Genetic Architecture of Grain Yield-Related Traits in Sorghum and Maize
Abstract
:1. Introduction
2. Increased Grain Yield through Crop Domestication
3. Genetic Dissection of Grain Yield-Related Traits in Sorghum and Maize
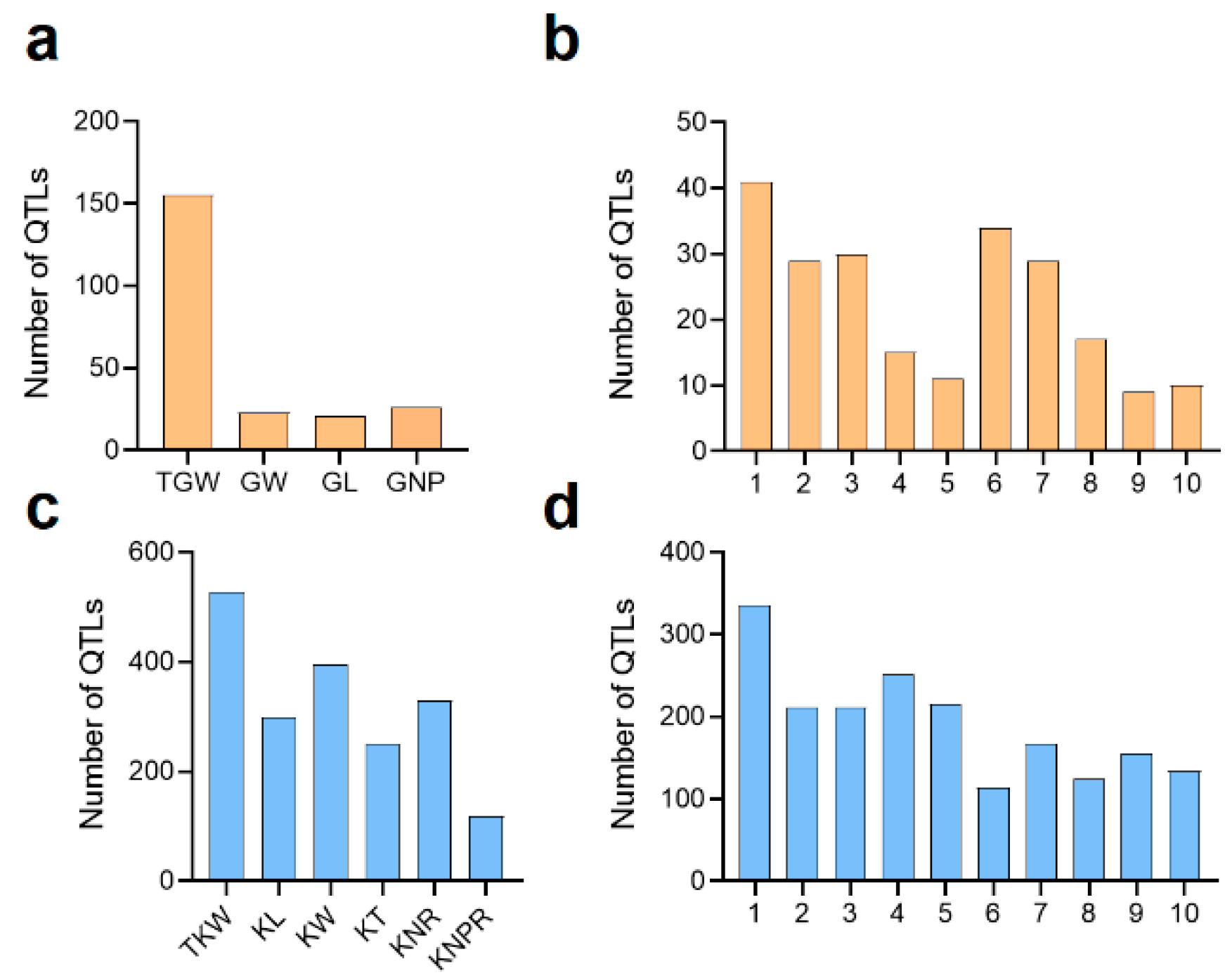
3.1. Sorghum
3.2. Maize
4. Functionally Characterized Genes Associated with Grain Yield-Related Traits in Sorghum and Maize
5. Conclusions and Prospective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Charles, H.G.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security, The Challenge of Feeding 9 Billion People. Science 2010, 327, 812. [Google Scholar]
- Schütz, H.J.M.; Verhoff, M. A How to Feed the World in 2050. Arch. Kriminol. 2011, 228, 151–159. [Google Scholar] [PubMed]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herridge, R.P.; Day, R.C.; Baldwin, S.; Macknight, R.C. Rapid analysis of seed size in Arabidopsis for mutant and QTL discovery. Plant Methods 2011, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.P.; Ramu, P.; Deshpande, S.P.; Hash, C.T.; Shah, T.; Upadhyaya, H.D.; Riera-Lizarazu, O.; Brown, P.J.; Acharya, C.B.; Mitchell, S.E.; et al. Population genomic and genome-wide association studies of agroclimatic traits in sorghum. Proc. Natl. Acad. Sci. USA 2013, 110, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Paterson, A.H. Genomics of sorghum. Int. J. Plant Genom. 2008, 2008, 362451. [Google Scholar] [CrossRef] [Green Version]
- Ranum, P.; Pena-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef]
- Paterson, A.H.; Bowers, J.E.; Bruggmann, R.; Dubchak, I.; Grimwood, J.; Gundlach, H.; Haberer, G.; Hellsten, U.; Mitros, T.; Poliakov, A.; et al. The Sorghum bicolor genome and the diversification of grasses. Nature 2009, 457, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield Trends Are Insufficient to Double Global Crop Production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [Green Version]
- Swigonova, Z.; Lai, J.; Ma, J.; Ramakrishna, W.; Llaca, V.; Bennetzen, J.L.; Messing, J. Close split of sorghum and maize genome progenitors. Genome Res. 2004, 14, 1916–1923. [Google Scholar] [CrossRef] [Green Version]
- Messmer, R.; Fracheboud, Y.; Banziger, M.; Vargas, M.; Stamp, P.; Ribaut, J.M. Drought stress and tropical maize: QTL-by-environment interactions and stability of QTLs across environments for yield components and secondary traits. Theor. Appl. Genet. 2009, 119, 913–930. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Li, Y.; Sun, B.; Peng, B.; Liu, C.; Liu, Z.; Yang, Z.; Li, Q.; Tan, W.; Zhang, Y.; et al. Quantitative trait loci mapping for yield components and kernel-related traits in multiple connected RIL populations in maize. Euphytica 2013, 193, 303–316. [Google Scholar] [CrossRef]
- Han, L.; Chen, J.; Mace, E.S.; Liu, Y.; Zhu, M.; Yuyama, N.; Jordan, D.R.; Cai, H. Fine mapping of qGW1, a major QTL for grain weight in sorghum. Theor. Appl. Genet. 2015, 128, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, Q.; Dong, L.; Wang, H.; Liu, F.; Weng, J.; Li, X.; Xie, C. Genetic architecture of the maize kernel row number revealed by combining QTL mapping using a high-density genetic map and bulked segregant RNA sequencing. BMC Genom. 2016, 17, 915. [Google Scholar] [CrossRef] [Green Version]
- Boyles, R.E.; Cooper, E.A.; Myers, M.T.; Brenton, Z.; Rauh, B.L.; Morris, G.P.; Kresovich, S. Genome-Wide Association Studies of Grain Yield Components in Diverse Sorghum Germplasm. Plant Genome 2016, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Lee, Y.K.; Gladman, N.; Chopra, R.; Christensen, S.A.; Regulski, M.; Burow, G.; Hayes, C.; Burke, J.; Ware, D.; et al. MSD1 regulates pedicellate spikelet fertility in sorghum through the jasmonic acid pathway. Nat. Commun. 2018, 9, 822. [Google Scholar] [CrossRef] [PubMed]
- Dampanaboina, L.; Jiao, Y.; Chen, J.; Gladman, N.; Chopra, R.; Burow, G.; Hayes, C.; Christensen, S.A.; Burke, J.; Ware, D.; et al. Sorghum MSD3 Encodes an omega-3 Fatty Acid Desaturase that Increases Grain Number by Reducing Jasmonic Acid Levels. Int. J. Mol. Sci. 2019, 20, 5359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladman, N.; Jiao, Y.; Lee, Y.K.; Zhang, L.; Chopra, R.; Regulski, M.; Burow, G.; Hayes, C.; Christensen, S.A.; Dampanaboina, L.; et al. Fertility of Pedicellate Spikelets in Sorghum Is Controlled by a Jasmonic Acid Regulatory Module. Int. J. Mol. Sci. 2019, 20, 4951. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; van Oosterom, E.J.; Jordan, D.R.; Hammer, G.L. Pre-anthesis ovary development determines genotypic differences in potential kernel weight in sorghum. J. Exp. Bot. 2009, 60, 1399–1408. [Google Scholar] [CrossRef]
- Silva Lda, C.; Wang, S.; Zeng, Z.B. Composite interval mapping and multiple interval mapping: Procedures and guidelines for using Windows QTL Cartographer. Methods Mol. Biol. 2012, 871, 75–119. [Google Scholar] [CrossRef]
- Mace, E.; Innes, D.; Hunt, C.; Wang, X.; Tao, Y.; Baxter, J.; Hassall, M.; Hathorn, A.; Jordan, D. The Sorghum QTL Atlas: A powerful tool for trait dissection, comparative genomics and crop improvement. Theor. Appl. Genet. 2019, 132, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Schaid, D.J.; Chen, W.; Larson, N.B. From genome-wide associations to candidate causal variants by statistical fine-mapping. Nat. Rev. Genet. 2018, 19, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Mace, E.S.; Jordan, D.R. Integrating sorghum whole genome sequence information with a compendium of sorghum QTL studies reveals uneven distribution of QTL and of gene-rich regions with significant implications for crop improvement. Theor. Appl. Genet. 2011, 123, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Hufford, M.B.; Lubinksy, P.; Pyhajarvi, T.; Devengenzo, M.T.; Ellstrand, N.C.; Ross-Ibarra, J. The genomic signature of crop-wild introgression in maize. PLoS Genet. 2013, 9, e1003477. [Google Scholar] [CrossRef]
- Wendorf, F.; Close, A.E.; Schild, R.; Wasylikowa, K.; Housley, R.A.; Harlan, J.R.; Królik, H. Saharan exploitation of plants 8000 years BP. Nature 1992, 359, 721–724. [Google Scholar] [CrossRef]
- van Heerwaarden, J.; Doebley, J.; Briggs, W.H.; Glaubitz, J.C.; Goodman, M.M.; de Jesus Sanchez Gonzalez, J.; Ross-Ibarra, J. Genetic signals of origin, spread, and introgression in a large sample of maize landraces. Proc. Natl. Acad. Sci. USA 2011, 108, 1088–1092. [Google Scholar] [CrossRef] [Green Version]
- Harlan, J.R.; De Wet, J.M.J.; Stemler, A. (Eds.) Origins of African Plant Domestication; De Gruyter Mouton: The Hague, Paris, 1976. [Google Scholar] [CrossRef]
- Matsuoka, Y.V.Y.; Goodman, M.M.; Sanchez, J.G.; Buckler, E.; Doebley, J. A single domestication for maize shown. Proc. Natl. Acad. Sci. USA 2002, 99, 6080–6084. [Google Scholar] [CrossRef] [Green Version]
- Zou, C.; Li, L.; Miki, D.; Li, D.; Tang, Q.; Xiao, L.; Rajput, S.; Deng, P.; Peng, L.; Jia, W.; et al. The genome of broomcorn millet. Nat. Commun. 2019, 10, 436. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.H.; Messing, J. Amplification of prolamin storage protein genes in different subfamilies of the Poaceae. Theor. Appl. Genet. 2009, 119, 1397–1412. [Google Scholar] [CrossRef]
- Allaby, R.G.; Fuller, D.Q.; Brown, T.A. The genetic expectations of a protracted model for the origins of domesticated crops. Proc. Natl. Acad. Sci. USA 2008, 105, 13982–13986. [Google Scholar] [CrossRef] [Green Version]
- Susko, D.J.; Lovett-Doust, L. Patterns of seed mass variation and their effects on seedling traits in Alliaria petiolata (Brassicaceae). Am. J. Bot. 2000, 87, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Flint-Garcia, S.A.; Bodnar, A.L.; Scott, M.P. Wide variability in kernel composition, seed characteristics, and zein profiles among diverse maize inbreds, landraces, and teosinte. Theor. Appl. Genet. 2009, 119, 1129–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takanashi, H.; Shichijo, M.; Sakamoto, L.; Kajiya-Kanegae, H.; Iwata, H.; Sakamoto, W.; Tsutsumi, N. Genetic dissection of QTLs associated with spikelet-related traits and grain size in sorghum. Sci. Rep. 2021, 11, 9398. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Samayoa, L.F.; Bradbury, P.J.; Olukolu, B.A.; Xue, W.; York, A.M.; Tuholski, M.R.; Wang, W.; Daskalska, L.L.; Neumeyer, M.A.; et al. The genetic architecture of teosinte catalyzed and constrained maize domestication. Proc. Natl. Acad. Sci. USA 2019, 116, 5643–5652. [Google Scholar] [CrossRef] [Green Version]
- Flint-Garcia, S.A. Genetics and consequences of crop domestication. J. Agric. Food Chem. 2013, 61, 1876–8267. [Google Scholar] [CrossRef]
- Bommert, N.S.-N.P.; Jackson, D.; Hirano, H.-Y. Genetics and evolution of inflorescence and flower development in grasses. Plant Cell Physiol. 2005, 46, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Smith, O.; Nicholson, W.V.; Kistler, L.; Mace, E.; Clapham, A.; Rose, P.; Stevens, C.; Ware, R.; Samavedam, S.; Barker, G.; et al. A domestication history of dynamic adaptation and genomic deterioration in Sorghum. Nat. Plants 2019, 5, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Lenser, T.; Theissen, G. Molecular mechanisms involved in convergent crop domestication. Trends Plant Sci. 2013, 18, 704–714. [Google Scholar] [CrossRef]
- Wang, C.F.S.Y.C.; Xing, Y. A causal C-A mutation in the second exon of GS3 highly associated with rice grain length and validated as a functional marker. Theor. Appl. Genet. 2009, 118, 465–472. [Google Scholar] [CrossRef]
- Sun, S.; Wang, L.; Mao, H.; Shao, L.; Li, X.; Xiao, J.; Ouyang, Y.; Zhang, Q. A G-protein pathway determines grain size in rice. Nat. Commun. 2018, 9, 851. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Yang, X.; Bai, G.; Warburton, M.L.; Mahuku, G.; Gore, M.; Dai, J.; Li, J.; Yan, J. Cloning and characterization of a putative GS3 ortholog involved in maize kernel development. Theor. Appl. Genet. 2010, 120, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Zhai, G.; Yan, S.; Li, S.; Zhou, L.; Ding, Y.; Liu, H.; Zhang, Z.; Zou, J.; Zhang, L.; et al. Sorghum qTGW1a encodes a G-protein subunit and acts as a negative regulator of grain size. J. Exp. Bot. 2020, 71, 5389–5401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cook, J.; Melia-Hancock, S.; Guill, K.; Bottoms, C.; Garcia, A.; Ott, O.; Nelson, R.; Recker, J.; Balint-Kurti, P.; et al. Expanding Maize Genetic Resources with Predomestication Alleles: Maize-Teosinte Introgression Populations. Plant Genome 2016, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Liu, H.; Zhou, L.; Lin, Z. Genetic Architecture of domestication- and improvement-related traits using a population derived from Sorghum virgatum and Sorghum bicolor. Plant Sci. 2019, 283, 135–146. [Google Scholar] [CrossRef]
- Nabukalu, P.; Kong, W.; Cox, T.S.; Pierce, G.J.; Compton, R.; Tang, H.; Paterson, A.H. Genetic variation underlying kernel size, shape, and color in two interspecific S. bicolor2 × S. halepense subpopulations. Genet. Resour. Crop Evol. 2021, 69, 1261–1281. [Google Scholar] [CrossRef]
- Tao, Y.; Mace, E.; George-Jaeggli, B.; Hunt, C.; Cruickshank, A.; Henzell, R.; Jordan, D. Novel Grain Weight Loci Revealed in a Cross between Cultivated and Wild Sorghum. Plant Genome 2018, 11, 170089. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; van Oosterom, E.J.; Jordan, D.R.; Doherty, A.; Hammer, G.L. Genetic Variation in Potential Kernel Size Affects Kernel Growth and Yield of Sorghum. Crop Sci. 2010, 50, 685–695. [Google Scholar] [CrossRef]
- Bai, C.; Wang, C.; Wang, P.; Zhu, Z.; Cong, L.; Li, D.; Liu, Y.; Zheng, W.; Lu, X. QTL mapping of agronomically important traits in sorghum (Sorghum bicolor L.). Euphytica 2017, 213, 285. [Google Scholar] [CrossRef]
- Boyles, R.E.; Pfeiffer, B.K.; Cooper, E.A.; Zielinski, K.J.; Myers, M.T.; Rooney, W.L.; Kresovich, S. Quantitative Trait Loci Mapping of Agronomic and Yield Traits in Two Grain Sorghum Biparental Families. Crop Sci. 2017, 57, 2443–2456. [Google Scholar] [CrossRef] [Green Version]
- Shehzad, T.; Okuno, K. QTL mapping for yield and yield-contributing traits in sorghum (Sorghum bicolor (L.) Moench) with genome-based SSR markers. Euphytica 2014, 203, 17–31. [Google Scholar] [CrossRef]
- Brown, P.J.; Klein, P.E.; Bortiri, E.; Acharya, C.B.; Rooney, W.L.; Kresovich, S. Inheritance of inflorescence architecture in sorghum. Theor. Appl. Genet. 2006, 113, 931–942. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, C. Mapping quantitative trait loci for yield-related traits and predicting candidate genes for grain weight in maize. Sci. Rep. 2019, 9, 16112. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.C.; Rooney, W.L.; Mitchell, S.E.; Sharma, A.; Klein, P.E.; Mullet, J.E.; Kresovich, S. Genetic Improvement of Sorghum as a Biofuel Feedstock: II. QTL for Stem and Leaf Structural Carbohydrates. Crop Sci. 2008, 48, 2180–2193. [Google Scholar] [CrossRef]
- Nagaraja Reddy, R.; Madhusudhana, R.; Murali Mohan, S.; Chakravarthi, D.V.; Mehtre, S.P.; Seetharama, N.; Patil, J.V. Mapping QTL for grain yield and other agronomic traits in post-rainy sorghum [Sorghum bicolor (L.) Moench]. Theor. Appl. Genet. 2013, 126, 1921–1939. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, G.; Satish, K.; Madhusudhana, R.; Reddy, R.N.; Mohan, S.M.; Seetharama, N. Identification of quantitative trait loci for agronomically important traits and their association with genic-microsatellite markers in sorghum. Theor. Appl. Genet. 2009, 118, 1439–1454. [Google Scholar] [CrossRef]
- Fakrudin, B.; Kavil, S.P.; Girma, Y.; Arun, S.S.; Dadakhalandar, D.; Gurusiddesh, B.H.; Patil, A.M.; Thudi, M.; Khadi, B.M.; Kamatar, M.Y.; et al. Molecular mapping of genomic regions harbouring QTLs for root and yield traits in sorghum (Sorghum bicolor L. Moench). Physiol. Mol. Biol. Plants 2013, 19, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Phuong, N.; Stützel, H.; Uptmoor, R. Quantitative trait loci associated to agronomic traits and yield components in a Sorghum bicolor L. Moench RIL population cultivated under pre-flowering drought and well-watered conditions. Agric. Sci. 2013, 4, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Said, A.A.; Uptmoor, R.; El-Soda, M. Mapping quantitative trait loci associated with yield and its related traits in Sorghum bicolor. Egypt. J. Agron. 2018, 40, 251–259. [Google Scholar] [CrossRef]
- Paterson, A.H.; Lin, Y.R.; Li, Z.; Schertz, K.F.; Doebley, J.F.; Pinson, S.R.; Liu, S.-C.; Stansel, J.W.; Irvine, J.E. Convergent Domestication of Cereal Crops by Independent Mutations at Corresponding Genetic Loci. Science 1995, 269, 1714–1718. [Google Scholar] [CrossRef]
- Tuinstra, M.R.; Grote, E.M.; Goldsbrough, P.B.; Ejeta, G. Genetic analysis of post-flowering drought tolerance and components of grain development in Sorghum bicolor (L.) Moench. Mol. Breed. 1997, 3, 439–448. [Google Scholar] [CrossRef]
- Guindo, D.; Teme, N.; Vaksmann, M.; Doumbia, M.; Vilmus, I.; Guitton, B.; Sissoko, A.; Mestres, C.; Davrieux, F.; Fliedel, G.; et al. Quantitative trait loci for sorghum grain morphology and quality traits: Toward breeding for a traditional food preparation of West-Africa. J. Cereal Sci. 2019, 85, 256–272. [Google Scholar] [CrossRef]
- Rami, J.F.; Dufour, P.; Trouche, G.; Fliedel, G.; Mestres, C.; Davrieux, F.; Blanchard, P.; Hamon, P. Quantitative trait loci for grain quality, productivity, morphological and agronomical traits in sorghum (Sorghum bicolor L. Moench). Theor. Appl. Genet. 1998, 97, 605–616. [Google Scholar] [CrossRef]
- Mocoeur, A.; Zhang, Y.M.; Liu, Z.Q.; Shen, X.; Zhang, L.M.; Rasmussen, S.K.; Jing, H.C. Stability and genetic control of morphological, biomass and biofuel traits under temperate maritime and continental conditions in sweet sorghum (Sorghum bicolour). Theor. Appl. Genet. 2015, 128, 1685–1701. [Google Scholar] [CrossRef] [PubMed]
- Feltus, F.A.; Hart, G.E.; Schertz, K.F.; Casa, A.M.; Kresovich, S.; Abraham, S.; Klein, P.E.; Brown, P.J.; Paterson, A.H. Alignment of Genetic Maps and QTLs between Inter-and Intra-Specific Sorghum Populations. Theor. Appl. Genet. 2006, 112, 1295–1305. [Google Scholar] [CrossRef]
- Mekonnen, T.B.; Dong, H.; Getinet, M.; Gabizew, A.; Paterson, A.; Bantte, K. QTL analysis in multiple sorghum mapping populations facilitates dissection of the genetic control of agronomic and yield-related traits in sorghum [Sorghum bicolor (Moench)]. Euphytica 2022, 218, 24. [Google Scholar] [CrossRef]
- Fan, C.; Xing, Y.; Mao, H.; Lu, T.; Han, B.; Xu, C.; Li, X.; Zhang, Q. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 2006, 112, 1164–1171. [Google Scholar] [CrossRef]
- Liu, H.; Li, Q.; Xing, Y. Genes Contributing to Domestication of Rice Seed Traits and Its Global Expansion. Genes 2018, 9, 489. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Zhao, X.; Wang, X.; Hathorn, A.; Hunt, C.; Cruickshank, A.W.; Oosterom, E.J.; Godwin, I.D.; Mace, E.S.; Jordan, D.R. Large-scale GWAS in sorghum reveals common genetic control of grain size among cereals. Plant Biotechnol. J. 2019, 18, 1093–1105. [Google Scholar] [CrossRef] [Green Version]
- Han, X.Q.Y.; Sandrine, A.M.N.; Qiu, F. Fine mapping of qKRN8, a QTL for maize kernel row number, and prediction of the candidate gene. Theor. Appl. Genet. 2020, 133, 3139–3150. [Google Scholar] [CrossRef]
- Nie, N.; Ding, X.; Chen, L.; Wu, X.; An, Y.; Li, C.; Song, Y.; Zhang, D.; Liu, Z.; Wang, T.; et al. Characterization and fine mapping of qkrnw4, a major QTL controlling kernel row number in maize. Theor. Appl. Genet. 2019, 132, 3321–3331. [Google Scholar] [CrossRef]
- Raihan, M.S.; Liu, J.; Huang, J.; Guo, H.; Pan, Q.; Yan, J. Multi-environment QTL analysis of grain morphology traits and fine mapping of a kernel-width QTL in Zheng58 x SK maize population. Theor. Appl. Genet. 2016, 129, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.-x.; Chen, L.; Wu, X.; Qin, W.; Song, Y.; Zhang, D.; Wang, T.; Li, Y.; Shi, Y. Fine mapping of qKW7, a major QTL for kernel weight and kernel width in maize, confirmed by the combined analytic approaches of linkage and association analysis. Euphytica 2016, 210, 221–232. [Google Scholar] [CrossRef]
- Jia, H.; Li, M.; Li, W.; Liu, L.; Jian, Y.; Yang, Z.; Shen, X.; Ning, Q.; Du, Y.; Zhao, R.; et al. A serine/threonine protein kinase encoding gene KERNEL NUMBER PER ROW6 regulates maize grain yield. Nat. Commun. 2020, 11, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Jia, H.; Cao, X.; Huang, J.; Li, F.; Tao, Y.; Qiu, F.; Zheng, Y.; Zhang, Z. Fine mapping and candidate gene prediction of a pleiotropic quantitative trait locus for yield-related trait in Zea mays. PLoS ONE 2012, 7, e49836. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Wang, X.; Wang, B.; Tian, Y.; Li, M.; Nie, Y.; Peng, Q.; Wang, Z. Fine Mapping a Major QTL for Kernel Number per Row under Different Phosphorus Regimes in Maize (Zea mays L.). Theor. Appl. Genet. 2013, 126, 1545–1553. [Google Scholar] [CrossRef]
- Calderón, C.I.; Yandell, B.S.; Doebley, J.F. Fine Mapping of a QTL Associated with Kernel Row Number on Chromosome 1 of Maize. PLoS ONE 2016, 11, e0150276. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Zhao, R.; Liu, L.; Zhu, C.; Li, M.; Du, H.; Zhang, Z. Identification of a candidate gene underlying qKRN5b for kernel row number in Zea mays L. Theor. Appl. Genet. 2019, 132, 3439–3448. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z.; Zhang, X.; Liu, H.; Zhou, L.; Zhong, S.; Li, Y.; Zhu, C.; Lin, Z. krn1, a major quantitative trait locus for kernel row number in maize. New Phytol. 2019, 223, 1634–1646. [Google Scholar] [CrossRef]
- Liu, L.; Du, Y.; Shen, X.; Li, M.; Sun, W.; Huang, J.; Liu, Z.; Tao, Y.; Zheng, Y.; Yan, J.; et al. KRN4 Controls Quantitative Variation in Maize Kernel Row Number. PLoS Genet. 2015, 11, e1005670. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.; Li, Y.-X.; Wu, X.; Li, X.; Chen, L.; Shi, Y.; Song, Y.; Zhang, D.; Wang, T.; Li, Y. Fine mapping of qKL1.07, a major QTL for kernel length in maize. Mol. Breed. 2016, 36, 8. [Google Scholar] [CrossRef]
- Gong, D.; Tan, Z.; Zhao, H.; Pan, Z.; Sun, Q.; Qiu, F. Fine mapping of a kernel length-related gene with potential value for maize breeding. Theor. Appl. Genet. 2021, 134, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhao, Y.; Mao, W.; Ma, X.; Su, C. QTL Analysis and Fine Mapping of a Major QTL Conferring Kernel Size in Maize (Zea mays). Front. Genet. 2020, 11, 603920. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Li, Y.; Mu, Z.; Chen, L.; Guo, H.; Chen, Z.; Li, C.; Liu, X.; Zhang, D.; Shi, Y.; et al. Fine mapping and candidate gene analysis of qKW7b, a major QTL for kernel width in maize. Mol. Breed. 2020, 40, 67. [Google Scholar] [CrossRef]
- Zhan, J.; Wang, F.; Xing, W.; Liu, J.; Fan, Z.; Tao, Y. Fine mapping and candidate gene prediction of a major QTL for kernel number per ear in maize. Mol. Breed. 2018, 38, 27. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.-X.; Li, C.; Wu, X.; Qin, W.; Li, X.; Jiao, F.; Zhang, X.; Zhang, D.; Shi, Y.; et al. Fine-Mapping of QGW4.05, a Major QTL for Kernel Weight and Size in Maize. BMC Plant Biol. 2016, 16, 81. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Bai, Q.; Zhan, W.; Ma, C.; Wang, S.; Feng, Y.; Zhang, M.; Zhu, Y.; Cheng, M.; Xi, Z. Fine mapping and candidate gene analysis of qhkw5-3, a major QTL for kernel weight in maize. Theor. Appl. Genet. 2019, 132, 2579–2589. [Google Scholar] [CrossRef]
- Zhou, Q.; Dong, Y.; Shi, Q.; Zhang, L.; Chen, H.; Hu, C.; Li, Y. Verification and fine mapping of qGW1.05, a major QTL for grain weight in maize (Zea mays L.). Mol. Genet. Genom. 2017, 292, 871–881. [Google Scholar] [CrossRef]
- Huang, J.; Lu, G.; Liu, L.; Raihan, M.S.; Xu, J.; Jian, L.; Zhao, L.; Tran, T.M.; Zhang, Q.; Liu, J.; et al. The Kernel Size-Related Quantitative Trait Locus qKW9 Encodes a Pentatricopeptide Repeat Protein That Aaffects Photosynthesis and Grain Filling. Plant Physiol. 2020, 183, 1696–1709. [Google Scholar] [CrossRef]
- Mace, E.S.; Tai, S.; Gilding, E.K.; Li, Y.; Prentis, P.J.; Bian, L.; Campbell, B.C.; Hu, W.; Innes, D.J.; Han, X.; et al. Whole-genome sequencing reveals untapped genetic potential in Africa’s indigenous cereal crop sorghum. Nat. Commun. 2013, 4, 2320. [Google Scholar] [CrossRef] [Green Version]
- Makita, Y.; Shimada, S.; Kawashima, M.; Kondou-Kuriyama, T.; Toyoda, T.; Matsui, M. MOROKOSHI: Transcriptome database in Sorghum bicolor. Plant Cell Physiol. 2015, 56, e6. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.L.; Li, X.H.; Li, J.Z.; Fu, J.F.; Wang, Y.Z.; Wei, M.G. Dent corn genetic background influences QTL detection for grain yield and yield components in high-oil maize. Euphytica 2009, 169, 273–284. [Google Scholar] [CrossRef]
- Peng, B.; Li, Y.; Wang, Y.; Liu, C.; Liu, Z.; Tan, W.; Zhang, Y.; Wang, D.; Shi, Y.; Li, Y.; et al. QTL analysis for yield components and kernel-related traits in maize across multi-environments. Theor. Appl. Genet. 2011, 122, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Sun, C.; Zhang, Z.; Zheng, Y.; Qiu, F. Genetic analysis and major QTL detection for maize kernel size and weight in multi-environments. Theor. Appl. Genet. 2014, 127, 1019–1037. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Rustgi, S.; Kumar, N. Genetic and molecular basis of grain size and grain number and its relevance to grain productivity in higher plants. Genome 2006, 49, 565–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yi, Q.; Hou, X.; Hu, Y.; Li, Y.; Yu, G.; Liu, H.; Zhang, J.; Huang, Y. Identification of quantitative trait loci for kernel-related traits and the heterosis for these traits in maize (Zea mays L.). Mol. Genet. Genom. 2020, 295, 121–133. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Li, L.; Schnable, J.; Gu, R.; Wang, J. QTL Identification and Epistatic Effect Analysis of Seed Size- and Weight-Related Traits in Zea mays L. Mol. Breed. 2019, 39, 67. [Google Scholar] [CrossRef]
- Lan, T.; He, K.; Chang, L.; Cui, T.; Zhao, Z.; Xue, J.; Liu, J. QTL mapping and genetic analysis for maize kernel size and weight in multi-environments. Euphytica 2018, 214, 119. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Z.; Yong, H.; Zhang, X.; Hao, Z.; Zhang, F.; Li, M.; Zhang, D.; Li, X.; Wang, Z.; et al. Analysis of the genetic architecture of maize ear and grain morphological traits by combined linkage and association mapping. Theor. Appl. Genet. 2017, 130, 1011–1029. [Google Scholar] [CrossRef]
- Shi, Z.; Song, W.; Xing, J.; Duan, M.; Wang, F.; Tian, H.; Xu, L.; Wang, S.; Su, A.; Li, C.; et al. Molecular mapping of quantitative trait loci for three kernel-related traits in maize using a double haploid population. Mol. Breed. 2017, 37, 108. [Google Scholar] [CrossRef]
- Su, C.; Wang, W.; Gong, S.; Zuo, J.; Li, S.; Xu, S. High Density Linkage Map Construction and Mapping of Yield Trait QTLs in Maize (Zea mays) Using the Genotyping-by-Sequencing (GBS) Technology. Front. Plant Sci. 2017, 8, 706. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Zhang, L.; Jia, A.; Rong, T. Identification of QTL for maize grain yield and kernel-related traits. J. Genet. 2016, 95, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, L.; Liu, S.; Li, Z.; Huang, R.; Li, Y.; Cheng, H.; Li, X.; Zhou, B.; Wu, S.; et al. The Genetic Basis of Natural Variation in Kernel Size and Related Traits Using a Four-Way Cross Population in Maize. PLoS ONE 2016, 11, e0153428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Lu, X.; Zhang, Z.; Xu, M.; Mao, K.; Li, W.; Wei, F.; Sun, P.; Tang, J. Genetic analysis of heterosis for maize grain yield and its components in a set of SSSL testcross populations. Euphytica 2016, 210, 181–193. [Google Scholar] [CrossRef]
- Huo, D.; Ning, Q.; Shen, X.; Liu, L.; Zhang, Z. QTL Mapping of Kernel Number-Related Traits and Validation of One Major QTL for Ear Length in Maize. PLoS ONE 2016, 11, e0155506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sa, K.J.; Park, J.Y.; Woo, S.Y.; Ramekar, R.V.; Jang, C.S.; Lee, J.K. Mapping of QTL traits in corn using a RIL population derived from a cross of dent corn × waxy corn. Genes Genom. 2015, 37, 1–14. [Google Scholar] [CrossRef]
- Alvarez Prado, S.; Sadras, V.O.; Borras, L. Independent genetic control of maize (Zea mays L.) kernel weight determination and its phenotypic plasticity. J. Exp. Bot. 2014, 65, 4479–4487. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Liu, Z.; Hu, Y.; Li, W.; Fu, Z.; Ding, D.; Li, H.; Qiao, M.; Tang, J. QTL analysis of Kernel-related traits in maize using an immortalized F2 population. PLoS ONE 2014, 9, e89645. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Jia, H.T.; Liu, L.; Zhang, C.X.; Liu, Z.J.; Zhang, Z.X. Quantitative trait loci mapping for kernel row number using chromosome segment substitution lines in maize. Genet. Mol. Res. 2014, 13, 1707–1716. [Google Scholar] [CrossRef]
- Tian, B.; Wang, J.; Wang, G.; Lübberstedt, T. Confirmation of a major QTL on chromosome 10 for maize kernel row number in different environments. Plant Breed. 2014, 133, 184–188. [Google Scholar] [CrossRef]
- Yang, G.; Li, Y.; Wang, Q.; Zhou, Y.; Zhou, Q.; Shen, B.; Zhang, F.; Liang, X. Detection and integration of quantitative trait loci for grain yield components and oil content in two connected recombinant inbred line populations of high-oil maize. Mol. Breed. 2011, 29, 313–333. [Google Scholar] [CrossRef]
- Cai, H.; Chu, Q.; Gu, R.; Yuan, L.; Liu, J.; Zhang, X.; Chen, F.; Mi, G.; Zhang, F. Identification of QTLs for plant height, ear height and grain yield in maize (Zea mays L.) in response to nitrogen and phosphorus supply. Plant Breed. 2012, 131, 502–510. [Google Scholar] [CrossRef]
- Lu, M.; Xie, C.-X.; Li, X.-H.; Hao, Z.-F.; Li, M.-S.; Weng, J.-F.; Zhang, D.-G.; Bai, L.; Zhang, S.-H. Mapping of quantitative trait loci for kernel row number in maize across seven environments. Mol. Breed. 2010, 28, 143–152. [Google Scholar] [CrossRef]
- Guo, J.; Chen, Z.; Liu, Z.; Wang, B.; Song, W.; Li, W.; Chen, J.; Dai, J.; Lai, J. Identification of genetic factors affecting plant density response through QTL mapping of yield component traits in maize (Zea mays L.). Euphytica 2011, 182, 409. [Google Scholar] [CrossRef]
- Li, J.Z.; Zhang, Z.W.; Li, Y.L.; Wang, Q.L.; Zhou, Y.G. QTL consistency and meta-analysis for grain yield components in three generations in maize. Theor. Appl. Genet. 2011, 122, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.Q.; Ma, J.L.; Zhang, C.R.; Dong, H.F.; Xi, Z.Y.; Xia, Z.L.; Wu, J.Y. Mapping for Test Weight by Using F 2:3 Population in Maize. J. Genet. 2011, 90, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yan, J.; Ma, X.; Teng, W.; Wu, W.; Dai, J.; Dhillon, B.S.; Melchinger, A.E.; Li, J. Dissection of the genetic basis of heterosis in an elite maize hybrid by QTL mapping in an immortalized F2 population. Theor. Appl. Genet. 2010, 120, 333–340. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Yang, X.; Warburton, M.L.; Bai, G.; Dai, J.; Li, J.; Yan, J. Relationship, evolutionary fate and function of two maize co-orthologs of rice GW2 associated with kernel size and weight. BMC Plant Biol. 2010, 10, 7. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.J.; Wu, A.Z.; Zheng, C.C.; Wang, Y.F.; Cai, R.; Shen, X.F.; Xu, R.R.; Liu, P.; Kong, L.J.; Dong, S.T. QTL mapping of maize (Zea mays) stay-green traits and their relationship to yield. Plant Breed. 2009, 128, 54–62. [Google Scholar] [CrossRef]
- Frascaroli, E.; Cane, M.A.; Landi, P.; Pea, G.; Gianfranceschi, L.; Villa, M.; Morgante, M.; Pe, M.E. Classical genetic and quantitative trait loci analyses of heterosis in a maize hybrid between two elite inbred lines. Genetics 2007, 176, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Coque, M.; Gallais, A. Genomic regions involved in response to grain yield selection at high and low nitrogen fertilization in maize. Theor. Appl. Genet. 2006, 112, 1205–1220. [Google Scholar] [CrossRef]
- Yan, J.-B.; Tang, H.; Huang, Y.-Q.; Zheng, Y.-L.; Li, J.-S. Quantitative trait loci mapping and epistatic analysis for grain yield and yield components using molecular markers with an elite maize hybrid. Euphytica 2006, 149, 121–131. [Google Scholar] [CrossRef]
- Xiao, Y.N.; Li, X.H.; George, M.L.; Li, M.S.; Zhang, S.H.; Zheng, Y.L. Quantitative Trait Locus Analysis of Drought Tolerance and Yield in Maize in China. Plant Mol. Biol. Rep. 2005, 23, 155–165. [Google Scholar] [CrossRef]
- Veldboom, L.R.; Lee, M. Molecular-marker-facilitated studies of morphological traits in maize. II: Determination of QTLs for grain yield and yield components. Theor. Appl. Genet. 1994, 89, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Garcia, A.; McMullen, M.D.; Flint-Garcia, S.A. Genetic Analysis of Kernel Traits in Maize-Teosinte Introgression Populations. G3 Genes Genomes Genet. 2016, 6, 2523–2530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Ren, Z.; Luo, B.; Zhong, H.; Ma, P.; Zhang, H.; Hu, H.; Wang, Y.; Liu, D.; Gao, S.; et al. Genetic architecture of maize yield traits dissected by QTL mapping and GWAS in maize. Crop J. 2021; in press. [Google Scholar] [CrossRef]
- Hao, D.; Xue, L.; Zhang, Z.; Cheng, Y.; Chen, G.; Zhou, G.; Li, P.; Yang, Z.; Xu, C. Combined linkage and association mapping reveal candidate loci for kernel size and weight in maize. Breed Sci. 2019, 69, 420–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Huang, J.; Guo, H.; Lan, L.; Wang, H.; Xu, Y.; Yang, X.; Li, W.; Tong, H.; Xiao, Y.; et al. The Conserved and Unique Genetic Architecture of Kernel Size and Weight in Maize and Rice. Plant Physiol. 2017, 175, 774–785. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Tan, X.; Yang, Y.; Liu, P.; Zhang, X.; Zhang, Y.; Wang, L.; Hu, Y.; Ma, L.; Li, Z.; et al. Analysis of the genetic architecture of maize kernel size traits by combined linkage and association mapping. Plant Biotechnol. J. 2020, 18, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Du, Y.; Huo, D.; Wang, M.; Shen, X.; Yue, B.; Qiu, F.; Zheng, Y.; Yan, J.; Zhang, Z. Genetic architecture of maize kernel row number and whole genome prediction. Theor. Appl. Genet. 2015, 128, 2243–2254. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liao, C.; Wang, X.; Yang, R.; Zhai, L.; Huang, J. Construction of maize–teosinte introgression line population and identification of major quantitative trait loci. Euphytica 2021, 217, 179. [Google Scholar] [CrossRef]
- Yu, F.; Li, J.; Huang, Y.; Liu, L.; Li, D.; Chen, L.; Luan, S. FERONIA receptor kinase controls seed size in Arabidopsis thaliana. Mol. Plant 2014, 7, 920–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, H.; Sun, S.; Yao, J.; Wang, C.; Yu, S.; Xu, C.; Li, X.; Zhang, Q. Linking differential domain functions of the GS3 protein to natural variation of grain size in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 19579–19584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Li, Y.; Sui, Z.; Lan, T.; Song, W.; Zhang, M.; Zhang, Y.; Xing, J. A C-terminal encoded peptide, ZmCEP1, is essential for kernel development in maize. J. Exp. Bot. 2021, 72, 5390–5406. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.M.; Ren, Z.J.; Wang, B.H.; Zhang, L.; Zhao, Y.J.; Wu, J.W.; Li, L.G.; Zhang, X.S.; Zhao, X.Y. A nitrate transporter encoded by ZmNPF7.9 is essential for maize seed development. Plant Sci. 2021, 308, 110901. [Google Scholar] [CrossRef] [PubMed]
- Sosso, D.; Luo, D.; Li, Q.B.; Sasse, J.; Yang, J.; Gendrot, G.; Suzuki, M.; Koch, K.E.; McCarty, D.R.; Chourey, P.S.; et al. Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat. Genet. 2015, 47, 1489–1493. [Google Scholar] [CrossRef]
- Yang, N.; Liu, J.; Gao, Q.; Gui, S.; Chen, L.; Yang, L.; Huang, J.; Deng, T.; Luo, J.; He, L.; et al. Genome assembly of a tropical maize inbred line provides insights into structural variation and crop improvement. Nat. Genet. 2019, 51, 1052–1059. [Google Scholar] [CrossRef] [Green Version]
- Sosso, D.; Mbelo, S.; Vernoud, V.; Gendrot, G.; Dedieu, A.; Chambrier, P.; Dauzat, M.; Heurtevin, L.; Guyon, V.; Takenaka, M.; et al. PPR2263, a DYW-Subgroup Pentatricopeptide repeat protein, is required for mitochondrial nad5 and cob transcript editing, mitochondrion biogenesis, and maize growth. Plant Cell 2012, 24, 676–691. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Marcos, J.F.; Dal Pra, M.; Giulini, A.; Costa, L.M.; Gavazzi, G.; Cordelier, S.; Sellam, O.; Tatout, C.; Paul, W.; Perez, P.; et al. empty pericarp4 encodes a mitochondrion-targeted pentatricopeptide repeat protein necessary for seed development and plant growth in maize. Plant Cell 2007, 19, 196–210. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.J.; Xiu, Z.H.; Meeley, R.; Tan, B.C. Empty pericarp5 encodes a pentatricopeptide repeat protein that is required for mitochondrial RNA editing and seed development in maize. Plant Cell 2013, 25, 868–883. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Wang, X.; Bonnard, G.; Shen, Y.; Xiu, Z.; Li, X.; Gao, D.; Zhang, Z.; Tan, B.C. Empty pericarp7 encodes a mitochondrial E-subgroup pentatricopeptide repeat protein that is required for ccmFN editing, mitochondrial function and seed development in maize. Plant J. 2015, 84, 283–295. [Google Scholar] [CrossRef]
- Cai, M.; Li, S.; Sun, F.; Sun, Q.; Zhao, H.; Ren, X.; Zhao, Y.; Tan, B.C.; Zhang, Z.; Qiu, F. Emp10 encodes a mitochondrial PPR protein that affects the cis-splicing of nad2 intron 1 and seed development in maize. Plant J. 2017, 91, 132–144. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.Z.; Ding, S.; Wang, H.C.; Sun, F.; Huang, W.L.; Song, S.; Xu, C.; Tan, B.C. The pentatricopeptide repeat protein EMP9 is required for mitochondrial ccmB and rps4 transcript editing, mitochondrial complex biogenesis and seed development in maize. New Phytol. 2017, 214, 782–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Pan, Z.; Zhao, H.; Zhao, J.; Cai, M.; Li, J.; Zhang, Z.; Qiu, F. EMPTY PERICARP11 serves as a factor for splicing of mitochondrial nad1 intron and is required to ensure proper seed development in maize. J. Exp. Bot. 2017, 68, 4571–4581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, F.; Xiu, Z.; Jiang, R.; Liu, Y.; Zhang, X.; Yang, Y.Z.; Li, X.; Zhang, X.; Wang, Y.; Tan, B.C. The mitochondrial pentatricopeptide repeat protein EMP12 is involved in the splicing of three nad2 introns and seed development in maize. J. Exp. Bot. 2019, 70, 963–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiu, Z.; Sun, F.; Shen, Y.; Zhang, X.; Jiang, R.; Bonnard, G.; Zhang, J.; Tan, B.C. EMPTY PERICARP16 is required for mitochondrial nad2 intron 4 cis-splicing, complex I assembly and seed development in maize. Plant J. 2016, 85, 507–519. [Google Scholar] [CrossRef]
- Qi, W.; Yang, Y.; Feng, X.; Zhang, M.; Song, R. Mitochondrial Function and Maize Kernel Development Requires Dek2, a Pentatricopeptide Repeat Protein Involved in nad1 mRNA Splicing. Genetics 2017, 205, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.; Liu, M.; Xiao, Z.; Ren, X.; Zhao, H.; Gong, D.; Liang, K.; Tan, Z.; Shao, Y.; Qiu, F. ZmSMK9, a pentatricopeptide repeat protein, is involved in the cis-splicing of nad5, kernel development and plant architecture in maize. Plant Sci. 2019, 288, 110205. [Google Scholar] [CrossRef]
- Qi, W.; Tian, Z.; Lu, L.; Chen, X.; Chen, X.; Zhang, W.; Song, R. Editing of Mitochondrial Transcripts nad3 and cox2 by Dek10 Is Essential for Mitochondrial Function and Maize Plant Development. Genetics 2017, 205, 1489–1501. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Feng, F.; Qi, W.; Xu, L.; Yao, D.; Wang, Q.; Song, R. Dek35 Encodes a PPR Protein that Affects cis-Splicing of Mitochondrial nad4 Intron 1 and Seed Development in Maize. Mol. Plant 2017, 10, 427–441. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhong, M.; Shuai, B.; Song, J.; Zhang, J.; Han, L.; Ling, H.; Tang, Y.; Wang, G.; Song, R. E+ subgroup PPR protein defective kernel 36 is required for multiple mitochondrial transcripts editing and seed development in maize and Arabidopsis. New Phytol. 2017, 214, 1563–1578. [Google Scholar] [CrossRef] [Green Version]
- Dai, D.; Luan, S.; Chen, X.; Wang, Q.; Feng, Y.; Zhu, C.; Qi, W.; Song, R. Maize Dek37 Encodes a P-type PPR Protein That Affects cis-Splicing of Mitochondrial nad2 Intron 1 and Seed Development. Genetics 2018, 208, 1069–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Gu, W.; Sun, S.; Chen, Z.; Chen, J.; Song, W.; Zhao, H.; Lai, J. Defective Kernel 39 encodes a PPR protein required for seed development in maize. J. Integr. Plant Biol. 2018, 60, 45–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.J.; Zhang, Y.F.; Hou, M.; Sun, F.; Shen, Y.; Xiu, Z.H.; Wang, X.; Chen, Z.L.; Sun, S.S.; Small, I.; et al. Small kernel 1 encodes a pentatricopeptide repeat protein required for mitochondrial nad7 transcript editing and seed development in maize (Zea mays) and rice (Oryza sativa). Plant J. 2014, 79, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Bommert, P.; Nagasawa, N.S.; Jackson, D. Quantitative variation in maize kernel row number is controlled by the FASCIATED EAR2 locus. Nat. Genet. 2013, 45, 334–337. [Google Scholar] [CrossRef]
- Burow, Z.X.G.; Hayes, C.; Burke, J. Characterization of a Multiseeded (msd1) Mutant of Sorghum for Increasing Grain Yield. Crop Sci. 2014, 54, 2030–2037. [Google Scholar] [CrossRef] [Green Version]
- Francis, A.; Dhaka, N.; Bakshi, M.; Jung, K.H.; Sharma, M.K.; Sharma, R. Comparative phylogenomic analysis provides insights into TCP gene functions in Sorghum. Sci. Rep. 2016, 6, 38488. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, L.; Comadran, J.; Druka, A.; Marshall, D.F.; Thomas, W.T.; Macaulay, M.; MacKenzie, K.; Simpson, C.; Fuller, J.; Bonar, N.; et al. INTERMEDIUM-C, a modifier of lateral spikelet fertility in barley, is an ortholog of the maize domestication gene TEOSINTE BRANCHED 1. Nat. Genet. 2011, 43, 169–172. [Google Scholar] [CrossRef]
- Browse, J. The power of mutants for investigating jasmonate biosynthesis and signaling. Phytochemistry 2009, 70, 1539–1546. [Google Scholar] [CrossRef]
- Goetz, S.; Hellwege, A.; Stenzel, I.; Kutter, C.; Hauptmann, V.; Forner, S.; McCaig, B.; Hause, G.; Miersch, O.; Wasternack, C.; et al. Role of cis-12-oxo-phytodienoic acid in tomato embryo development. Plant Physiol. 2012, 158, 1715–1727. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhao, Y.; McCaig, B.C.; Wingerd, B.A.; Wang, J.; Whalon, M.E.; Pichersky, E.; Howe, G.A. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 2004, 16, 126–143. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.; Yuan, Z.; Chen, M.; Yin, C.; Luo, Z.; Zhao, X.; Liang, W.; Hu, J.; Zhang, D. Jasmonic acid regulates spikelet development in rice. Nat. Commun. 2014, 5, 3476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLong, A.; Calderon-Urrea, A.; Dellaporta, S.L. Sex determination gene TASSELSEED2 of maize encodes a short-chain alcohol dehydrogenase required for stage.specific floral organ abortion Rapid sequence evolution of the mammalian sex-determining gene SRY Rapid evolution of the sex determining locus in Old World mice and rats. Cell 1993, 74, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Lunde, C.; Kimberlin, A.; Leiboff, S.; Koo, A.J.; Hake, S. Tasselseed5 overexpresses a wound-inducible enzyme, ZmCYP94B1, that affects jasmonate catabolism, sex determination, and plant architecture in maize. Commun. Biol. 2019, 2, 114. [Google Scholar] [CrossRef]
- Acosta, I.F.; Laparra, H.; Romero, S.P.; Schmelz, E.; Hamberg, M.; Mottinger, J.P.; Moreno, M.A.; Dellaporta, S.L. Tasselseed1 Is a Lipoxygenase Affecting Jasmonic Acid Signaling in Sex Determination of Maize. Science 2009, 323, 262–265. [Google Scholar] [CrossRef] [Green Version]
- Youssef, H.M.; Eggert, K.; Koppolu, R.; Alqudah, A.M.; Poursarebani, N.; Fazeli, A.; Sakuma, S.; Tagiri, A.; Rutten, T.; Govind, G.; et al. VRS2 regulates hormone-mediated inflorescence patterning in barley. Nat. Genet. 2017, 49, 157–161. [Google Scholar] [CrossRef]
- AuBuchon-Elder, T.; Coneva, V.; Goad, D.M.; Jenkins, L.M.; Yu, Y.; Allen, D.K.; Kellogg, E.A. Sterile Spikelets Contribute to Yield in Sorghum and Related Grasses. Plant Cell 2020, 32, 3500–3518. [Google Scholar] [CrossRef] [PubMed]
- Doll, N.M.; Royek, S.; Fujita, S.; Okuda, S.; Chamot, S.; Stintzi, A.; Widiez, T.; Hothorn, M.; Schaller, A.; Ingram, G. A two-way molecular dialogue between embryo and endosperm is required for seed development. Science 2020, 367, 431–435. [Google Scholar] [CrossRef]
- Guan, H.-y.; Dong, Y.-b.; Lu, S.-p.; Liu, T.-s.; He, C.-m.; Liu, C.-x.; Liu, Q.; Dong, R.; Wang, J.; Li, Y.-l.; et al. Characterization and map-based cloning of miniature2-m1, a gene controlling kernel size in maize. J. Integr. Agric. 2020, 19, 1961–1973. [Google Scholar] [CrossRef]
- Sui, Z.; Wang, T.; Li, H.; Zhang, M.; Li, Y.; Xu, R.; Xing, G.; Ni, Z.; Xin, M. Overexpression of Peptide-Encoding OsCEP6.1 Results in Pleiotropic Effects on Growth in Rice (Oryza. sativa). Front. Plant Sci. 2016, 7, 228. [Google Scholar] [CrossRef] [Green Version]
- Nimchuk, Z.L.; Zhou, Y.; Tarr, P.T.; Peterson, B.A.; Meyerowitz, E.M. Plant stem cell maintenance by transcriptional cross-regulation of related receptor kinases. Development 2015, 142, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Somssich, M.; Je, B.I.; Simon, R.; Jackson, D. CLAVATA-WUSCHEL signaling in the shoot meristem. Development 2016, 143, 3238–3248. [Google Scholar] [CrossRef] [Green Version]
- Janocha, D.; Lohmann, J.U. From signals to stem cells and back again. Curr. Opin. Plant Biol. 2018, 45, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Y.X.; Li, C.; Shi, Y.; Song, Y.; Zhang, D.; Wang, H.; Li, Y.; Wang, T. The retromer protein ZmVPS29 regulates maize kernel morphology likely through an auxin-dependent process(es). Plant Biotechnol. J. 2020, 18, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Han, L.; Hymes, M.; Denver, R.; Clark, S.E. CLAVATA2 forms a distinct CLE-binding receptor complex regulating Arabidopsis stem cell specification. Plant J. 2010, 63, 889–900. [Google Scholar] [CrossRef] [Green Version]
- Schoof, H.; Lenhard, M.; Haecker, A.; Mayer, K.F.; Jürgens, G.; Laux, T. The Stem Cell Population of Arabidopsis Shoot Meristems Is Maintained by a Regulatory Loop between the CLAVATA and WUSCHEL Genes. Cell 2000, 100, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Sun, W.; Singh, R.; Zheng, Y.; Cao, Z.; Li, M.; Lunde, C.; Hake, S.; Zhang, Z. GRF-interacting factor1 Regulates Shoot Architecture and Meristem Determinacy in Maize. Plant Cell 2018, 30, 360–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wang, X.; Pan, Q.; Li, P.; Liu, Y.; Lu, X.; Zhong, W.; Li, M.; Han, L.; Li, J.; et al. QTG-Seq Accelerates QTL Fine Mapping through QTL Partitioning and Whole-Genome Sequencing of Bulked Segregant Samples. Mol. Plant 2019, 12, 426–437. [Google Scholar] [CrossRef] [Green Version]
- Takagi, H.; Abe, A.; Yoshida, K.; Kosugi, S.; Natsume, S.; Mitsuoka, C.; Uemura, A.; Utsushi, H.; Tamiru, M.; Takuno, S.; et al. QTL-seq: Rapid mapping of quantitative trait loci in rice by whole genome resequencing of DNA from two bulked populations. Plant J. 2013, 74, 174–183. [Google Scholar] [CrossRef]
- Wu, S.; Qiu, J.; Gao, Q. QTL-BSA: A Bulked Segregant Analysis and Visualization Pipeline for QTL-seq. Interdiscip. Sci. 2019, 11, 730–737. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, X.; Miao, H.; Chu, Y.; Cui, F.; Yang, W.; Wang, C.; Shen, Y.; Xu, T.; Zhao, L.; et al. QTL identification for seed weight and size based on a high-density SLAF-seq genetic map in peanut (Arachis hypogaea L.). BMC Plant Biol. 2019, 19, 537. [Google Scholar] [CrossRef]
- Rankenberg, T.; Geldhof, B.; van Veen, H.; Holsteens, K.; Van de Poel, B.; Sasidharan, R. Age-Dependent Abiotic Stress Resilience in Plants. Trends Plant Sci. 2021, 26, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, A.N.; Govindarajulu, R.; Hawkins, J.S. QTL mapping in an interspecific sorghum population uncovers candidate regulators of salinity tolerance. Plant Stress 2021, 2, 100024. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, B.; Yang, Z.; Liu, Y.; Yang, S.; Shi, Y.; Jiang, C.; Qin, F. Manipulating ZmEXPA4 expression ameliorates the drought-induced prolonged anthesis and silking interval in maize. Plant Cell 2021, 33, 2058–2071. [Google Scholar] [CrossRef]
- Murray, S.C.; Sharma, A.; Rooney, W.L.; Klein, P.E.; Mullet, J.E.; Mitchell, S.E.; Kresovich, S. Genetic Improvement of Sorghum as a Biofuel Feedstock: I. QTL for Stem Sugar and Grain Nonstructural Carbohydrates. Crop Sci. 2008, 48, 2165–2179. [Google Scholar] [CrossRef]
- Mushtaq, M.; Ahmad Dar, A.; Skalicky, M.; Tyagi, A.; Bhagat, N.; Basu, U.; Bhat, B.A.; Zaid, A.; Ali, S.; Dar, T.U.; et al. CRISPR-Based Genome Editing Tools: Insights into Technological Breakthroughs and Future Challenges. Genes 2021, 12, 797. [Google Scholar] [CrossRef] [PubMed]
| Crop | Trait a | QTL | Chr b | Marker Interval | Distance c | Candi d | Reference |
|---|---|---|---|---|---|---|---|
| Sorghum | GW | qGW1 | 1 | SB00037–SB00219 | 101 kb | 13 | [13] |
| GW | qTGW1a | 1 | SM010165–SM010171 | 33 kb | 3 | [43] | |
| Maize | KRN | qKRN8 | 8 | umc2571–umc2593 | 520 kb | 6 | [70] |
| KRN | qkrnw4 | 4 | Ch4.200–Ch4.K-2 | 33 kb | 2 | [71] | |
| KW | qKW-9.2 | 9 | FSR6–MSR36 | 630 kb | 28 | [72] | |
| KW | qKW7 | 7 | 7H-16–7F-5 | 647 kb | 4 | [73] | |
| KNPR | qKNR6 | 6 | M6–M8 | 110 kb | 2 | [74] | |
| KNPR | qKNPR6 | 6 | N6M19–umc1257 | 198 kb | 6 | [75] | |
| KNPR | qKN | 10 | bnlg1360–umc1645 | 480 kb | 1 | [76] | |
| KRN | KRN1.4 | 1 | umc1737–cic001 | 203 kb | 7 | [77] | |
| KRN | qKRN5b | 5 | umc1365–umc2512 | 147.2 kb | 3 | [78] | |
| KRN | krn1 | 1 | SNP1–SNP2 | 6.6 kb | 1 | [79] | |
| KRN | KRN4 | 4 | M6–M8 | 3 kb | 2 | [80] | |
| KL | qKL1.07 | 1 | ML194–ML162 | 1.6 Mb | 1 | [81] | |
| KL | qKL9 | 9 | C9-54–C9-58 | 942 kb | 24 | [82] | |
| KL | qKL-2 | 9 | mk3106–mk3114 | 1.95 Mb | 40 | [83] | |
| KW | qKW7b | 7 | M115.8–M116.7 | 59 kb | 1 | [84] | |
| KRN | KNE4 | 4 | umc1086–M5 | 440 kb | 14 | [85] | |
| GW | qGW4.05 | 4 | ND16–ND19 | 279.6 kb | 2 | [86] | |
| GW | qhkw5-3 | 5 | InYM20–InYM36 | 125.3 kb | 6 | [87] | |
| GW | qGW1.05 | 1 | umc1601–umc1754 | 1.11 Mb | 30 | [88] | |
| GW | qKW9 | 9 | M3484–M3506 | 20 kb | 3 | [89] |
| Crop | Gene | Trait a | Annotation | Variations | Ref b |
|---|---|---|---|---|---|
| Sorghum | MSD1 | GNP | TCP-domain TF | Missense mutation in msd1-1/2 | [16] |
| MSD2 | GNP | lipoxygenase (LOX) | Nonsense mutation in msd2-1, missense mutation in msd2-2, nonsense mutation in msd2-3 | [18] | |
| MSD3 | GNP | ω-3 fatty acid desaturase enzyme | in msd3-2, nonsense mutation in msd3-3, alternative splicing in msd3-1 and msd3-4 | [17] | |
| qTGW1a | GW | G-protein γ subunit | 5 bp insertion, frame shift | [43] | |
| Maize | ZmCEP1 | KS | C-terminal encoded peptide | Two frameshift mutations (1 bp insertion, 1 bp deletion) in zmcep1 | [134] |
| KNR6 | KNPR | Protein kinase | substitution mutations | [74] | |
| ZmNPF7.9 | KS | Nitrate transporter | Single nucleotide mutation (G to A) | [135] | |
| ids1/Ts6 | KRN | AP2-domain TF | 5 kb indel | [79] | |
| ZmSWEET4c | SF | Hexose transporter | Insertion in zmsweet4c | [136] | |
| ZmBAM1d | KW | CLV1/BAM receptor kinase | Insertion in zmbam1d | [137] | |
| qKW9 | KW | DYW-PPR protein | Deletion in qkw9 | [89] | |
| PPR2263 | KS | DYW-PPR protein | Insertion in ppr2263 | [138] | |
| Emp4 | KS | PLS- PPR proteins | Insertions in the emp4 | [139] | |
| Emp5 | KS | PLS- PPR proteins | 1.4 kb insertion in emp5 | [140] | |
| Emp7 | KS | PLS- PPR proteins | Insertion in emp7 | [141] | |
| Emp10 | KS | P-type PPR protein | 431 bp deletion in emp10 | [142] | |
| Emp9 | KS | P-type PPR protein | Insertion in emp9 | [143] | |
| Emp11 | KS | P-type PPR protein | Insertion in emp11 | [144] | |
| Emp12 | KS | PPR protein | Insertion in emp12 | [145] | |
| Emp16 | KS | P-type PPR protein | Insertion in emp16 | [146] | |
| Dek2 | KS | P-type PPR protein | Insertion in dek2 | [147] | |
| Zmsmk9 | KS | P-type PPR protein | Frameshift mutation in zmsmk9 | [148] | |
| Dek10 | KS | E-subgroup PPR protein | 5 bp insertion in dek10 | [149] | |
| Dek35 | KS | P-type PPR protein | Insertion in dek35 | [150] | |
| Dek36 | KS | E+ subgroup PPR | Insertion in dek36 | [151] | |
| Dek37 | KS | P-type PPR protein | Insertion in dek37 | [152] | |
| Dek39 | KS | PLS-PPR protein | Nonsense mutation in dek39 | [153] | |
| Smk1 | KS | PPR-E class protein | Missense, insertions in smk1 | [154] | |
| FEA2 | KRN | Leucine-rich repeat (LRR) receptor-like protein | Expression differences in fea2 | [155] | |
| UB3 | KRN | SBP-box TF | 2 kb transposon-containing indel | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baye, W.; Xie, Q.; Xie, P. Genetic Architecture of Grain Yield-Related Traits in Sorghum and Maize. Int. J. Mol. Sci. 2022, 23, 2405. https://doi.org/10.3390/ijms23052405
Baye W, Xie Q, Xie P. Genetic Architecture of Grain Yield-Related Traits in Sorghum and Maize. International Journal of Molecular Sciences. 2022; 23(5):2405. https://doi.org/10.3390/ijms23052405
Chicago/Turabian StyleBaye, Wodajo, Qi Xie, and Peng Xie. 2022. "Genetic Architecture of Grain Yield-Related Traits in Sorghum and Maize" International Journal of Molecular Sciences 23, no. 5: 2405. https://doi.org/10.3390/ijms23052405
APA StyleBaye, W., Xie, Q., & Xie, P. (2022). Genetic Architecture of Grain Yield-Related Traits in Sorghum and Maize. International Journal of Molecular Sciences, 23(5), 2405. https://doi.org/10.3390/ijms23052405







