Impact of Fluid Dynamics on the Viability and Differentiation Capacity of 3D-Cultured Jaw Periosteal Cells
Abstract
:1. Introduction
2. Results
2.1. Cell Proliferation within JPC-Seeded Scaffolds Cultured in the Perfusion System
2.2. Visualization and Quantitative Analysis of Cell Distribution within β-TCP Scaffolds Cultured under Static and Perfusion Conditions by Crystal Violet Staining
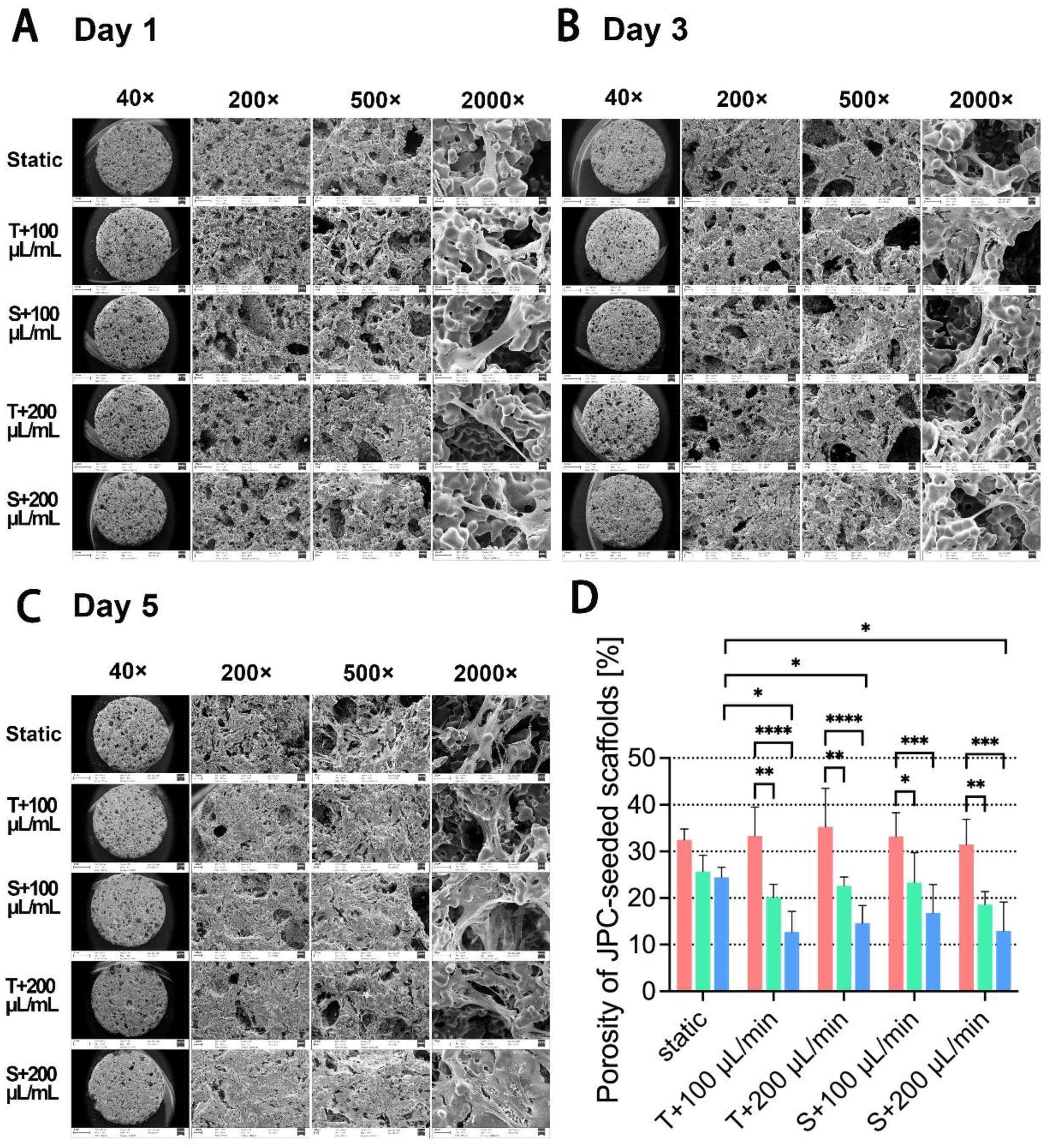
2.3. Visualization of Cell Morphology on JPC-Seeded β-TCP Scaffolds and Quantification of Scaffold Porosity by Scanning Electron Microscopy
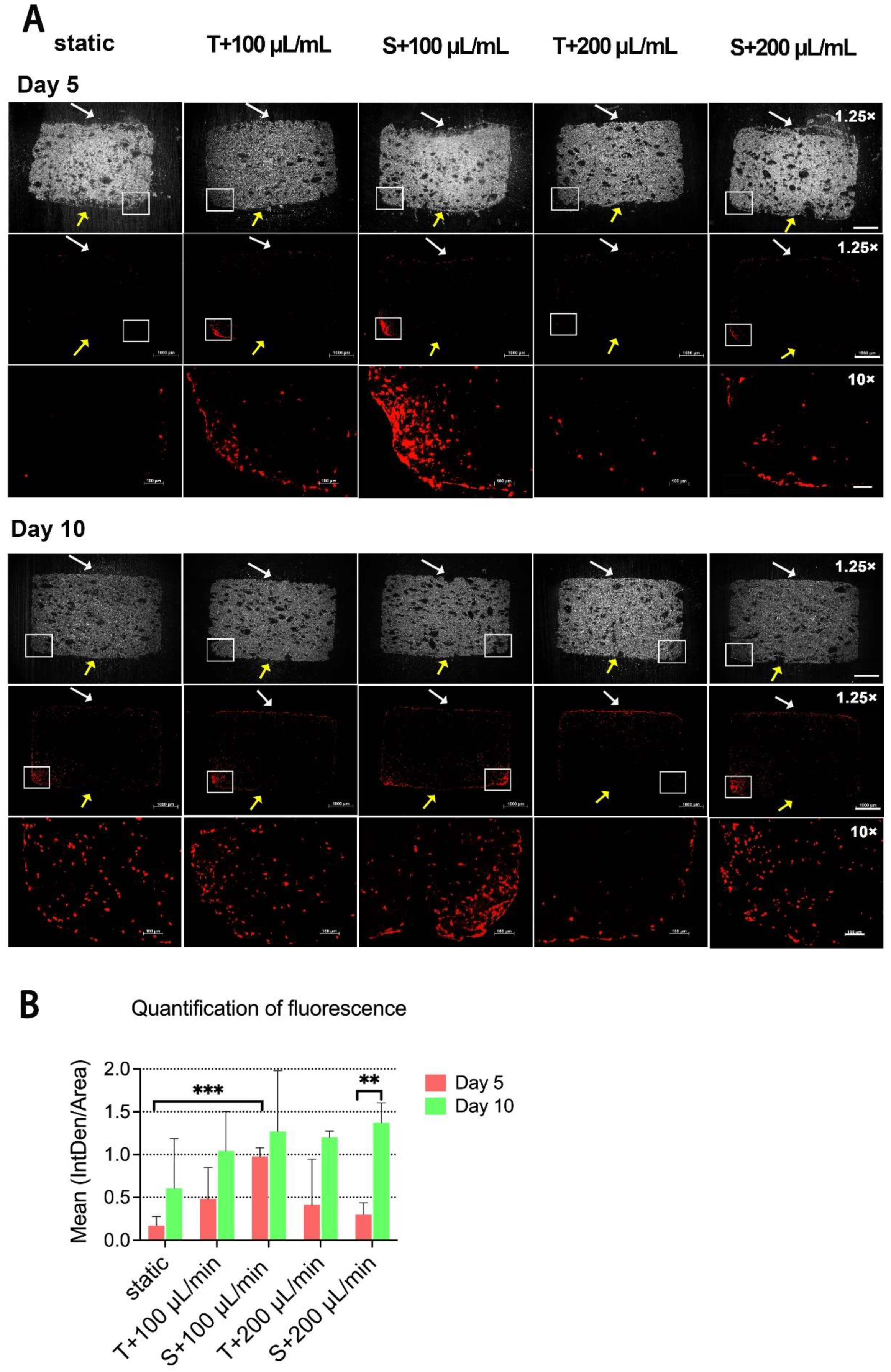
2.4. Visualization of Cell Density and Distribution within Scaffolds by Fluorescent Staining and Microscopy
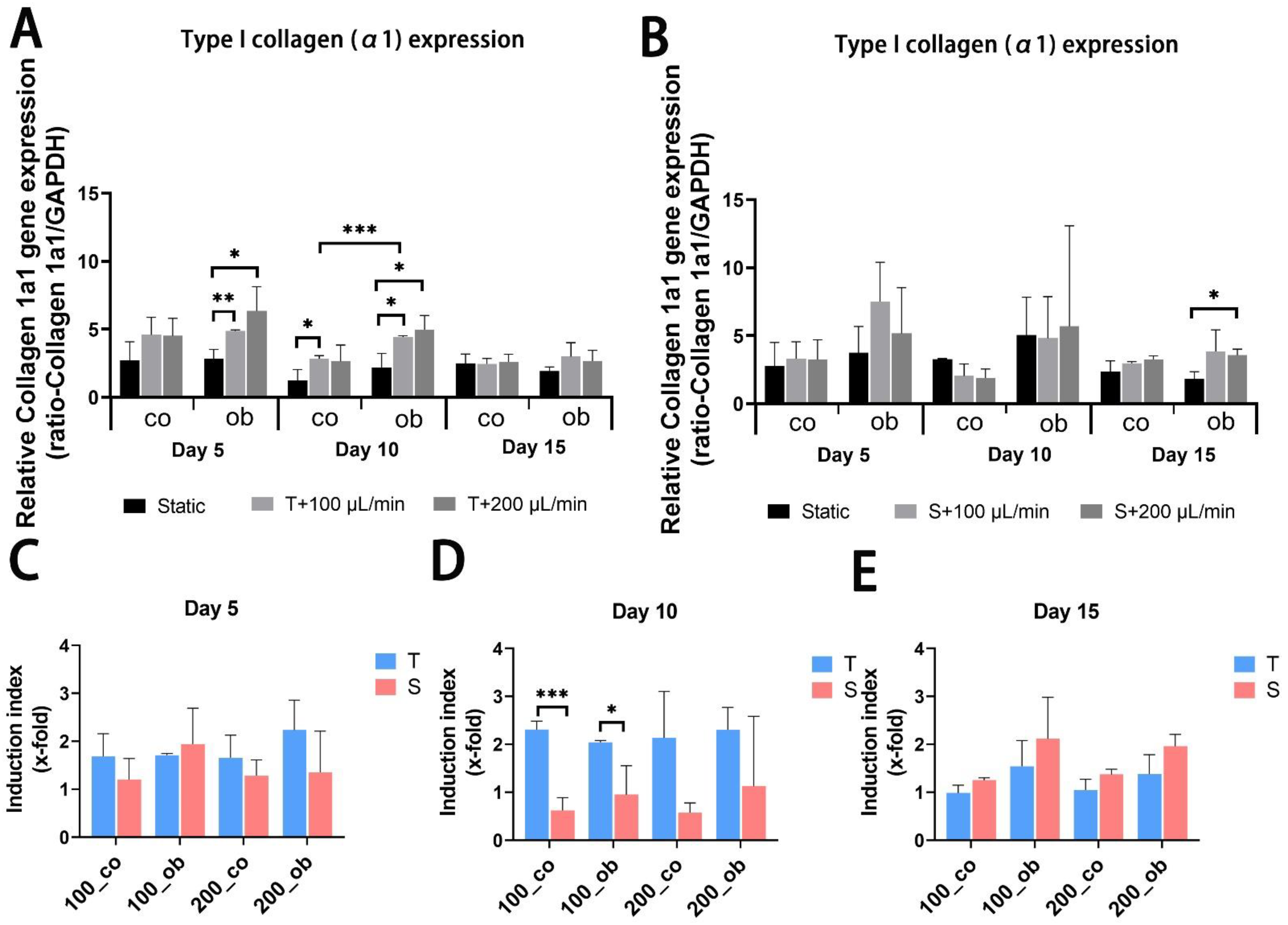
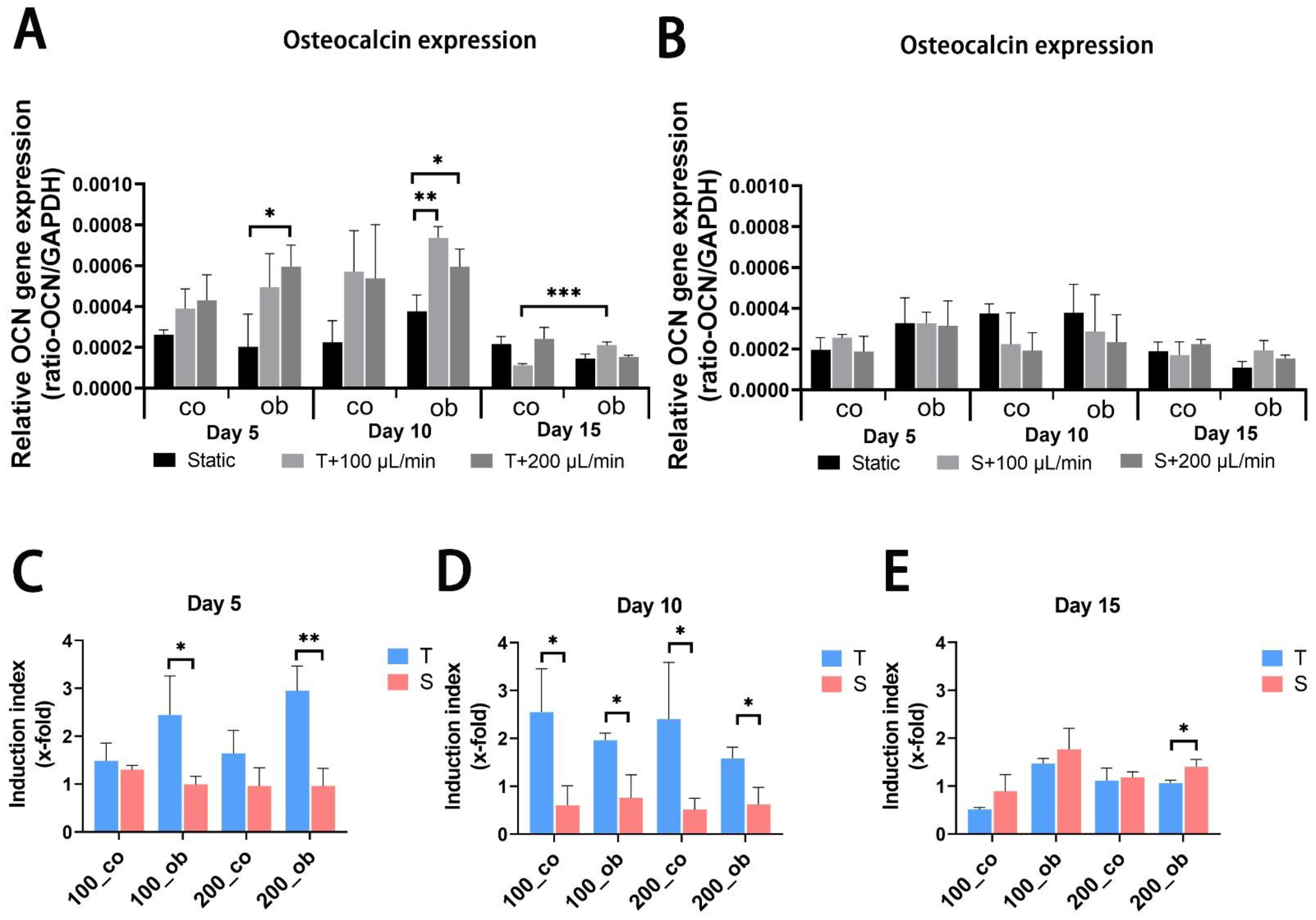
2.5. Gene Expression Analyses of Osteogenic Markers in 3D-Cultured JPCs Growing under Perfusion Conditions
3. Discussion
4. Materials and Methods
4.1. Isolation and Expansion of Jaw Periosteal Cells (JPCs)
4.2. Cell Seeding of β-TCP Scaffolds
4.3. Cultivation and Configuration of the Perfusion Bioreactor
4.4. Cell Metabolic Activity Assay
4.5. Crystal Violet Staining and Quantification
4.6. Scanning Electron Microscope (SEM) Analysis of JPC-Seeded Scaffolds
4.7. Embedding and Microtome Sectioning of Embedded JPC-Seeded Scaffolds
4.8. Fluorescent Cell Labeling with Sytox Orange
4.9. Gene Expression Analyses by Quantitative PCR
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, J.S.; Chen, P.Y.; Tsuang, Y.H.; Chen, M.H.; Chen, P.Q. Vitamin-D binding protein does not enhance healing in rat bone defects: A pilot study. Clin. Orthop. Relat. Res. 2009, 467, 3156–3164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartmell, S.H.; Porter, B.D.; Garcia, A.J.; Guldberg, R.E. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 2003, 9, 1197–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leclerc, E.; David, B.; Griscom, L.; Lepioufle, B.; Fujii, T.; Layrolle, P.; Legallaisa, C. Study of osteoblastic cells in a microfluidic environment. Biomaterials 2006, 27, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.; Hoffmann, J.; Munz, A.; Friedrich, B.; Geis-Gerstorfer, J.; Reinert, S. Analysis of OPLA scaffolds for bone engineering constructs using human jaw periosteal cells. J. Mater. Sci. Mater. Med. 2008, 19, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Ke, C.J.; Yen, K.C.; Hsieh, H.C.; Sun, J.S.; Lin, F.H. 3D porous calcium-alginate scaffolds cell culture system improved human osteoblast cell clusters for cell therapy. Theranostics 2015, 5, 643–655. [Google Scholar] [CrossRef] [Green Version]
- Ardjomandi, N.; Huth, J.; Stamov, D.R.; Henrich, A.; Klein, C.; Wendel, H.P.; Reinert, S.; Alexander, D. Surface biofunctionalization of beta-TCP blocks using aptamer 74 for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 267–275. [Google Scholar] [CrossRef]
- Filipowska, J.; Reilly, G.C.; Osyczka, A.M. A single short session of media perfusion induces osteogenesis in hBMSCs cultured in porous scaffolds, dependent on cell differentiation stage. Biotechnol. Bioeng. 2016, 113, 1814–1824. [Google Scholar] [CrossRef]
- Liu, Y.; Chan, J.K.; Teoh, S.H. Review of vascularised bone tissue-engineering strategies with a focus on co-culture systems. J. Tissue Eng. Regen. Med. 2015, 9, 85–105. [Google Scholar] [CrossRef]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef]
- Kanczler, J.M.; Oreffo, R.O. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cell Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Le, A.V.; Mendez, J.J.; Chang, J.; Niklason, L.E.; Steinbacher, D.M. Osteogenic performance of donor-matched human adipose and bone marrow mesenchymal cells under dynamic culture. Tissue Eng. Part A 2015, 21, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Perera, P.; Rath, B.; Agarwal, S. Dynamic regulation of bone morphogenetic proteins in engineered osteochondral constructs by biomechanical stimulation. Tissue Eng. Part A 2013, 19, 783–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.Y.; Teoh, S.H.; Chong, W.S.; Foo, T.T.; Chng, Y.C.; Choolani, M.; Chan, J. A biaxial rotating bioreactor for the culture of fetal mesenchymal stem cells for bone tissue engineering. Biomaterials 2009, 30, 2694–2704. [Google Scholar] [CrossRef] [PubMed]
- Bruder, S.P.; Kurth, A.A.; Shea, M.; Hayes, W.C.; Jaiswal, N.; Kadiyala, S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J. Orthop. Res. 1998, 16, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.N.; Ko, H.; Moriarty, R.A.; Etheridge, J.M.; Fisher, J.P. Dynamic Bioreactor Culture of High Volume Engineered Bone Tissue. Tissue Eng. Part A 2016, 22, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, A.; Liu, Y.; Teoh, S.H. Review: Bioreactor design towards generation of relevant engineered tissues: Focus on clinical translation. J. Tissue Eng. Regen. Med. 2018, 12, e7–e22. [Google Scholar] [CrossRef]
- Rauh, J.; Milan, F.; Gunther, K.P.; Stiehler, M. Bioreactor systems for bone tissue engineering. Tissue Eng. Part B Rev. 2011, 17, 263–280. [Google Scholar] [CrossRef] [Green Version]
- Eggli, P.S.; Muller, W.; Schenk, R.K. Porous hydroxyapatite and tricalcium phosphate cylinders with two different pore size ranges implanted in the cancellous bone of rabbits. A comparative histomorphometric and histologic study of bony ingrowth and implant substitution. Clin. Orthop. Relat. Res. 1988, 232, 127–138. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Dobelin, N. Beta-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Dai, J.; Umrath, F.; Reinert, S.; Alexander, D. Jaw Periosteal Cells Seeded in Beta-Tricalcium Phosphate Inhibit Dendritic Cell Maturation. Biomolecules 2020, 10, 887. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Umrath, F.; Steinle, H.; Schmitt, L.F.; Yu, L.T.; Schlensak, C.; Wendel, H.P.; Reinert, S.; Alexander, D.; Avci-Adali, M. Influence of Human Jaw Periosteal Cells Seeded beta-Tricalcium Phosphate Scaffolds on Blood Coagulation. Int. J. Mol. Sci. 2021, 22, 9942. [Google Scholar] [CrossRef] [PubMed]
- Ardjomandi, N.; Henrich, A.; Huth, J.; Klein, C.; Schweizer, E.; Scheideler, L.; Rupp, F.; Reinert, S.; Alexander, D. Coating of ss-tricalcium phosphate scaffolds-a comparison between graphene oxide and poly-lactic-co-glycolic acid. Biomed. Mater. 2015, 10, 045018. [Google Scholar] [CrossRef] [PubMed]
- Brauchle, E.; Carvajal Berrio, D.; Rieger, M.; Schenke-Layland, K.; Reinert, S.; Alexander, D. Raman Spectroscopic Analyses of Jaw Periosteal Cell Mineralization. Stem Cells Int. 2017, 2017, 1651376. [Google Scholar] [CrossRef] [PubMed]
- Danalache, M.; Kliesch, S.M.; Munz, M.; Naros, A.; Reinert, S.; Alexander, D. Quality Analysis of Minerals Formed by Jaw Periosteal Cells under Different Culture Conditions. Int. J. Mol. Sci. 2019, 20, 4193. [Google Scholar] [CrossRef] [Green Version]
- Barthes, J.; Ozcelik, H.; Hindie, M.; Ndreu-Halili, A.; Hasan, A.; Vrana, N.E. Cell microenvironment engineering and monitoring for tissue engineering and regenerative medicine: The recent advances. Biomed Res. Int. 2014, 2014, 921905. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.K.; Kao, S.W.; Roux, B.M.; Rodriguez, R.A.; Tang, S.J.; Fisher, J.P.; Cheng, M.H.; Brey, E.M. Perfusion Bioreactor Culture of Bone Marrow Stromal Cells Enhances Cranial Defect Regeneration. Plast. Reconstr. Surg. 2019, 143, 993e–1002e. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Miyamoto, Y.; Tsuchiya, A.; Hayashi, K.; Tsuru, K.; Ohe, G. Physical and Histological Comparison of Hydroxyapatite, Carbonate Apatite, and beta-Tricalcium Phosphate Bone Substitutes. Materials 2018, 11, 1993. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.L.; Iannotti, J.P.; Ham, A.; Bleuit, J.; Chen, J.H. Effects of fluid shear stress on bone cells. Biorheology 1994, 31, 163–170. [Google Scholar] [CrossRef]
- McCoy, R.J.; O’Brien, F.J. Influence of shear stress in perfusion bioreactor cultures for the development of three-dimensional bone tissue constructs: A review. Tissue Eng. Part B Rev. 2010, 16, 587–601. [Google Scholar] [CrossRef]
- Wittkowske, C.; Reilly, G.C.; Lacroix, D.; Perrault, C.M. In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation. Front. Bioeng. Biotechnol. 2016, 4, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurkan, U.A.; Akkus, O. The mechanical environment of bone marrow: A review. Ann. Biomed. Eng. 2008, 36, 1978–1991. [Google Scholar] [CrossRef]
- Hoemann, C.D.; El-Gabalawy, H.; McKee, M.D. In vitro osteogenesis assays: Influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol. Biol. 2009, 57, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Farley, J.R.; Wergedal, J.E.; Baylink, D.J. Fluoride directly stimulates proliferation and alkaline phosphatase activity of bone-forming cells. Science 1983, 222, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Mygind, T.; Stiehler, M.; Baatrup, A.; Li, H.; Zou, X.; Flyvbjerg, A.; Kassem, M.; Bunger, C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials 2007, 28, 1036–1047. [Google Scholar] [CrossRef]
- Zeng, L.; Wei, J.; Han, D.; Liu, H.; Liu, Y.; Zhao, N.; Sun, S.; Wang, Y.; Feng, H. Functional analysis of novel RUNX2 mutations in cleidocranial dysplasia. Mutagenesis 2017, 32, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.J.; Bae, H.S.; Ryoo, H.M.; Baek, S.H. A novel RUNX2 mutation in exon 8, G462X, in a patient with Cleidocranial Dysplasia. J. Cell Biochem. 2018, 119, 1152–1162. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cen, W.; Wang, S.; Umrath, F.; Reinert, S.; Alexander, D. Impact of Fluid Dynamics on the Viability and Differentiation Capacity of 3D-Cultured Jaw Periosteal Cells. Int. J. Mol. Sci. 2022, 23, 4682. https://doi.org/10.3390/ijms23094682
Cen W, Wang S, Umrath F, Reinert S, Alexander D. Impact of Fluid Dynamics on the Viability and Differentiation Capacity of 3D-Cultured Jaw Periosteal Cells. International Journal of Molecular Sciences. 2022; 23(9):4682. https://doi.org/10.3390/ijms23094682
Chicago/Turabian StyleCen, Wanjing, Suya Wang, Felix Umrath, Siegmar Reinert, and Dorothea Alexander. 2022. "Impact of Fluid Dynamics on the Viability and Differentiation Capacity of 3D-Cultured Jaw Periosteal Cells" International Journal of Molecular Sciences 23, no. 9: 4682. https://doi.org/10.3390/ijms23094682





