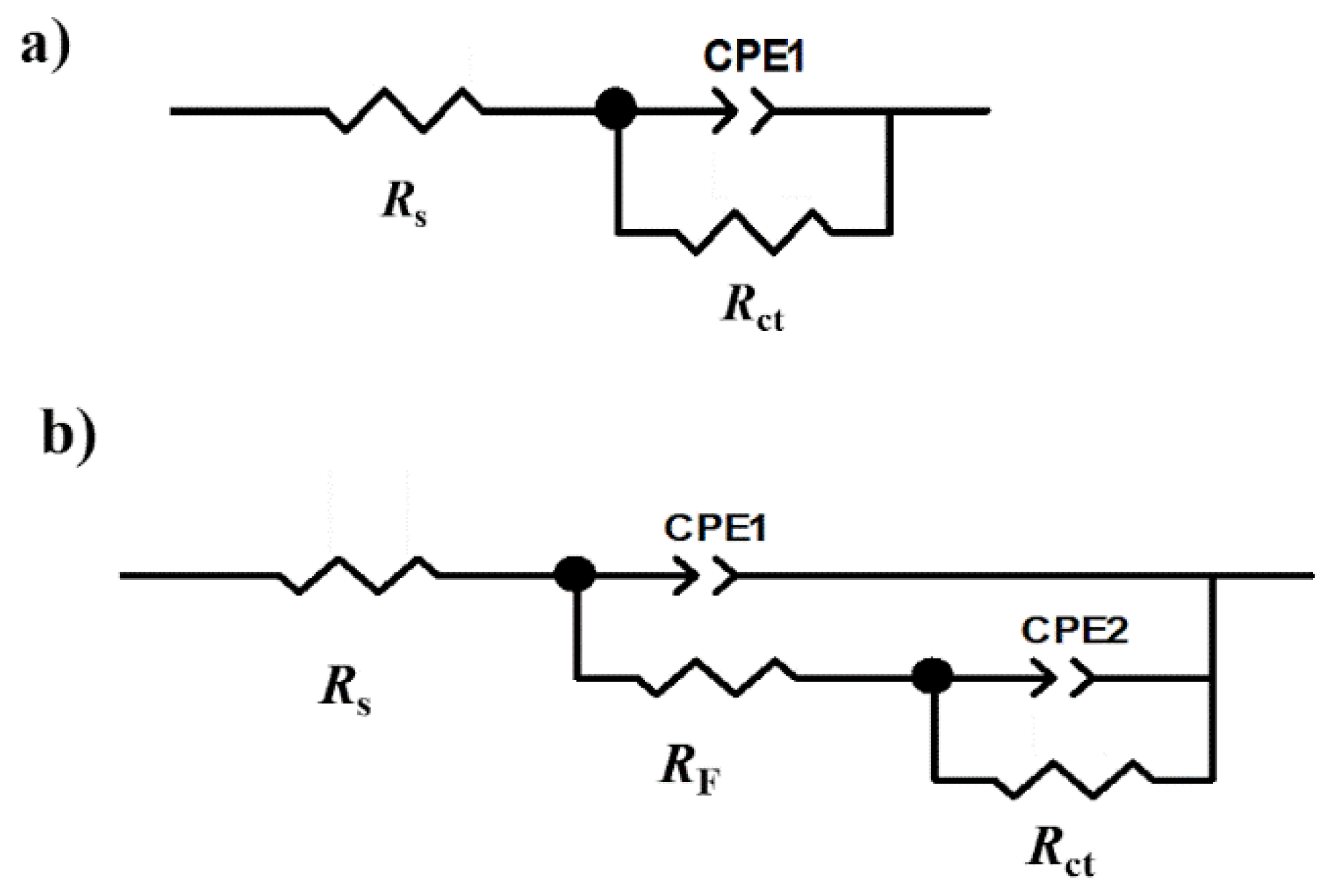
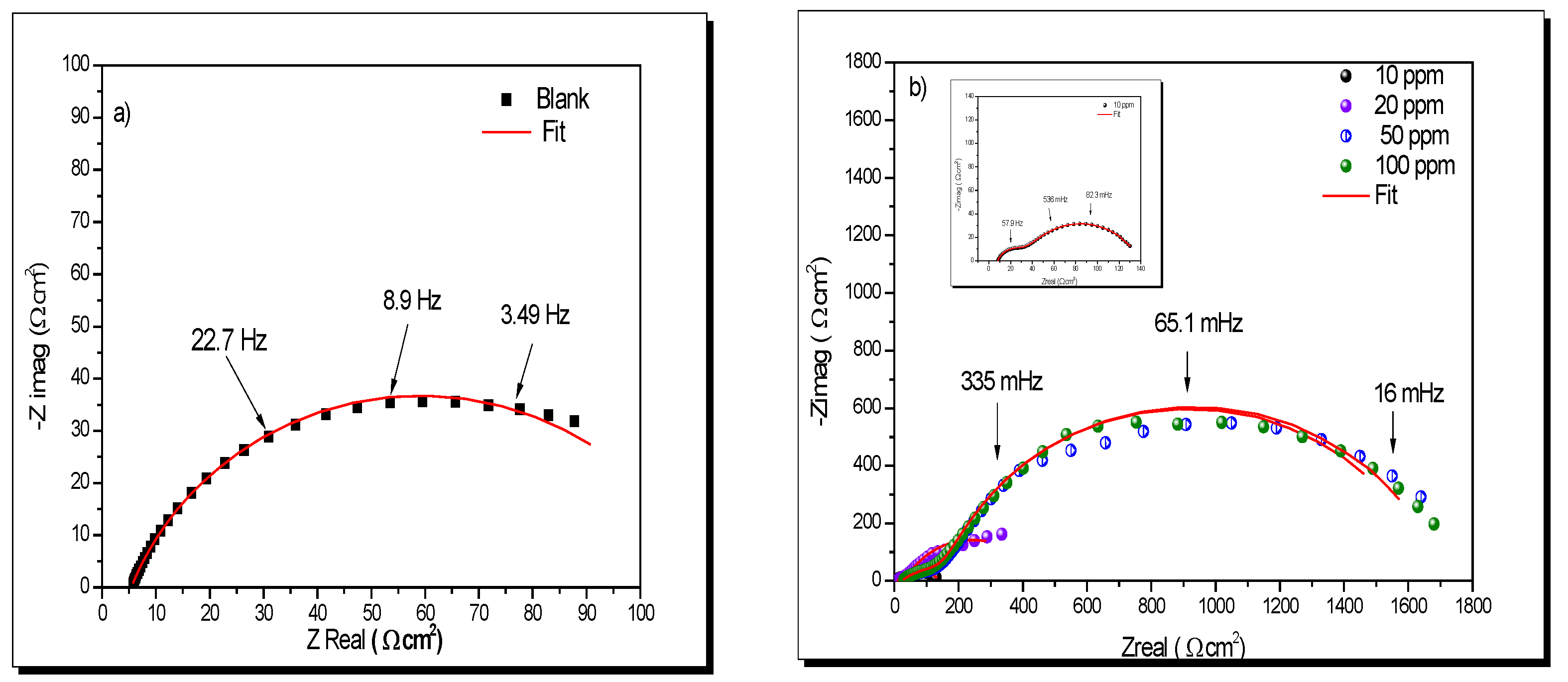
| xi | Descriptor | Symbol | Units | Reference | Parameter | ERR (%) |
|---|---|---|---|---|---|---|
| x1 | Molecular weight | MW | Da | [69,70] | - | - |
| x2 | Acid dissociation constant | pKa | - | - | 0.5287 | 0.0600 |
| x3 | Octanol-water partition coefficient | log P | - | [71,72] | - | - |
| x4 | Water solubility | log S | - | - | - | - |
| x5 | Polar surface area | PSA | Å2 | [38,71,72] | - | - |
| x6 | Polarizability | α | Å3 | [72] | - | - |
| x7 | Energy of HOMO | EHOMO | eV | [38,69,71,72] | 812.1748 | 97.8259 |
| x8 | Energy of LUMO | ELUMO | eV | [38,69,71,72] | 823.4630 | 0.1034 |
| x9 | Electrophilicity | ω | eV | [44,72] | 6579.0080 | 0.0688 |
| x10 | The fraction of electrons shared | ΔN | - | [44,70,72] | 33.1669 | 1.3933 |
| Sum of ERR | 99.4514 | |||||
| Drug | pKa | EHOMO | ELUMO | ω | ΔN | IE% | Common Use | 2D Structure |
|---|---|---|---|---|---|---|---|---|
| Deserpidine | 6.68 | −4.92 | −2.42 | 0.92 | 1.33 | 95.29 | Antihypertensive and antipsychotic |  |
| Daunorubicin | 8.20 | −5.87 | −4.01 | 1.23 | 1.11 | 96.67 | Cancer treatment |  |
| Dipyridamole | 6.40 | −4.34 | −1.83 | 0.77 | 1.55 | 97.28 | Anticoagulant |  |
| Doxorubicin | 9.46 | −5.86 | −4.00 | 1.23 | 1.12 | 97.40 | Cancer treatment |  |
| Amphotericin B | 3.58 | −5.27 | −3.29 | 1.07 | 1.37 | 97.55 | Antibiotic and fungicide |  |
| Minocycline | 2.30 | −5.32 | −3.42 | 1.09 | 1.38 | 97.58 | Antibiotic |  |
| Acepromazine | 9.30 | −4.92 | −2.53 | 0.93 | 1.37 | 97.73 | Antipsychotic |  |
| Cephaloridine | 3.40 | −5.31 | −3.43 | 1.09 | 1.40 | 98.57 | Antibiotic |  |
| Mercaptopurine | 7.80 | −5.03 | −2.83 | 0.98 | 1.40 | 98.66 | Cancer treatment | 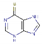 |
| Rifampicin | 1.70 | −4.86 | −2.81 | 0.96 | 1.55 | 98.71 | Antibiotic | 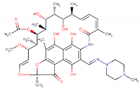 |
| C (ppm) | Rs (Ω cm2) | n | Cdl (µF/cm2) | Rct (Ω cm2) | CF (µF/cm2) | n2 | Rmol (Ω cm2) | Rtotal (Ω cm2) | IE% (%) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 6 | 0.800 | 2960 | 127 | - | - | - | - | - |
| 10 | 8.24 | 0.80 | 181.3 | 102.00 | 4034.0 | 0.8 | 28.70 | 130.70 | 3.2 |
| 20 | 10.53 | 0.77 | 187.5 | 404.10 | 622.2 | 0.52 | 337.90 | 742.00 | 83.0 |
| 50 | 24.66 | 0.85 | 90.3 | 1493.00 | 40.7 | 0.49 | 151.70 | 1644.70 | 92.3 |
| 100 | 24.29 | 0.84 | 51.9 | 1522.00 | 26.0 | 0.48 | 157.00 | 1679.00 | 92.5 |
| C (ppm) | Ecorr (mV) vs. Ag/AgCl sat | icorr (µA/cm2) | ba (mV/dec) | −bc (mV/dec) | IE% (%) |
|---|---|---|---|---|---|
| 0 | −804.7 | 67.4 | 159.5 | 173 | - |
| 10 | −909.7 | 65.0 | 146.6 | 161.5 | 3.4 |
| 20 | −709.6 | 4.9 | 104.5 | 204.1 | 92.6 |
| 50 | −907.7 | 7.4 | 170.5 | 60.3 | 89.0 |
| 100 | −916.5 | 8.2 | 187.8 | 68.2 | 87.4 |
| AFM image | Ra (nm) | Rq (nm) |
|---|---|---|
| a | 142 | 181 |
| b | 30.5 | 45 |
| c | 3.4 | 4.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltran-Perez, C.; Serrano, A.A.A.; Solís-Rosas, G.; Martínez-Jiménez, A.; Orozco-Cruz, R.; Espinoza-Vázquez, A.; Miralrio, A. A General Use QSAR-ARX Model to Predict the Corrosion Inhibition Efficiency of Drugs in Terms of Quantum Mechanical Descriptors and Experimental Comparison for Lidocaine. Int. J. Mol. Sci. 2022, 23, 5086. https://doi.org/10.3390/ijms23095086
Beltran-Perez C, Serrano AAA, Solís-Rosas G, Martínez-Jiménez A, Orozco-Cruz R, Espinoza-Vázquez A, Miralrio A. A General Use QSAR-ARX Model to Predict the Corrosion Inhibition Efficiency of Drugs in Terms of Quantum Mechanical Descriptors and Experimental Comparison for Lidocaine. International Journal of Molecular Sciences. 2022; 23(9):5086. https://doi.org/10.3390/ijms23095086
Chicago/Turabian StyleBeltran-Perez, Carlos, Andrés A. A. Serrano, Gilberto Solís-Rosas, Anatolio Martínez-Jiménez, Ricardo Orozco-Cruz, Araceli Espinoza-Vázquez, and Alan Miralrio. 2022. "A General Use QSAR-ARX Model to Predict the Corrosion Inhibition Efficiency of Drugs in Terms of Quantum Mechanical Descriptors and Experimental Comparison for Lidocaine" International Journal of Molecular Sciences 23, no. 9: 5086. https://doi.org/10.3390/ijms23095086