JAK2 Inhibitor, Fedratinib, Inhibits P-gp Activity and Co-Treatment Induces Cytotoxicity in Antimitotic Drug-Treated P-gp Overexpressing Resistant KBV20C Cancer Cells
Abstract
:1. Introduction
2. Results
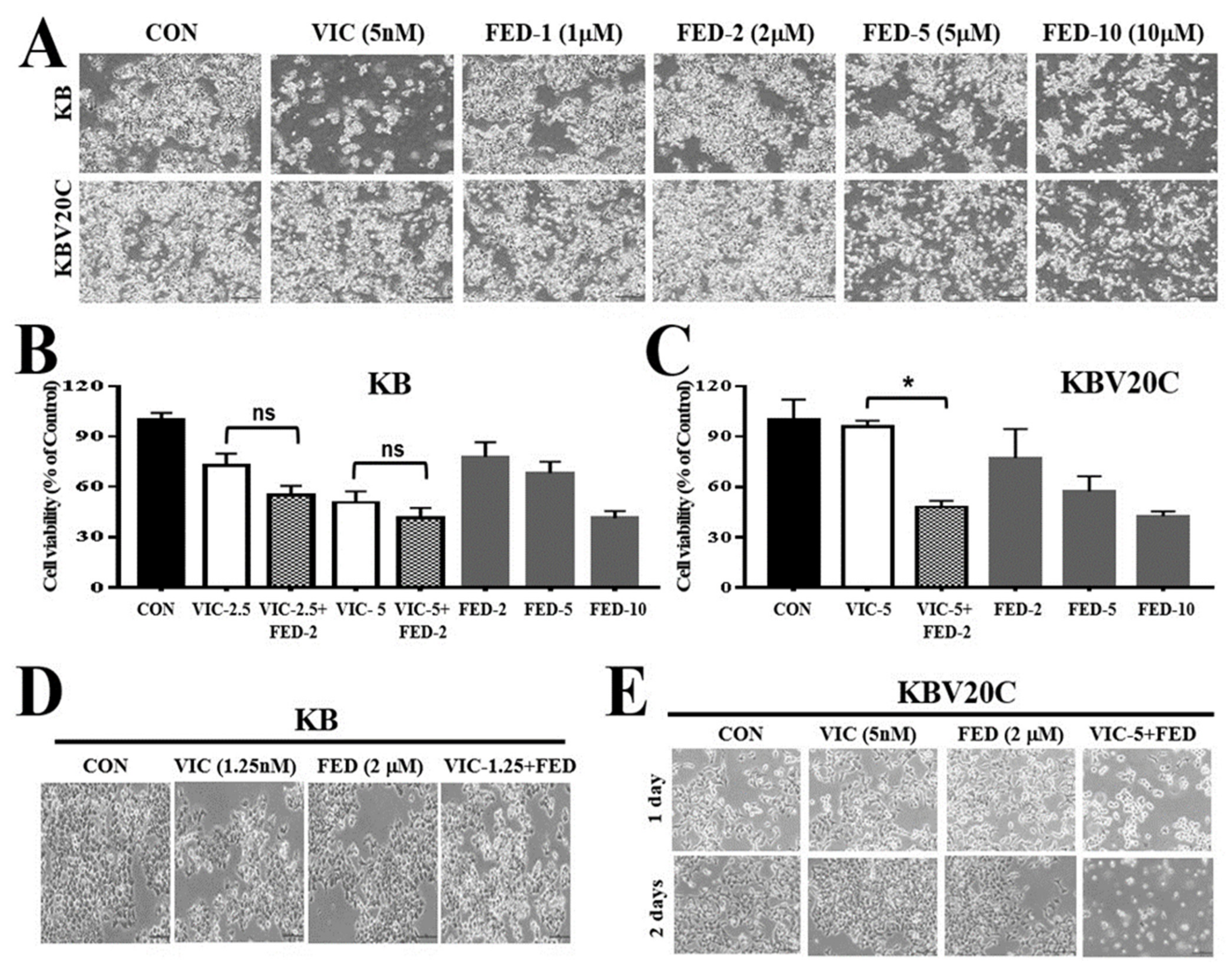
2.1. Single Treatment of Fedratinib Displays Similar Cytotoxic Effects in Drug-Sensitive Parent KB and Drug-Resistant KBV20C Cells
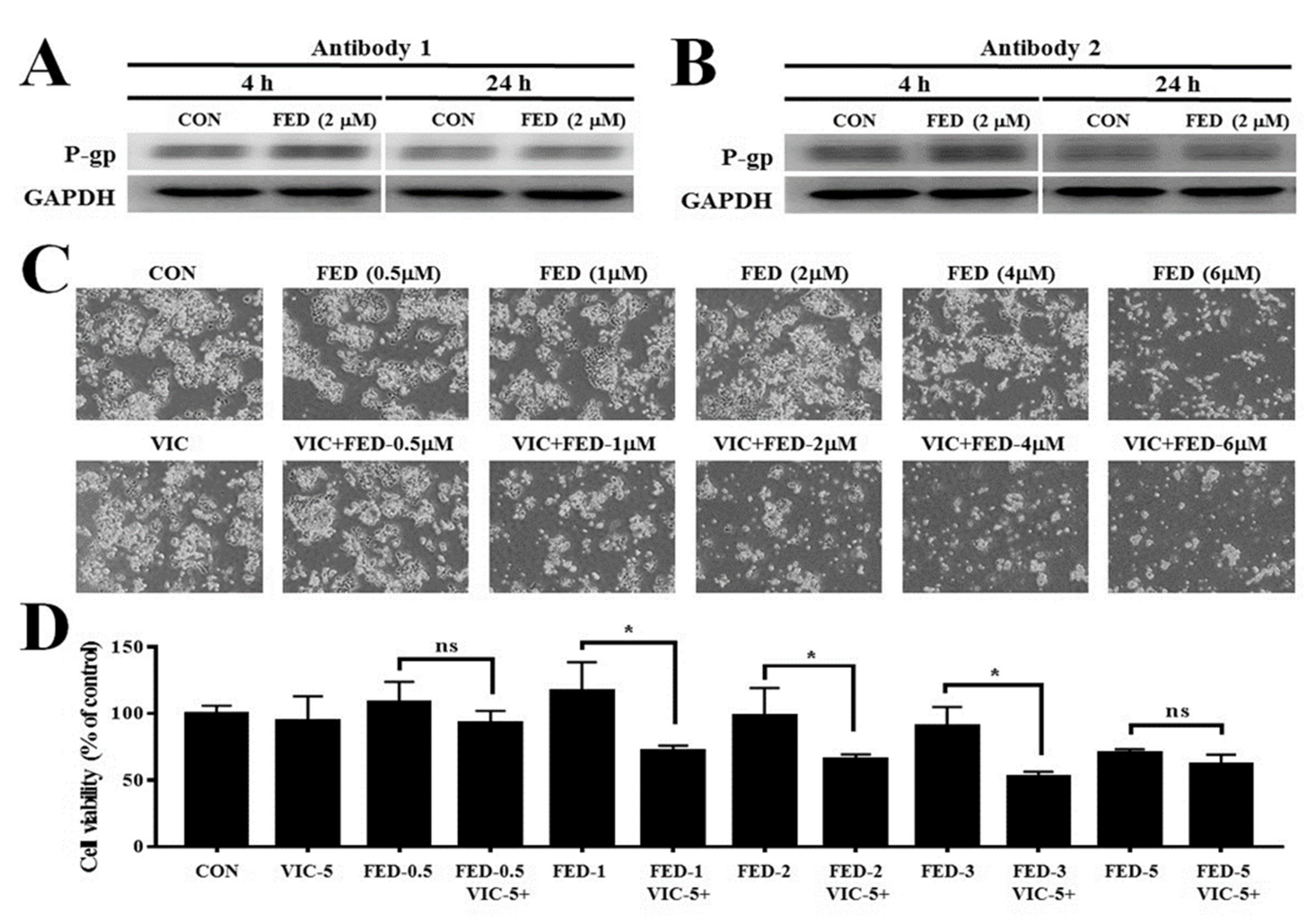
2.2. VIC–Fedratinib Co-Treatment Increases Cytotoxicity in Drug-Resistant KBV20C Cancer Cells
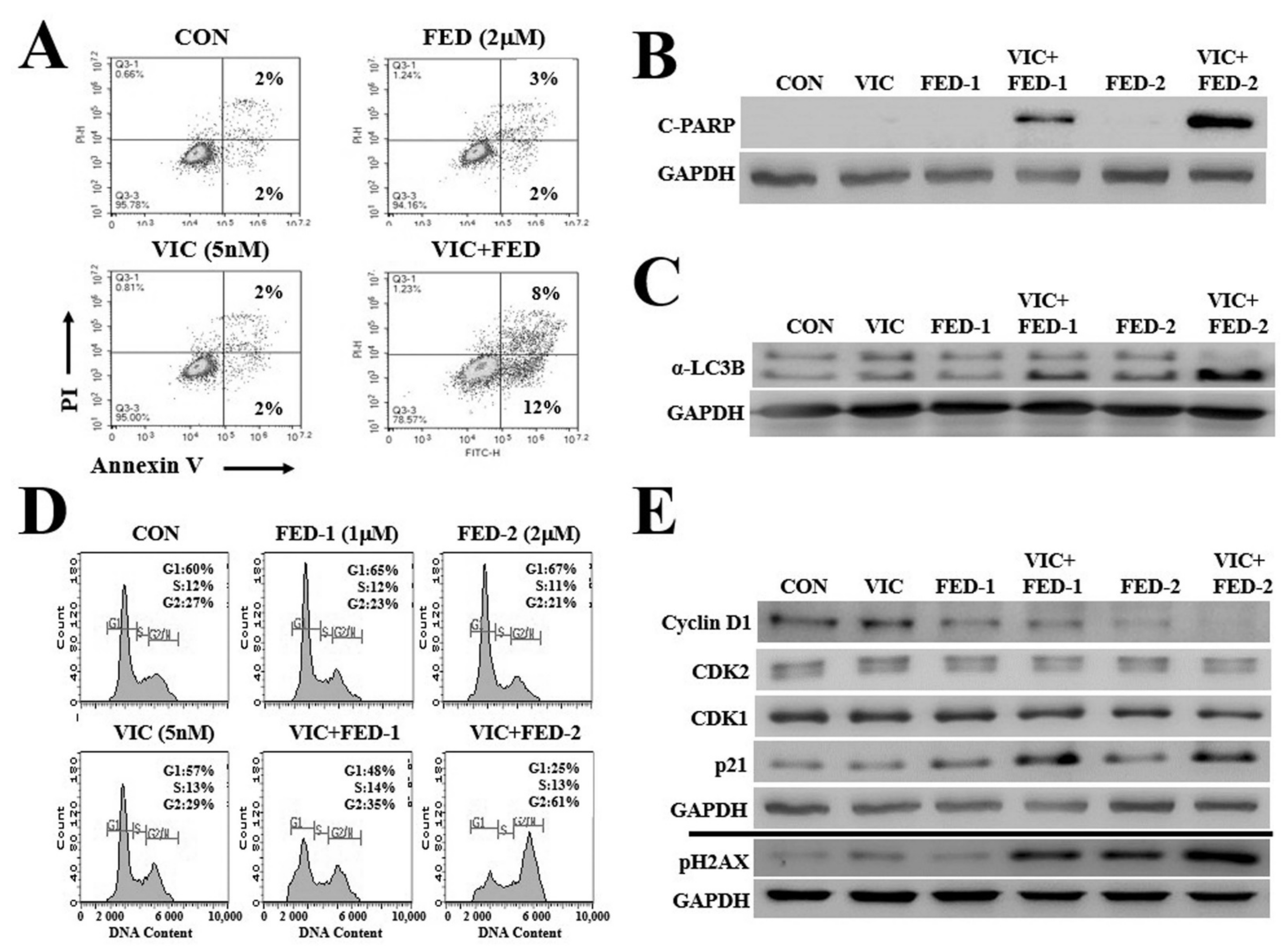
2.3. VIC–Fedratinib Co-Treatment Increases Apoptosis in KBV20C Cells in a Dose-Dependent Manner
2.4. VIC–Fedratinib Co-Treatment Induces G2-Arrest and Increases DNA Damage in KBV20C Cells
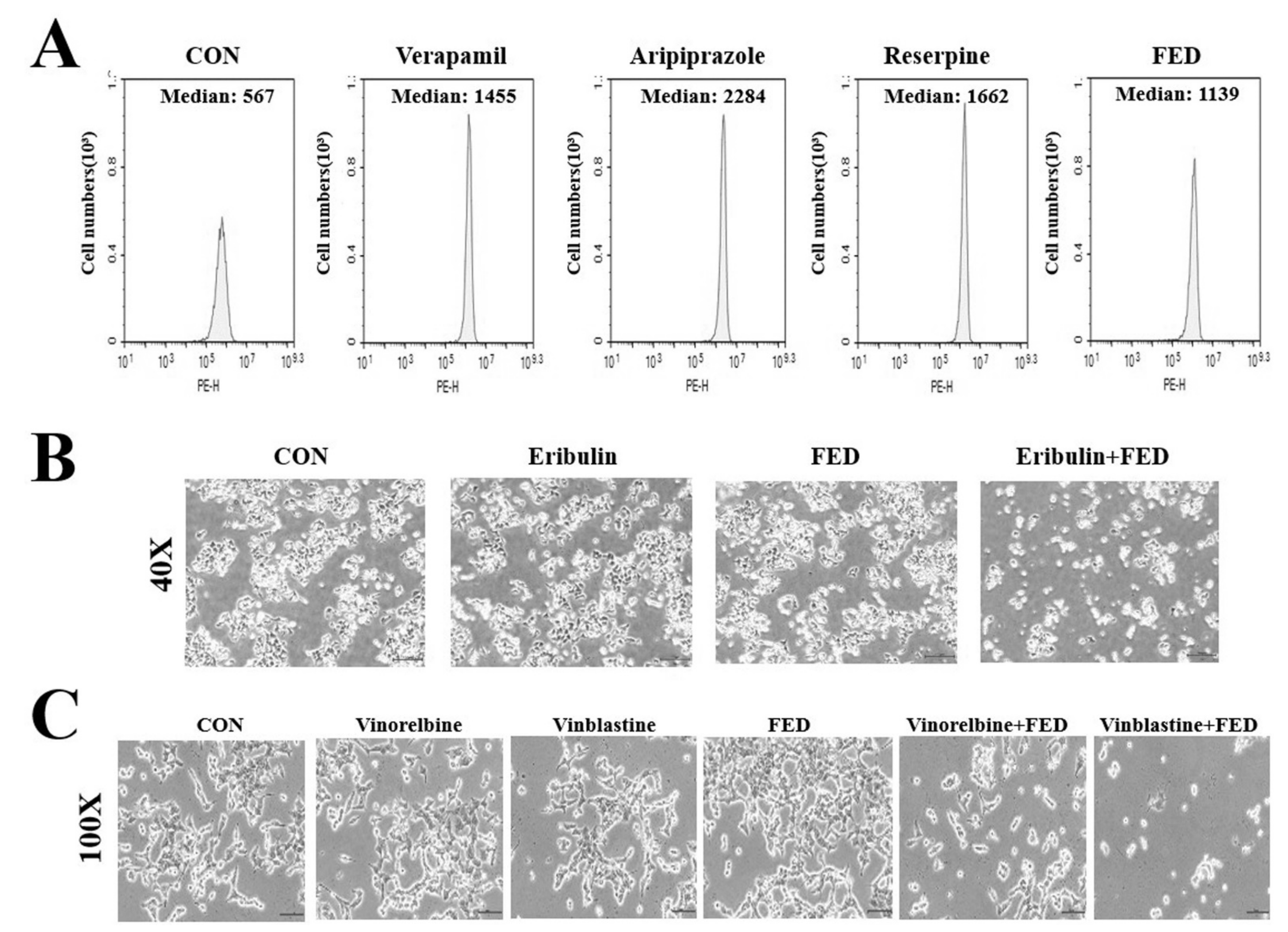
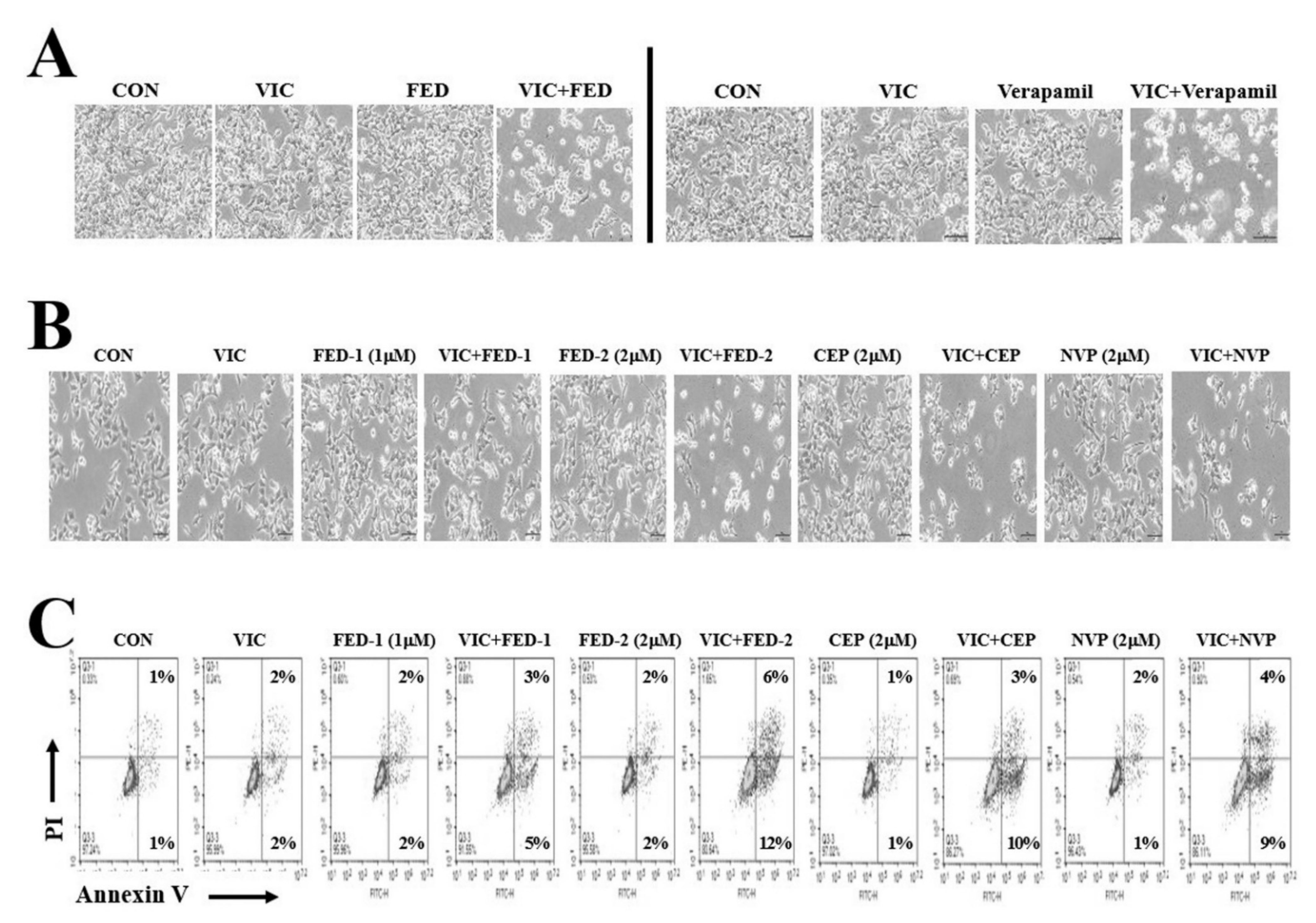
2.5. Fedratinib Demonstrates P-gp Inhibiting Activity
2.6. Fedratinib Treatment Complements the Cytotoxic Effects of Other Antimitotic Drugs in KBV20C Cells
2.7. JAK2 Inhibitors (Fedratinib, CEP-33779, and NVP-BSK805) Increase Cytotoxicity in VIC-Treated KBV20C Cells through Similar Mechanisms of Action
3. Discussion
4. Materials and Methods
4.1. Reagents and Cell Culture
4.2. Microscopic Observation
4.3. Cell Viability Assay
4.4. Fluorescence-Activated Cell Sorting (FACS) Analysis
4.5. Annexin V Analysis
4.6. Rhodamine Uptake Tests
4.7. Western Blot Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| MDR | Multidrug resistance |
| P-gp | P-glycoprotein |
| JAK2 | Janus kinase 2 |
| FED | Fedratinib |
| CEP | CEP-33779 |
| NVP | NVP-BSK805 |
| VIC | Vincristine |
| ERI | Eribulin |
| VIO | Vinorelbine |
| VIB | Vinblastine |
| VER | Verapamil |
| DMSO | Dimethylsulfoxide |
| C-PARP | Cleaved ploy ADP ribose polymerase |
| FACS | Fluorescence-activated cell sorting |
| FDA | The United States Food and Drug Administration |
References
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoo, H.I.; Kang, H.S.; Ro, J.; Yoon, S. Salinomycin sensitizes antimitotic drugs-treated cancer cells by increasing apoptosis via the prevention of G2 arrest. Biochem. Biophys. Res. Commun. 2012, 418, 98–103. [Google Scholar] [CrossRef] [PubMed]
- McGrogan, B.T.; Gilmartin, B.; Carney, D.N.; McCann, A. Taxanes, microtubules and chemoresistant breast cancer. Biochim. Biophys. Acta 2008, 1785, 96–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016, 370, 153–164. [Google Scholar] [CrossRef]
- Shukla, S.; Wu, C.P.; Ambudkar, S.V. Development of inhibitors of ATP-binding cassette drug transporters: Present status and challenges. Expert Opin. Drug Metab. Toxicol. 2008, 4, 205–223. [Google Scholar] [CrossRef]
- Kathawala, R.J.; Wang, Y.J.; Ashby, C.R., Jr.; Chen, Z.S. Recent advances regarding the role of ABC subfamily C member 10 (ABCC10) in the efflux of antitumor drugs. Chin. J. Cancer 2014, 33, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Chufan, E.E.; Kapoor, K.; Ambudkar, S.V. Drug-protein hydrogen bonds govern the inhibition of the ATP hydrolysis of the multidrug transporter P-glycoprotein. Biochem. Pharmacol. 2016, 101, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Libby, E.; Hromas, R. Dismounting the MDR horse. Blood 2010, 116, 4037–4038. [Google Scholar] [CrossRef]
- Yang, K.; Wu, J.; Li, X. Recent advances in the research of P-glycoprotein inhibitors. Biosci. Trends 2008, 2, 137–146. [Google Scholar]
- Clark, K.B. New therapeutic bearings for repositioned drugs. Curr. Top Med. Chem. 2013, 13, 2281–2282. [Google Scholar] [CrossRef]
- Pantziarka, P.; Cairns, L. Recycling existing drugs for cancer therapy: Delivering low cost cancer care. Ecancermedicalscience 2014, 8, ed40. [Google Scholar]
- Yoon, S.; Wang, X.; Vongpunsawad, S.; Tromp, G.; Kuivaniemi, H. Editorial: FDA-Approved drug repositioning for P-glycoprotein overexpressing resistant cancer. Front. Oncol. 2021, 11, 632657. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Wu, J.; Zhong, S.; Li, H. Inhibition of JAK2 reverses paclitaxel resistance in human ovarian cancer cells. Int. J. Gynecol. Cancer 2015, 25, 1557–1564. [Google Scholar] [CrossRef]
- Balko, J.M.; Schwarz, L.J.; Luo, N.; Estrada, M.V.; Giltnane, J.M.; Davila-Gonzalez, D.; Wang, K.; Sanchez, V.; Dean, P.T.; Combs, S.E.; et al. Triple-negative breast cancers with amplification of JAK2 at the 9p24 locus demonstrate JAK2-specific dependence. Sci. Transl. Med. 2016, 8, 334ra53. [Google Scholar] [CrossRef] [Green Version]
- Barrett, M.T.; Anderson, K.S.; Lenkiewicz, E.; Andreozzi, M.; Cunliffe, H.E.; Klassen, C.L.; Dueck, A.C.; McCullough, A.E.; Reddy, S.K.; Ramanathan, R.K.; et al. Genomic amplification of 9p24.1 targeting JAK2, PD-L1, and PD-L2 is enriched in high-risk triple negative breast cancer. Oncotarget 2015, 6, 26483–26493. [Google Scholar] [CrossRef] [Green Version]
- Buchert, M.; Burns, C.J.; Ernst, M. Targeting JAK kinase in solid tumors: Emerging opportunities and challenges. Oncogene 2016, 35, 939–951. [Google Scholar] [CrossRef]
- Bose, P.; Verstovsek, S. JAK inhibition for the treatment of myelofibrosis: Limitations and future perspectives. Hemasphere 2020, 4, e424. [Google Scholar] [CrossRef]
- Raivola, J.; Haikarainen, T.; Abraham, B.G.; Silvennoinen, O. Janus Kinases in Leukemia. Cancers 2021, 13, 800. [Google Scholar] [CrossRef]
- Tang, S.J.; Chen, L.K.; Wang, F.; Zhang, Y.K.; Huang, Z.C.; To, K.K.; Wang, X.K.; Talele, T.T.; Chen, Z.S.; Chen, W.Q.; et al. CEP-33779 antagonizes ATP-binding cassette subfamily B member 1 mediated multidrug resistance by inhibiting its transport function. Biochem. Pharmacol. 2014, 91, 144–156. [Google Scholar] [CrossRef]
- Cheon, J.H.; Kim, J.Y.; Lee, B.M.; Kim, H.S.; Yoon, S. P-gp inhibition by XL019, a JAK2 inhibitor, Increases apoptosis of vincristine-treated resistant KBV20C cells with increased p21 and pH2AX expression. Anticancer Res. 2017, 37, 6761–6769. [Google Scholar]
- Cheon, J.H.; Kim, K.S.; Yadav, D.K.; Kim, M.; Kim, H.S.; Yoon, S. The JAK2 inhibitors CEP-33779 and NVP-BSK805 have high P-gp inhibitory activity and sensitize drug-resistant cancer cells to vincristine. Biochem. Biophys. Res. Commun. 2017, 490, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Ragheb, M.; Harrison, C.N.; McLornan, D.P. Current and future role of fedratinib in the treatment of myelofibrosis. Future Oncol. 2020, 16, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Talpaz, M.; Kiladjian, J.J. Fedratinib, a newly approved treatment for patients with myeloproliferative neoplasm-associated myelofibrosis. Leukemia 2021, 35, 1–17. [Google Scholar] [CrossRef]
- Florian, S.; Mitchison, T.J. Anti-Microtubule Drugs. Methods Mol. Biol. 2016, 1413, 403–421. [Google Scholar] [PubMed]
- Jiang, C.; Lee, S.H.; Park, J.H.; Lee, J.S.; Park, J.W.; Kim, J.R.; Lee, S.H.; Kim, H.S.; Yoon, S. A low dose of aripiprazole has the strongest sensitization effect among 19 repositioned bipolar drugs in P-gp-overexpressing drug-resistant cancer cells. Anticancer Res. 2021, 41, 687–697. [Google Scholar] [CrossRef]
- Kim, K.S.; Jiang, C.; Kim, J.Y.; Park, J.H.; Kim, H.R.; Lee, S.H.; Kim, H.S.; Yoon, S. Low-dose crizotinib, a tyrosine kinase inhibitor, highly and specifically sensitizes P-glycoprotein-overexpressing chemoresistant cancer cells through induction of late apoptosis in vivo and in vitro. Front. Oncol. 2020, 10, 696. [Google Scholar] [CrossRef]
- Park, J.H.; Kundu, A.; Lee, S.H.; Jiang, C.; Lee, S.H.; Kim, Y.S.; Kyung, S.Y.; Park, S.H.; Kim, H.S. Specific pyruvate kinase M2 inhibitor, compound 3K, Induces autophagic cell death through disruption of the glycolysis pathway in ovarian cancer cells. Int. J. Biol. Sci. 2021, 17, 1895–1908. [Google Scholar] [CrossRef]
- Wang, L.G.; Liu, X.M.; Kreis, W.; Budman, D.R. The effect of antimicrotubule agents on signal transduction pathways of apoptosis: A review. Cancer Chemother. Pharmacol. 1999, 44, 355–461. [Google Scholar] [CrossRef]
- Lim, J.S.; Park, Y.; Lee, B.M.; Kim, H.S.; Yoon, S. Co-treatment with celecoxib or NS398 strongly sensitizes resistant cancer cells to antimitotic drugs independent of P-gp inhibition. Anticancer Res. 2016, 36, 5063–5070. [Google Scholar]
- Kim, J.Y.; Tae, I.H.; Lee, B.M.; Kim, H.S.; Yoon, S. Low doses of the anti-psychotic drug aripiprazole have strong P-gp-inhibitory activity and sensitize anti-mitotic drug-resistant cancer cells. Anticancer Res. 2018, 38, 5101–5108. [Google Scholar] [CrossRef]
- Sarver, J.G.; Klis, W.A.; Byers, J.P.; Erhardt, P.W. Microplate screening of the differential effects of test agents on Hoechst 33342, rhodamine 123, and rhodamine 6G accumulation in breast cancer cells that overexpress P-glycoprotein. J. Biomol. Screen 2002, 7, 29–34. [Google Scholar]
- Kim, J.Y.; Park, Y.; Lee, B.M.; Kim, H.S.; Yoon, S. P-gp inhibition by the anti-psychotic drug pimozide increases apoptosis, as well as expression of pRb and pH2AX in highly drug-resistant KBV20C cells. Anticancer Res. 2018, 38, 5685–5692. [Google Scholar] [CrossRef]
- Park, Y.; Son, J.Y.; Lee, B.M.; Kim, H.S.; Yoon, S. Highly eribulin-resistant KBV20C oral cancer cells can be sensitized by co-treatment with the third-generation P-glycoprotein inhibitor, elacridar, at a low dose. Anticancer Res. 2017, 37, 4139–4146. [Google Scholar]
- Cheon, J.H.; Lee, B.M.; Kim, H.S.; Yoon, S. Highly halaven-resistant KBV20C cancer cells can be sensitized by co-treatment with fluphenazine. Anticancer Res. 2016, 36, 5867–5874. [Google Scholar] [CrossRef] [Green Version]
- Choi, A.R.; Kim, J.H.; Woo, Y.H.; Kim, H.S.; Yoon, S. Anti-malarial drugs primaquine and chloroquine have different sensitization effects with anti-mitotic drugs in resistant cancer cells. Anticancer Res. 2016, 36, 1641–1648. [Google Scholar] [CrossRef]
- Vainchenker, W.; Leroy, E.; Gilles, L.; Marty, C.; Plo, I.; Constantinescu, S.N. JAK inhibitors for the treatment of myeloproliferative neoplasms and other disorders. F1000Res 2018, 7, 82. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.A.; Odenike, O. The next generation of JAK inhibitors: An update on fedratinib, momelotonib, and pacritinib. Curr. Hematol. Malig. Rep. 2020, 15, 409–418. [Google Scholar] [CrossRef]
- Oh, Y.; Lee, J.S.; Lee, J.S.; Park, J.H.; Kim, H.S.; Yoon, S. Co-treatment of low dose pacritinib, a phase III JAK2 inhibitor, greatly increases apoptosis of P-gp over-expessing cancer cells with multidrug resistance. Anticancer Res. 2022, in press.
- Kim, J.Y.; Kim, H.S.; Yoon, S. Tyrosine kinase inhibitors imatinib and erlotinib increase apoptosis of antimitotic drug-resistant KBV20C cells without inhibiting P-gp. Anticancer Res. 2019, 39, 3785–3793. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.S.; Kim, I.S.; Yoon, S. Histamine receptor antagonists, loratadine and azelastine, sensitize P-gp-overexpressing antimitotic drug-resistant KBV20C cells through different molecular mechanisms. Anticancer Res. 2019, 39, 3767–3775. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, Y.J.; Lee, B.M.; Yoon, S. Co-treatment with HIV protease inhibitor nelfinavir creatly increases late-phase apoptosis of drug-resistant KBV20C cancer cells independently of P-glycoprotein inhibition. Anticancer Res. 2019, 39, 3757–3765. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.N.; Park, Y.S.; Kim, N.H.; Han, J.W.; Lee, H.Y.; Kim, Y.K. HDAC inhibitors downregulate MRP2 expression in multidrug resistant cancer cells: Implication for chemosensitization. Int. J. Oncol. 2011, 38, 807–812. [Google Scholar] [CrossRef]
- Jiang, C.; Zheng, T.; Park, J.H.; Lee, J.S.; Oh, Y.; Kundu, A.; Kim, H.S.; Yoon, S. Sensitization effects of pepurposed blood pressure-regulating drugs on drug-resistant cancer cells. Anticancer Res. 2021, 41, 6179–6190. [Google Scholar] [CrossRef]
- Kim, J.Y.; Son, J.Y.; Lee, B.M.; Kim, H.S.; Yoon, S. Aging-related repositioned drugs, donepezil and sildenafil citrate, increase apoptosis of anti-mitotic drug-resistant KBV20C cells through different molecular mechanisms. Anticancer Res. 2018, 38, 5149–5157. [Google Scholar] [CrossRef]
| JAK2 Inhibitors | Clinical Phase (Year) | P-gp-Inhibitory Activity (Year) |
|---|---|---|
| CEP-33779 | Mouse model (2011) | Yes (2014) |
| NVP-BSK805 | Mouse model (2020) | Yes (2017) |
| XL019 | Phase I (2014) | Yes (2017) |
| Pacritinib | Closer to FDA-approved (2021) | Yes (In press) |
| Fedratinib | FDA-approved (2019) | Yes (In this study) |
| Ruxolitinib | FDA-approved (2011) | Not determined |
| Baricitinib | FDA-approved (2018) | Not determined |
| Momelotinib | Fast track designation by FDA (2019) | No (In press) |
| Gandotinib | Phase II (2021) | Not determined |
| AZD1480 | Phase I (2015) | Not determined |
| NS-018 | Phase I/2 (2017) | Not determined |
| TG101209 | Mouse model (2013) | Not determined |
| FLLL32 | Mouse model (2010) | Not determined |
| AT9283 | Mouse model (2009) | Not determined |
| WP1066 | Mouse model (2010) | No (In press) |
| AG-490 | Mouse model (2002) | Not determined |
| AZ960 | Cell lines (2009) | Not determined |
| TG89 | Cell lines (2019) | Not determined |
| NSC 42834 (Z3) | Cell lines (2008) | Not determined |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, Y.; Lee, J.-S.; Lee, J.S.; Park, J.H.; Kim, H.S.; Yoon, S. JAK2 Inhibitor, Fedratinib, Inhibits P-gp Activity and Co-Treatment Induces Cytotoxicity in Antimitotic Drug-Treated P-gp Overexpressing Resistant KBV20C Cancer Cells. Int. J. Mol. Sci. 2022, 23, 4597. https://doi.org/10.3390/ijms23094597
Oh Y, Lee J-S, Lee JS, Park JH, Kim HS, Yoon S. JAK2 Inhibitor, Fedratinib, Inhibits P-gp Activity and Co-Treatment Induces Cytotoxicity in Antimitotic Drug-Treated P-gp Overexpressing Resistant KBV20C Cancer Cells. International Journal of Molecular Sciences. 2022; 23(9):4597. https://doi.org/10.3390/ijms23094597
Chicago/Turabian StyleOh, Yunmoon, Jin-Sol Lee, Ji Sun Lee, Jae Hyeon Park, Hyung Sik Kim, and Sungpil Yoon. 2022. "JAK2 Inhibitor, Fedratinib, Inhibits P-gp Activity and Co-Treatment Induces Cytotoxicity in Antimitotic Drug-Treated P-gp Overexpressing Resistant KBV20C Cancer Cells" International Journal of Molecular Sciences 23, no. 9: 4597. https://doi.org/10.3390/ijms23094597







