Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects
Abstract
:1. Introduction
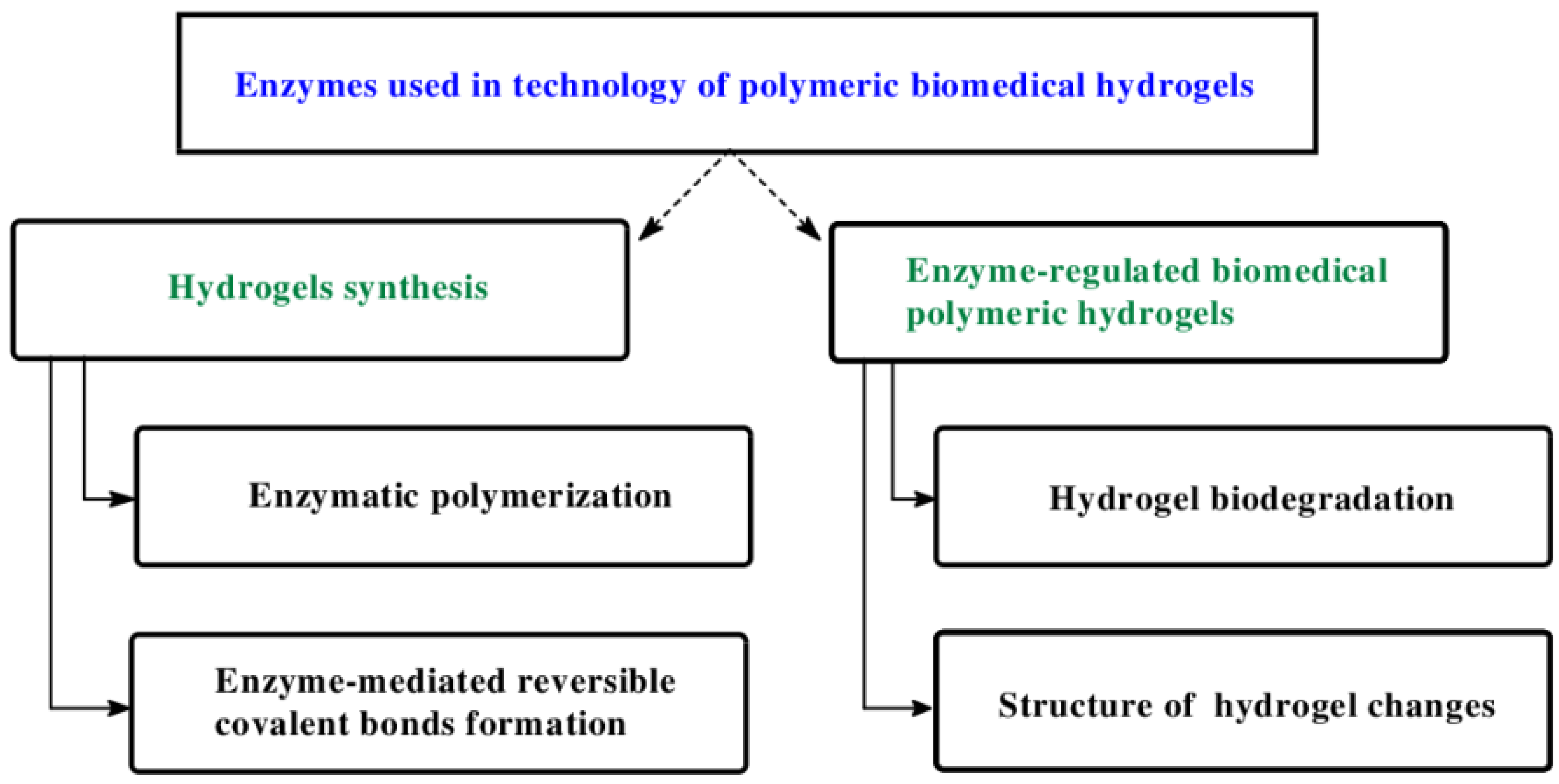
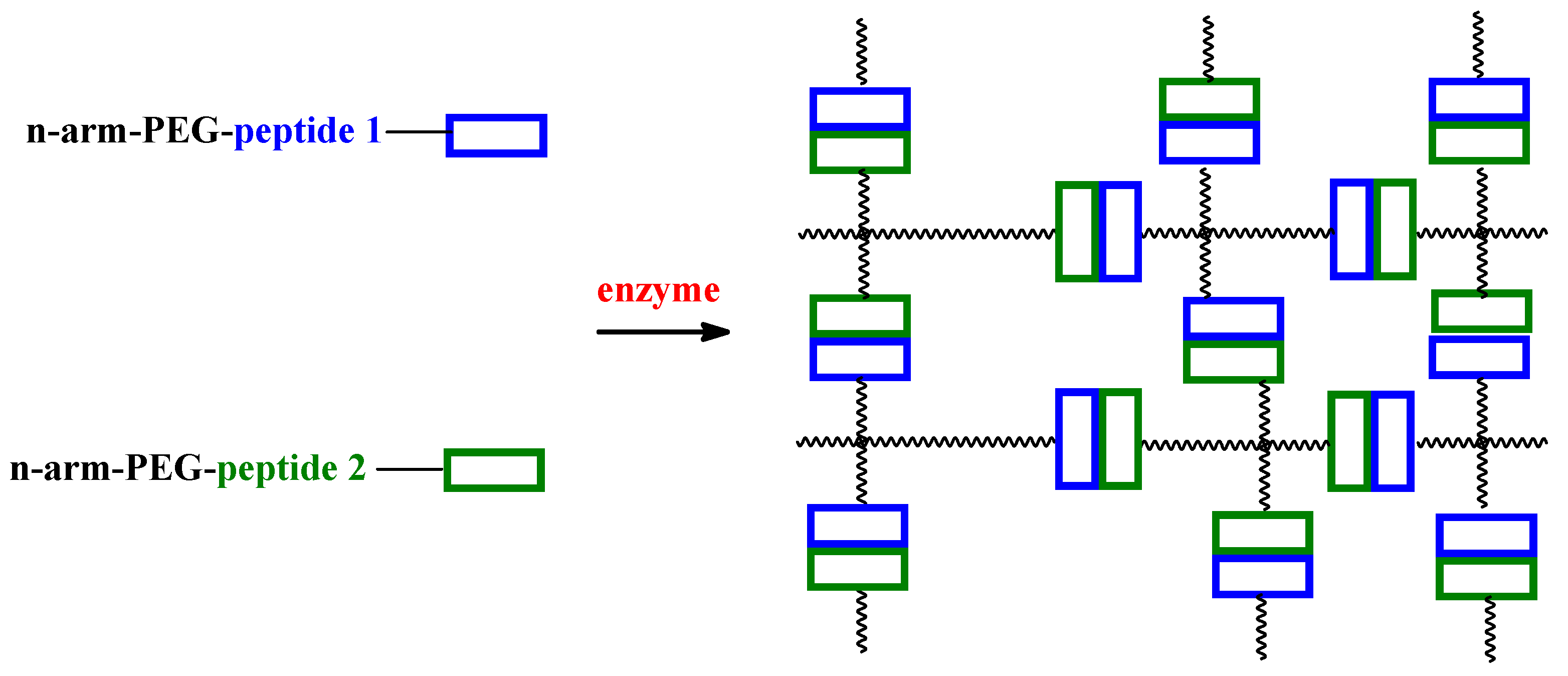
2. Enzyme Used in Biomedical Hydrogels Synthesis
3. Hydrogel Biodegradation
4. Enzyme-Responsive Hydrogels as Drug Delivery Systems
- -
- the hydrogel network must contain a chemical moiety being a substrate for the enzyme,
- -
- chemical moieties need to be accessible to enzymatic active center,
- -
- enzymatic reaction must cause significant change of hydrogel properties,
- -
4.1. Enzyme-Responsive Hydrogels as Insulin Delivery Systems
4.2. Enzyme-Responsive Hydrogels as Anticancer-Drug Delivery Systems
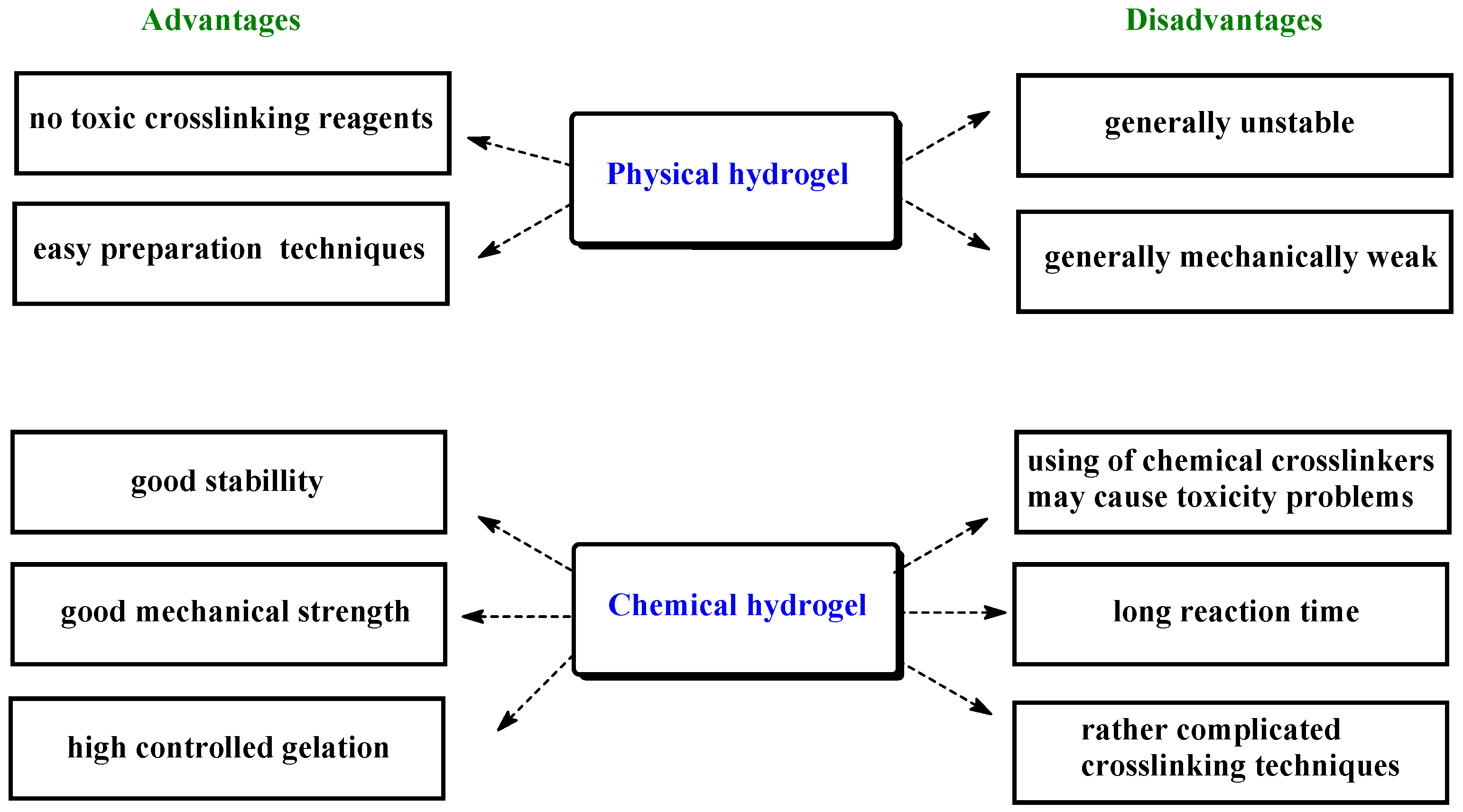
5. Conclusions, Challenges and Prospects
- -
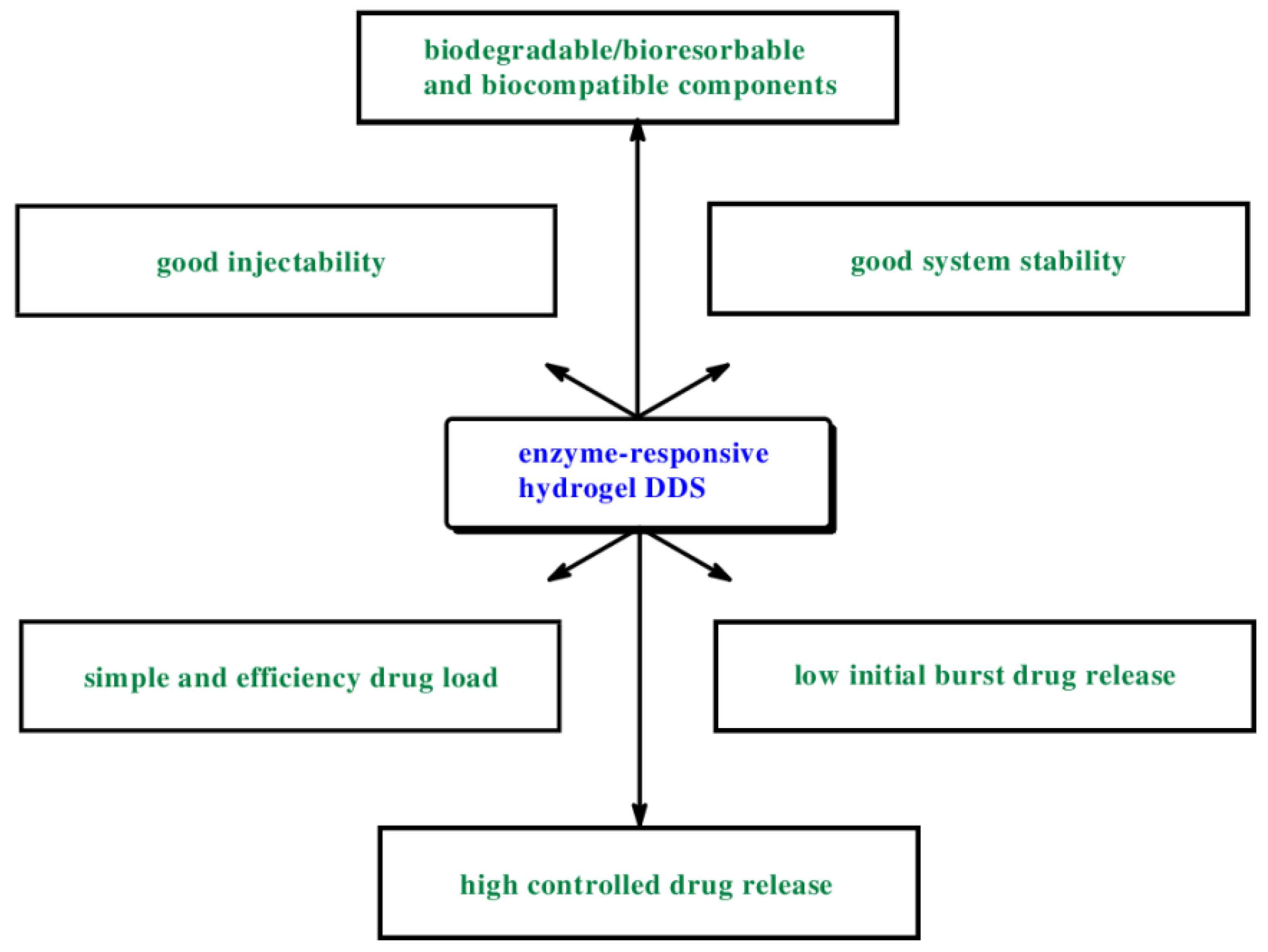
- possess a low viscosity of the hydrogel solutions to ensure a good injectability,
- -
- allow a simple and efficient drug load,
- -
- contain only biodegradable or bioresorbable and biocompatible components,
- -
- possess good system stability,
- -
- yield a low variability of drug release with a low initial burst,
- -
- characterized high controlled drug release.
- -
- sometimes lack of complete drug release control,
- -
- relatively frequent occurrence of the phenomenon of the drug burst release,
- -
- sometime a high degree of complexity of the synthesis methods,
- -
- relatively high cost of obtaining these biomaterials,
- -
- sometimes the toxicity of the matrix forming materials and solvents used.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADA—alginate dialdehyde |
| ADA-Gel—oxidized ALG, ADA and Gel hydrogel systems |
| ALG—alginate |
| Azored—azoreductases |
| CAT—catechol |
| α-Chym—α-chymotrypsin |
| DDS, DDSs—drug delivery system(s) |
| DEX—dextran |
| Dextr—dextranase |
| DOX—doxorubicin |
| EPT—hydrogel enzyme-prodrug systems |
| ERH—enzyme-responsive hydrogels |
| 5-FU—5-flurouracil |
| β-gal—β-galactosidase |
| Gel—gelatin |
| Gluc—glucose |
| Gluc acid—gluconic acid |
| GO—glucose oxidase |
| GP—glutathione peroxidase |
| HPR—horseradish peroxidase |
| HSBF—hydrogels sensitive to bioactive substances |
| Ins—insulin |
| IPN—interpenetrating polymer network |
| MMP-1—metalloprotease 1 |
| MMP2—metalloprotease 2 |
| MMP13—metalloprotease 13 |
| MMPs—metalloproteinases |
| mTG—microbial trans-glutaminase |
| NGs – nanogels |
| ox-ALG—oxidized alginate |
| PEG—poly(ethylene glycol) |
| γ-PGA—poly(γ-glutamic acid) |
| PGADA—poly(γ-glutamic acid)-dopamine |
| PHEMA—2-Hydroxyethyl methacrylate |
| PEGA—poly(ethylene glycol acrylamide) |
| Plas—plasmin |
| pNGs—peptide-crosslinked nanogels |
| pNIPAM—poly(N-isopro-pylacrylamide) |
| pNIPAM-co-PAAc—hydrogels composed of N-isopropylacrylamide (NIPAAm) and acrylic acid (AAc) with peptide cross-linkers |
| PLGA−PEG−PLGA—poly(DL-lactide-co-glycolide)-b-poly(ethylene glycol)-b-poly(DL-lactide-co-glycolide) copolymer |
| RCF—rat colonic fluid |
| SRH—stimuli-responsive hydrogels |
| TGlu—trans-glutaminase |
| Ttrans—sol-gel transition temperature |
| TRYP—trypsin |
| Tyr—tyrosinase |
| Ty-GG—tyramine-modified gellan gum |
References
- Vyas, D.; Patel, M.; Wairkar, S. Strategies for active tumor targeting-an update. Eur. J. Pharmacol. 2022, 915, 174512. [Google Scholar] [CrossRef] [PubMed]
- Mulas, K.; Stefanowicz, Z.; Oledzka, E.; Sobczak, M. Current state of the polymeric delivery systems of fluoroquinolones—A review. J. Control. Release 2019, 294, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Lim, S.I. Bioinspired tunable hydrogels: An update on methods of preparation, classification, and biomedical and therapeutic applications. Int. J. Pharmacol. 2022, 612, 121368. [Google Scholar] [CrossRef] [PubMed]
- Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart hydrogels—Synthetic stimuli-responsive antitumor drug release systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef]
- Kost, J.; Langer, R. Responsive polymeric delivery systems. Adv. Drug Deliv. Rev. 2012, 64, 327–341. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Siegel, R.A. Stimuli sensitive polymers and self-regulate drug delivery systems a very partial review. J. Control. Release 2014, 190, 337–351. [Google Scholar] [CrossRef] [Green Version]
- Ono, K.; Hashimoto, H.; Katayama, T.; Ueda, N.; Nagahama, K. Injectable biocatalytic nanocomposite hydrogel factories for focal enzyme-prodrug cancer therapy. Biomacromolecules 2021, 22, 4217–4227. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Lim, K.H.; Gonuguntla, S.; Lim, K.W.; Pranantyo, D.; Wai Pong Yong, W.P.; Yam, W.J.T.; Low, Z.; Teo, W.J.; et al. The pathway to intelligence: Using stimuli-responsive materials as building blocks for constructing smart and functional systems. Adv. Healthc. Mater. 2019, 31, 1804540. [Google Scholar] [CrossRef]
- Sharifzadeh, G.; Hosseinkhani, H. Biomolecule-responsive hydrogels in medicine. Adv. Healthc. Mater. 2017, 6, 1700801. [Google Scholar] [CrossRef]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016, 23, 748–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, W.-F.; He, Z.-D. Design and fabrication of hydrogel-based nanoparticulate systems for in vivo drug delivery. J. Control. Release 2016, 243, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, B.; Taranejoo, S.; Monemian, S.A.; Moghaddam, Z.S.; Daliri, K.; Derakhshankhah, H.; Derakhshani, Z. Classification of stimuli–responsive polymers as anticancer drug delivery systems. Drug Deliv. 2015, 22, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Miyata, T.; Uragami, T.; Nakamae, K. Biomolecule-sensitive hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 79–98. [Google Scholar] [CrossRef]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical applications of hydrogels in drug delivery system: An update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremião, M.P.D. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 49–60. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Zelzer, M.; Todd, S.J.; Hirst, A.R.; McDonald, T.O.; Ulijn, R.V. Enzyme responsive materials: Design strategies and future developments. Biomater. Sci. 2013, 1, 11. [Google Scholar] [CrossRef]
- Jin, R.; Teixeira, L.S.M.; Dijkstra, P.J.; Van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Enzymatically-crosslinked injectable hydrogels based on biomimetic dextran–hyaluronic acid conjugates for cartilage tissue engineering. Biomaterials 2010, 31, 3103–3113. [Google Scholar] [CrossRef] [PubMed]
- Garripelli, V.K.; Kim, J.K.; Son, S.; Kim, W.J.; Repka, M.A.; Jo, S. Matrix metalloproteinase-sensitive thermogelling polymer for bioresponsive local drug delivery. Acta Biomater. 2011, 7, 1984–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornton, P.D.; Heise, A. Bio-functionalisation to enzymatically control the solution properties of a self-supporting polymeric material. Chem. Commun. 2011, 47, 3108–3110. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Kawakami, K. Synthesis and characterization of both ionically and enzymatically cross-linkable alginate. Acta Biomater. 2007, 3, 495–501. [Google Scholar] [CrossRef]
- Jin, R.; Hiemstra, C.; Zhong, Z.Y.; Feijen, J. Enzyme-mediated fast in situ formation of hydrogels from dextran-tyramine conjugates. Biomaterials 2007, 28, 2791–2800. [Google Scholar] [CrossRef]
- Ogushi, Y.; Sakai, S.; Kawakami, K. Synthesis of enzymatically-gellable carboxymethylcellulose for biomedical applications. J. Biosci. Bioeng. 2007, 104, 30–33. [Google Scholar] [CrossRef]
- Sanborn, T.J.; Messersmith, P.B.; Barron, A.E. In situ crosslinking of a biomimetic peptide-PEG hydrogel via thermally triggered activation of factor XIII. Biomaterials 2002, 23, 2703–2710. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, J.N.; Lee, J.; Lee, H.; Lee, H.; Park, W.H. Enzymatically cross-linked poly (γ-glutamic acid) hydrogel with enhanced tissue adhesive property. ACS Biomater. Sci. Eng. 2020, 6, 3103–311311. [Google Scholar] [CrossRef]
- Distler, T.; McDonald, K.; Heid, S.; Karakaya, E.; Detsch, R.; Boccaccini, A.R. Ionically and enzymatically dual cross-linked oxidized alginate gelatin hydrogels with tunable stiffness and degradation behavior for tissue engineering. ACS Biomater. Sci. Eng. 2020, 6, 3899–3914. [Google Scholar] [CrossRef]
- Fuchsbauer, H.L.; Gerber, U.; Engelmann, U.J.; Seeger, T.; Sinks, C.; Hecht, T. Influence of gelatin matrices cross-linked with transglutaminase on the properties of an enclosed bioactive material using β-galactosidase as model system. Biomaterials 1996, 17, 1481–1488. [Google Scholar] [CrossRef]
- Ehrbar, M.; Rizzi, S.C.; Schoenmakers, R.G.; San Miguel, B.; Hubbell, J.A.; Weber, F.E.; Lutolf, M.P. Biomolecular hydrogels formed and degraded via site-specific enzymatic reactions. Biomacromolecules 2007, 8, 3000–3007. [Google Scholar] [CrossRef] [PubMed]
- Sperinde, J.J.; Griffith, L.G. Synthesis and characterization of enzymatically-cross-linked poly (ethylene glycol) hydrogels. Macromolecules 1997, 30, 5255–5264. [Google Scholar] [CrossRef]
- Chen, T.H.; Embree, H.D.; Wu, L.Q.; Payne, G.F. In vitro protein-polysaccharide conjugation: Tyrosinase-catalyzed conjugation of gelatin and chitosan. Biopolymers 2002, 64, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Embree, H.D.; Brown, E.M.; Taylor, M.M.; Payne, G.F. Enzyme-catalyzed gel formation of gelatin and chitosan: Potential for in situ applications. Biomaterials 2003, 24, 2831–2841. [Google Scholar] [CrossRef]
- Kurisawa, M.; Chung, J.E.; Yang, Y.Y.; Gao, S.J.; Uyama, H. Injectable biodegradable hydrogels composed of hyaluronic acid–tyramine conjugates for drug delivery and tissue engineering. Chem. Comm. 2005, 34, 4312–4314. [Google Scholar] [CrossRef]
- Oliveira, I.M.; Gonçalves, C.; Shin, M.E.; Lee, S.; Reis, R.L.; Khang, G.; Oliveira, J.M. Enzymatically crosslinked tyramine-gellan gum hydrogels as drug delivery system for rheumatoid arthritis treatment. Drug Deliv. Transl. Res. 2021, 11, 1288–1300. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Baptista-Silva, S.; Borges, S.; Costa-Pinto, A.R.; Costa, R.; Amorim, M.; Dias, J.R.; Ramos, O.; Alves, P.; Granja, P.L.; et al. In situ forming silk sericin-based hydrogel: A novel wound healing biomaterial. ACS Biomater. Sci. Eng. 2021, 7, 1573–158612. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kurisawa, M.; Yui, N. Double-stimuli-responsive degradable hydrogels: Interpenetrating polymer networks consisting of gelatin and dextran with different phase separation. Macromol. Rapid Com. 1996, 17, 313–318. [Google Scholar] [CrossRef]
- Kurisawa, M.; Terano, M.; Yui, N. Double-stimuli-responsive degradation of hydrogels consisting of oligopeptide-terminated poly (ethylene glycol) and dextran with an interpenetrating polymer network. J. Biomater. Sci. Polym. Ed. 1997, 8, 691–708. [Google Scholar] [CrossRef]
- Hovgaard, L.; Brondsted, H. Dextran hydrogels for colon-specific drug delivery. J. Control. Release 1995, 36, 159–166. [Google Scholar] [CrossRef]
- Plunkett, K.N.; Berkowski, K.L.; Moore, J.S. Chymotrypsin responsive hydrogel: Application of a disulfide exchange protocol for the preparation of methacrylamide containing peptides. Biomacromolecules 2005, 6, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Mann, B.K.; Gobin, A.S.; Tsai, A.T.; Schmedlen, R.H.; West, J.L. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: Synthetic ECM analogs for tissue engineering. Biomaterials 2001, 22, 3045–3051. [Google Scholar] [CrossRef]
- Kumashiro, Y.; Lee, T.K.; Ooya, T.; Yui, N. Enzymatic degradation of semi-IPN hydrogels based on n-isopropylacrylamide and dextran at a specific temperature range. Macromol. Rapid Com. 2002, 23, 407–410. [Google Scholar] [CrossRef]
- West, J.L.; Hubbell, J.A. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules 1999, 32, 241–244. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthesis and physicochemical characterization of end-linked poly (ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules 2003, 4, 713–722. [Google Scholar] [CrossRef]
- Kim, S.; Healy, K.E. Synthesis and characterization of injectable poly (N-isopropylacrylamide-co-acrylic acid) hydrogels with proteolytically degradable cross-links. Biomacromolecules 2003, 4, 1214–1223. [Google Scholar] [CrossRef]
- Yang, J.Y.; Jacobsen, M.T.; Pan, H.Z.; Kopecek, J. Synthesis and characterization of enzymatically degradable peg-based peptide-containing hydrogels. Macromol. Biosci. 2010, 10, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Kurisawa, M.; Matsuo, Y.; Yui, N. Modulated degradation of hydrogels with thermo-responsive network in relation to their swelling behawior. Macromol. Chem. Phys. 1998, 199, 705–709. [Google Scholar] [CrossRef]
- Van Dijk, M.; Van Nostrum, C.F.; Hennink, W.E.; Rijkers, D.T.S.; Liskamp, R.M.J. Synthesis and characterization of enzymatically biodegradable peg and peptide-based hydrogels prepared by click chemistry. Biomacromolecules 2010, 11, 1608–1614. [Google Scholar] [CrossRef]
- Khelfallah, N.S.; Decher, G.; Mesini, P.J. Synthesis of a new PHEMA/PEO enzymatically biodegradable hydrogel. Macromol. Rapid Com. 2006, 27, 1004–1008. [Google Scholar] [CrossRef]
- Szilágyi, B.Á.; Némethy, Á.; Magyar, A.; Szabó, I.; Bősze, S.; Gyarmati, B.; Szilágyi, A. Amino acid based hydrogels with enzymatically degradable cross-links. React. Funct. Polym. 2018, 133, 21–28. [Google Scholar] [CrossRef]
- Thornton, P.D.; Mart, R.J.; Webb, S.J.; Ulijn, R.V. Enzyme-responsive hydrogel particles for the controlled release of proteins: Designing peptide actuators to match payload. Soft Matter. 2008, 4, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Patrick, A.G.; Ulijn, R.V. Hydrogels for the detection and management of protease levels. Macromol. Biosci. 2010, 10, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Perlin, L.; MacNeil, S.; Rimmer, S. Cell adhesive hydrogels synthesised by copolymerisation of Arg-protected Gly-Arg-Gly-Asp-Ser methacrylate monomers and enzymatic deprotection. Chem. Comm. 2008, 45, 5951–5953. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.O.; Qu, H.L.; Saunders, B.R.; Ulijn, R.V. Branched peptide actuators for enzyme responsive hydrogel particles. Soft Matter. 2009, 5, 1728–1734. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Tirelli, N.; Cerritelli, S.; Cavalli, L.; Hubbell, J.A. Systematic modulation of Michael-type reactivity of thiols through the use of charged amino acids. Bioconjug. Chem. 2001, 12, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Klinger, D.; Aschenbrenner, E.M.; Weiss, C.K.; Landfester, K. Enzymatically degradable nanogels by inverse miniemulsion copolymerization of acrylamide with dextran methacrylates as crosslinkers. Polym. Chem. 2012, 3, 204–216. [Google Scholar] [CrossRef]
- Chandrawati, R. Enzyme-responsive polymer hydrogels for therapeutic delivery. Exp. Biol. Med. 2016, 241, 972–979. [Google Scholar] [CrossRef]
- Wang, Y.; Byrne, J.D.; Napier, M.E.; DeSimone, J.M. Engineering nanomedicines using stimuli-responsive biomaterials. Adv. Drug Deliv. Rev. 2012, 64, 1021–1030. [Google Scholar] [CrossRef] [Green Version]
- Webber, M.J.; Anderson, D.G. Smart approaches to glucose-responsive drug delivery. J. Drug Target. 2015, 23, 651–655. [Google Scholar] [CrossRef] [Green Version]
- Najmeddine, A.A.; Saeed, M.; Beadham, I.G.; ElShaer, A. Efficacy and safety of glucose sensors for delivery of insulin: A systematic review. PharmaNutrition 2021, 18, 100280. [Google Scholar] [CrossRef]
- Cartier, S.; Horbett, T.A.; Ratner, B.D. Glucose-sensitive commembrane coated porous filters for control of hydraulic permeability and insulin delivery from a pressurized reservoir. J. Membr. Sci. 1995, 106, 17–24. [Google Scholar] [CrossRef]
- Parker, R.S.; Doyle, F.J.; Peppas, N.A. A model-based algorithm for blood glucose control in type I diabetic patients. IEEE Trans. Biomed. 1999, 46, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Aimetti, A.A.; Wang, Q.; Dang, T.T.; Zhang, Y.; Veiseh, O.; Cheng, H.; Langer, R.S.; Anderson, D.G. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano 2013, 7, 4194–4201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, C.-C.; Du, F.-S.; Li, Z.-C. Supersensitive oxidation-responsive biodegradable PEG hydrogels for glucose-triggered insulin delivery. ACS Appl. Mater. Interfaces 2017, 9, 25905–25914. [Google Scholar] [CrossRef] [PubMed]
- Podual, K.; Doyle, F.J.; Peppas, N.A. Glucose-sensitivity of glucose oxidase-containing cationic copolymer hydrogels having poly (ethylene glycol) grafts. J. Control. Release 2000, 67, 9–17. [Google Scholar] [CrossRef]
- Kang, S.I.; Bae, Y.H. A sulfonamide based glucose-responsive hydrogel with covalently immobilized glucose oxidase and catalase. J. Control. Release 2003, 86, 115–121. [Google Scholar] [CrossRef]
- Ishihara, K.; Kobayashi, M.; Ishimaru, N.; Shinohara, I. Glucose induced permeation control of insulin through a complex membrane consisting of immobilized glucose oxidase and a poly (amine). Polym. J. 1984, 16, 625–631. [Google Scholar] [CrossRef] [Green Version]
- Nazli, C.; Demirer, G.S.; Yar, Y.; Acar, H.Y.; Kizilel, S. Targeted delivery of doxorubicin into tumor cells via MMP-sensitive PEG hydrogel-coated magnetic iron oxide nanoparticles (MIONPs). Colloids Surf. B. 2014, 122, 674–683. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, B.; Puperi, D.S.; Yonezawa, A.L.; Wu, Y.; Tseng, H.; Cuchiara, M.L.; West, J.L.; Grande-Allen, K.J. Integrating valve-inspired design features into poly (ethylene glycol) hydrogel scaffolds for heart valve tissue engineering. Acta Biomat. 2015, 14, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Nagel, G.; Sousa-Herves, A.; Wedepohl, S.; Calderón, M. Matrix metalloproteinase-sensitive multistage nanogels promote drug transport in 3d tumor model. Theranostics 2020, 10, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Duro-Castano, A.; Sousa-Herves, A.; Arminan, A.; Charbonnier, D.; Arroyo-Crespo, J.J.; Wedepohl, S.; Calderon, M.; Vicent, M.J. Polyglutamic acid-based crosslinked doxorubicin nanogels as an anti-metastatic treatment for triple negative breast cancer. J. Control. Release 2021, 332, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Palmese, L.L.; Fan, M.; Scott, R.A.; Tan, H.; Kiick, K.L. Multi-stimuli-responsive, liposome-crosslinked poly (ethylene glycol) hydrogels for drug delivery. J. Biomater. Sci. Polym. Ed. 2020, 32, 635–656. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Behl, G.; Kaur, S.; Yadav, N.; Liu, B.; Chhikara, A. Tumor microenvironment responsive nanogels as a smart triggered release platform for enhanced intracellular delivery of doxorubicin. J. Biomater. Sci. Polym. Ed. 2021, 32, 385–404. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, R.; Wang, X.; Gao, J.; Zheng, Y.; Sun, Z. Enzyme and Ph responsive 5-flurouracil (5-FU) loaded hydrogels based on olsalazine derivatives for colon-specific drug delivery. Eur. Polym. J. 2019, 118, 64–70. [Google Scholar] [CrossRef]
- Zhao, Z.; Shen, J.; Zhang, L.; Wang, L.; Xu, H.; Han, Y.; Jia, J.; Lu, Y.; Yu, R.; Liu, H. Injectable postoperative enzyme-responsive hydrogels for reversing temozolomide resistance and reducing local recurrence after glioma operation. Biomater. Sci. 2020, 8, 5306–5316. [Google Scholar] [CrossRef]
- Roy, R.; Yang, J.; Moses, M.A. Matrix metalloproteinases as novel biomarker s and potential therapeutic targets in human cancer. J. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef] [Green Version]
- Vartak, D.G.; Gemeinhart, R.A. Matrix metalloproteases: Underutilized targets for drug delivery. J. Drug Target. 2007, 15, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Nultsch, K.; Germershaus, O. Matrix metalloprotease triggered bioresponsive drug delivery systems—Design, synthesis and application. Eur. J. Pharm. Biopharmacol. 2018, 131, 189–202. [Google Scholar] [CrossRef]
- Yeh, P.Y.; Kopeckova, P.; Kopecek, J. Biodegradable and pH-sensitive hydrogels: Synthesis by crosslinking of N, N dimethylacrylamide copolymer precursors. J. Polym. Sci. A Polym. Chem. 1994, 32, 1627–1637. [Google Scholar] [CrossRef]
- Yeh, P.Y.; Kopeckova, P.; Kopecek, J. Degradability of hydrogels containing azoaromatic crosslinks. Macromol. Chem. Phys. 1995, 196, 2183–2202. [Google Scholar] [CrossRef]
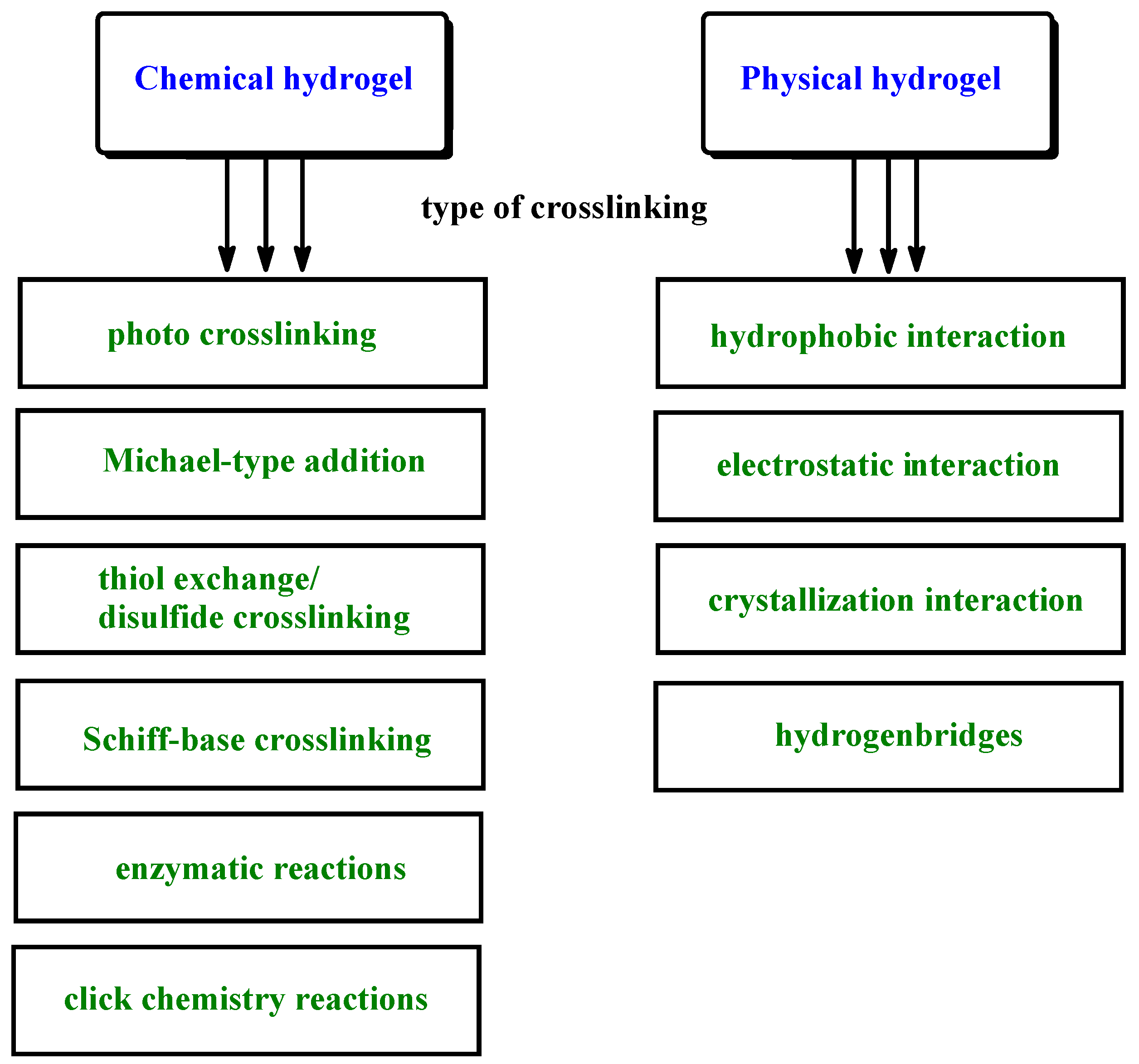
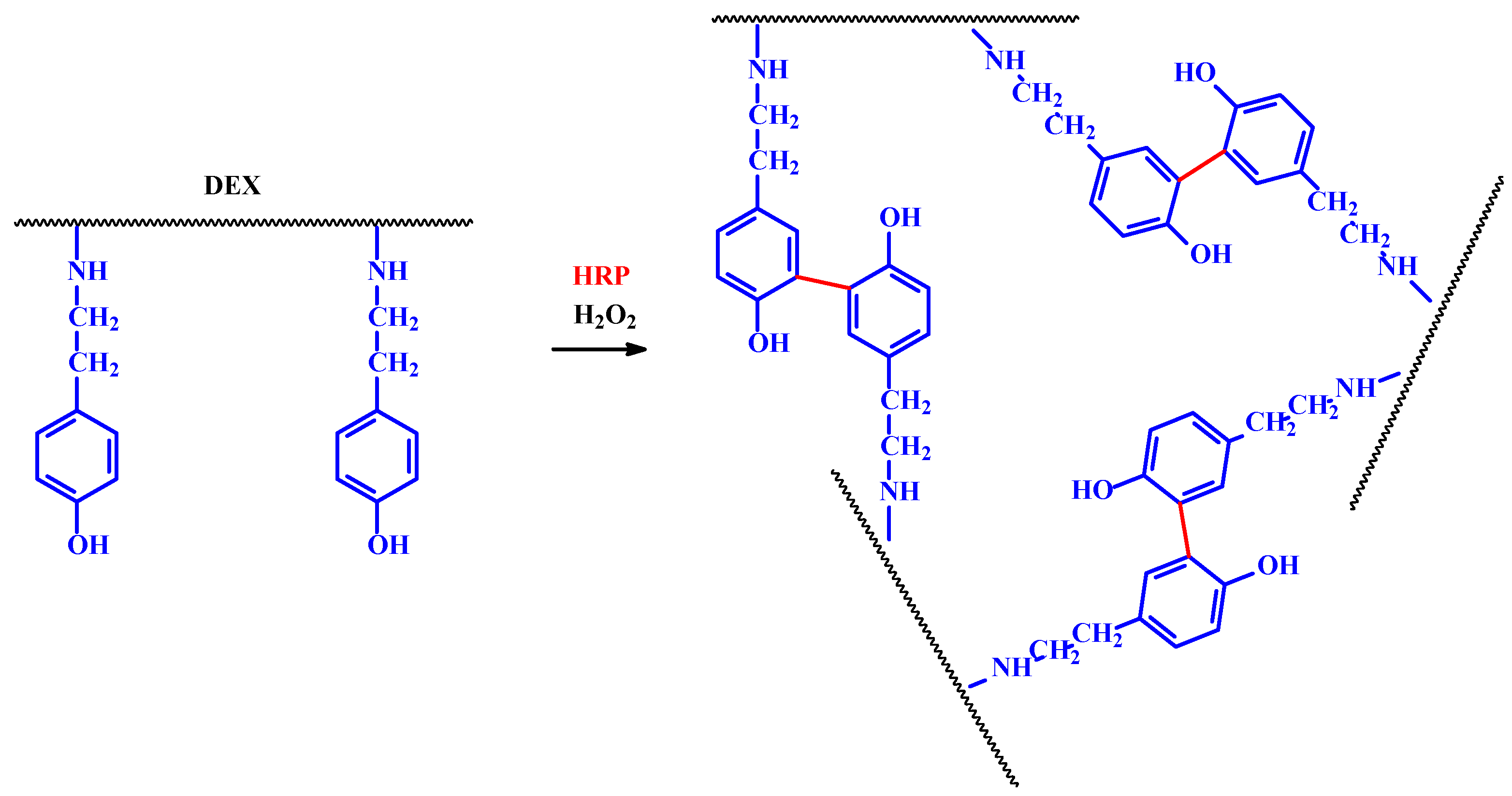

| Type of Polymeric Hydrogel | Enzymes | Refs |
|---|---|---|
| poly(allylamine) with acetyl protected dialanine | Elastase | [23] |
| hyaluronic acid/tyramine conjugate | HRP | [24] |
| DEX-tyramine linked by a urethane bond | HRP | [25] |
| DEX-tyramine linked by ester-containing diglycolic group | HRP | [25] |
| carboxymethylcellulose with phenol moieties by covalently incorporating tyramine | HRP | [26] |
| ALG with phenol moieties/tyramine | HRP | [24] |
| four-armed PEG terminated by 20-mer peptide | HRP | [27] |
| PGADA | HRP | [28] |
| marine derived oxidized ox-ALG, ADA, and Gel system/cross-linked Ca2+ and mTG | mTG | [29] |
| Gel | TGlu | [30] |
| multi-arm PEG | TGlu | [31] |
| cross-linked PEG | Tyr | [32] |
| Gel/chitosan conjugates | Tyr | [33,34] |
| Type of Hydrogel | Effect of Action | Enzymes | Refs |
|---|---|---|---|
| Gel and DEX | Degradation of the polymer | α-Chym and Dextr | [38] |
| DEX cross-linked with diisocyanate | Degradation of the polymer | Dextr | [38,39,40] |
| Hydrogels consisting oligopeptide-terminated PEG and DEX | Degradation of the polymer | Dextr and Papain | [39] |
| poly(acrylamide) | Degradation by cleavage of cross-links | α-Chym | [41] |
| PEG | Degradation by cleavage of cross-links | Collagenase | [42] |
| pNIPAM grafted on DEX and a pNIPAM–N,N-dimethylacrylamide copolymer | Degradation by cleavage of cross-links | Dextr | [43] |
| PEG | Degradation by cleavage of cross-links | Elastase | [42] |
| PEG-oligopeptide-PEG telechelic block copolymers | Degradation by cleavage of cross-links | MMP-1 | [44] |
| Multiarm-PEG | Degradation by cleavage of cross-links | MMP-1 | [31] |
| multiarm vinyl sulfone-terminated PEG macromers and alpha-omega cysteine oligopeptides | Degradation by cleavage of cross-links | MMP-1 | [45] |
| Pluronic and octapeptide multiblock copolymer | Degradation by cleavage of cross-links | MMP-2 | [18] |
| pNIPAM-co-PAAc | Degradation by cleavage of cross-links | MMP-13 | [46] |
| 4-arm azido-terminated PEG and [alkyne]-GFLGK-[alkyne] or ([alkyne]-GFLG)2K peptide | Degradation by cleavage of cross-links | Papain | [47] |
| PEG | Degradation of the polymer | Papain | [39,48] |
| pNIPAM grafted on DEX and a pNIPAM–N,N-dimethylacrylamide copolymer | Degradation of the polymer | Papain | [39,48] |
| PEG | Degradation by cleavage of cross-links | Plas | [44] |
| PEG | Degradation by cleavage of cross-links | Plas | [49] |
| PHEMA/PEO and Gly-Gly-Leu tripeptyde | Degradation by cleavage of cross-links | Subtilisin | [50] |
| PEG | Degradation by cleavage of cross-links | TRYP | [49] |
| natural amino acid/aspartic acid copolymers cross-linked by tetrapeptide | Degradation by cleavage of cross-links | TRYP | [51] |
| PEGA | Morphology control | Dextr | [52] |
| PEGA | Morphology control | Elastase | [53] |
| Gly-Arg-Gly-Asp-Ser functionalised hydrogels | Morphology control | Glutathione-S- transferase | [54] |
| PEGA | Morphology control | MMP-1/12 | [53] |
| PEGA | Morphology control | Thermolysin | [52] |
| PEGA | Morphology control | Thermolysin | [55] |
| PEGA | Morphology control | TRYP | [52] |
| Drug | Type of Hydrogel | Enzymes | The Main Conclusions | Refs |
|---|---|---|---|---|
| Ins | N,N-diethylaminoethyl methacrylate and 2-hydroxypropyl methacrylate cross-linked with a polyacrylamide membrane | GO | The low pH of the membrane caused ionization of the amino groups present in the DDS, which led to the swelling of the HSBF and the membrane permeability to insulin increased. | [62,63] |
| Ins | DEX/chitosan | GO | In vitro insulin release can be modulated in a pulsatile profile in response to Gluc concentrations. In vivo studies validated that these formulations provided improved Gluc control in type 1 diabetic mice subcutaneously administered with a degradable nano-network. A single injection of DDS facilitated stabilization of the blood Gluc levels in the normoglycemic state for up to 10 days. | [64] |
| Ins | 4-arm-PEG acrylic macromonomer | GO | The kinetics of hydrogel degradation and insulin release could be finely manipulated by tuning the concentration of H2O2 or changing the GO content and Gluc concentration. Of importance, an extremely low GO content was sufficient to afford a moderate insulin release at the hyperglycemic level, which would be beneficial for potential in vivo application. | [65] |
| Ins | poly(diethylaminoethyl-g-ethylene glycol) | GO | HSBF showed pulsatile reversible volume change when Gluc concentration varied between 0 and 2 g/L | [66] |
| Ins | poly(sulfadimethoxine) | GO | In Gluc concentration range of 0–300 mg/dl the ERH showed reversible sugar dependent swelling without hysteresis. | [67] |
| DOX | PEG-coated magnetic iron oxide nanoparticles | MMP | ERH were taken into cancer cells 11 times more efficiently than uncoated ones. These targeted nanocarriers were efficiently delivered and released DOX into the nuclei of HeLa cells within 2 h. | [69] |
| DOX | acrylate-PEG-PQ–PEG-acrylate conjugates, PEG-diacrylate and acrylate-PEG-RGDS | MMP | DOX loading efficacy was more than 97%. Drug in vitro release from obtained DDS was 60% and 36% after 4 days. | [70] |
| DOX | peptide-crosslinked nanogels (pNGs) -based on a dendritic polyglycerol | MMP | Stable conjugation of DOX at physiological pH and controlled drug release from pNGs were observed. | [71] |
| DOX | injectable polyamino acid-based nanogels (NGs) | Cathepsin B | DDSs were characterized with ~100 nm in size and 25 wt% drug loading. They content that became rapidly internalized in TNBC cell lines and displayed IC50 values comparable to the free form of DOX. NGs significantly inhibited lung metastases (in mouse model). | [72] |
| DOX | poly(ethylene glycol) hydrogel crosslinked via thiol-maleimide reactions | MMP | The hydrogel responded to both thermal and enzymatic stimuli in a local environment. DOX was loaded in the DDS with a high encapsulation efficiency. | [73] |
| DOX | disulfide cross-linked copolymer of 2-(dimethyl amino) ethyl methacrylate and PEG | GP | A relatively higher release of DOX was observed from the nanogels at pH 5.0 than at pH 7.4. DOX release was further accelerated in tumor simulated environment of pH 5.0 and GP. | [74] |
| 5-FU | product polymerization of olsalazine-AC/ HEMA/ MAA | Enzymes contain in the rat colonic fluid | 5-FU is locally released in colon part and the local high concentration of 5-FU induces necroptosis of colon cancer cells and circumvents cancer drug resistance. | [75] |
| 5-FU | PLGA−PEG−PLGA | β-gal | A single local injection of SRH and a prodrug 5-FU-β-gal provided long-lasting antitumor activity in vivo without observable side effects. | [8] |
| Temozolomide | Triglycerol monostearate | MMP | Hydrogels effectively reduced the recurrence of temozolomide -resistant glioma after surgery and significantly enhanced the efficiency of temozolomide to inhibit glioma growth. | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobczak, M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects. Int. J. Mol. Sci. 2022, 23, 4421. https://doi.org/10.3390/ijms23084421
Sobczak M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects. International Journal of Molecular Sciences. 2022; 23(8):4421. https://doi.org/10.3390/ijms23084421
Chicago/Turabian StyleSobczak, Marcin. 2022. "Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects" International Journal of Molecular Sciences 23, no. 8: 4421. https://doi.org/10.3390/ijms23084421






