An Innovative Customized Biomimetic Hydrogel for Drug Screening Application Potential: Biocompatibility and Cell Invasion Ability
Abstract
:1. Introduction
2. Results
2.1. Spheroid Forming Evaluation
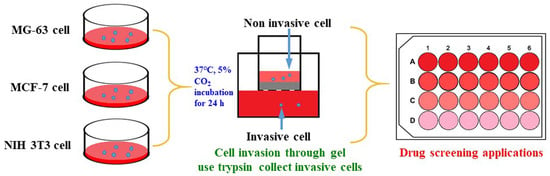
2.2. Cell Invasion Evaluation
3. Discussion
4. Materials and Methods
4.1. Materials Preparation
4.2. Cell Culture
4.3. Labeling Cells with Lipophilic Fluorescent Dyes
4.4. Cell Invasion Assay
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mimoto, R.; Yogosawa, S.; Saijo, H.; Fushimi, A.; Nogi, H.; Asakura, T.; Yoshida, K.; Takeyama, H. Clinical implications of drug-screening assay for recurrent metastatic hormone receptor-positive, human epidermal receptor 2-negative breast cancer using conditionally reprogrammed cells. Sci. Rep. 2019, 9, 13405. [Google Scholar] [CrossRef] [PubMed]
- Cavo, M.; Caria, M.; Pulsoni, I.; Beltrame, F.; Fato, M.; Scaglione, S. A new cell-laden 3D Alginate-Matrigel hydrogel resembles human breast cancer cell malignant morphology, spread and invasion capability observed “in vivo”. Sci. Rep. 2018, 8, 5333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, N.-E.; Lee, S.-H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Wang, F.; Zhang, J.; Sun, X.; Yan, Y.; Wang, Y.; Ouyang, J.; Zhang, J.; Honore, T.; Ge, J.; et al. Study on Development of Composite Hydrogels With Tunable Structures and Properties for Tumor-on-a-Chip Research. Front. Bioeng. Biotechnol. 2020, 8, 611796. [Google Scholar] [CrossRef]
- Fang, G.; Lu, H.; Law, A.; Gallego-Ortega, D.; Jin, D.; Lin, G. Gradient-sized control of tumor spheroids on a single chip. Lab Chip 2019, 19, 4093–4103. [Google Scholar] [CrossRef]
- Gong, X.; Lin, C.; Cheng, J.; Su, J.; Zhao, H.; Liu, T.; Wen, X.; Zhao, P. Generation of Multicellular Tumor Spheroids with Microwell-Based Agarose Scaffolds for Drug Testing. PLoS ONE 2015, 10, e0130348. [Google Scholar] [CrossRef] [Green Version]
- Białkowska, K.; Komorowski, P.; Bryszewska, M.; Miłowska, K. Spheroids as a Type of Three-Dimensional Cell Cultures—Examples of Methods of Preparation and the Most Important Application. Int. J. Mol. Sci. 2020, 21, 6225. [Google Scholar] [CrossRef]
- Galler, K.M.; Aulisa, L.; Regan, K.R.; D’Souza, R.N.; Hartgerink, J.D. Self-Assembling Multidomain Peptide Hydrogels: Designed Susceptibility to Enzymatic Cleavage Allows Enhanced Cell Migration and Spreading. J. Am. Chem. Soc. 2010, 132, 3217–3223. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.-C.; Huang, H.-T.; Lan, W.-C.; Huang, M.-S.; Saito, T.; Huang, B.-H.; Tsai, C.-H.; Fan, F.-Y.; Ou, K.-L. The Potential of a Tailored Biomimetic Hydrogel for In Vitro Cell Culture Applications: Characterization and Biocompatibility. Appl. Sci. 2020, 10, 9035. [Google Scholar] [CrossRef]
- E Matthew, J.; Nazario, Y.L.; Roberts, S.C.; Bhatia, S.R. Effect of mammalian cell culture medium on the gelation properties of Pluronic® F127. Biomaterials 2002, 23, 4615–4619. [Google Scholar] [CrossRef]
- Diniz, I.M.A.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F-127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2015, 26, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khattak, S.F.; Bhatia, S.R.; Roberts, S.C. Pluronic F127 as a Cell Encapsulation Material: Utilization of Membrane-Stabilizing Agents. Tissue Eng. 2005, 11, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Coyle, R.C.; Richards, D.J.; Berry, C.L.; Barrs, R.; Biggs, J.; Chou, C.J.; Trusk, T.C.; Mei, Y. Development of peptide-functionalized synthetic hydrogel microarrays for stem cell and tissue engineering applications. Acta Biomater. 2016, 45, 110–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.M.; Joung, Y.K.; Park, K.D. RGD-Conjugated Chitosan-Pluronic Hydrogels as a Cell Supported Scaffold for Articular Cartilage Regeneration. Macromol. Res. 2008, 16, 7. [Google Scholar] [CrossRef]
- Li, Y.; Kumacheva, E. Hydrogel microenvironments for cancer spheroid growth and drug screening. Sci. Adv. 2018, 4, eaas8998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shlark, R.A.; Prestwich, G.D. Attachment and spreading of fibroblasts on an RGD peptide–modified injectable hyaluronan hydrogel. J. Biomed. Mater. Res. 2004, 68A, 11. [Google Scholar]
- Morales, X.; Cortés-Domínguez, I.; Ortiz-De-Solorzano, C. Modeling the Mechanobiology of Cancer Cell Migration Using 3D Biomimetic Hydrogels. Gels 2021, 7, 17. [Google Scholar] [CrossRef]
- Boo, L.; Ho, W.Y.; Ali, N.M.; Yeap, S.K.; Ky, H.; Chan, K.G.; Yin, W.F.; Satharasinghe, D.A.; Liew, W.C.; Tan, S.W.; et al. MiRNA Transcriptome Profiling of Spheroid-Enriched Cells with Cancer Stem Cell Properties in Human Breast MCF-7 Cell Line. Int. J. Biol. Sci. 2016, 12, 427–445. [Google Scholar] [CrossRef]
- Cui, X.; Hartanto, Y.; Zhang, H. Advances in multicellular spheroids formation. J. R. Soc. Interface 2017, 14, 20160877. [Google Scholar] [CrossRef]
- Lin, R.-Z.; Chang, H.-Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef]
- Badea, M.A.; Balas, M.; Hermenean, A.; Ciceu, A.; Herman, H.; Ionita, D.; Dinischiotu, A. Influence of Matrigel on Single- and Multiple-Spheroid Cultures in Breast Cancer Research. SLAS Discov. Adv. Life Sci. R&D 2019, 24, 563–578. [Google Scholar] [CrossRef]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021, 21, 1–19. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Syam, S.; Wu, C.-J.; Lan, W.-C.; Ou, K.-L.; Huang, B.-H.; Lin, Y.-Y.; Saito, T.; Tsai, H.-Y.; Chuo, Y.-C.; Yen, M.-L.; et al. The Potential of a Surface-Modified Titanium Implant with Tetrapeptide for Osseointegration Enhancement. Appl. Sci. 2021, 11, 2616. [Google Scholar] [CrossRef]
- Perlot, R.L.; Shapiro, I.M.; Mansfield, K.; Adams, C.S. Matrix Regulation of Skeletal Cell Apoptosis II: Role of Arg-Gly-Asp-Containing Peptides. J. Bone Miner. Res. 2002, 17, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Hulkower, K.I.; Herber, R.L. Cell Migration and Invasion Assays as Tools for Drug Discovery. Pharmaceutics 2011, 3, 107–124. [Google Scholar] [CrossRef] [Green Version]
- Anguiano, M.; Morales, X.; Castilla, C.; Pena, A.R.; Ederra, C.; Martínez, M.; Ariz, M.; Esparza, M.; Amaveda, H.; Mora, M.; et al. The use of mixed collagen-Matrigel matrices of increasing complexity recapitulates the biphasic role of cell adhesion in cancer cell migration: ECM sensing, remodeling and forces at the leading edge of cancer invasion. PLoS ONE 2020, 15, e0220019. [Google Scholar] [CrossRef]
- Ravid-Hermesh, O.; Zurgil, N.; Shafran, Y.; Afrimzon, E.; Sobolev, M.; Hakuk, Y.; Eizig, Z.B.-O.; Deutsch, M. Analysis of Cancer Cell Invasion and Anti-metastatic Drug Screening Using Hydrogel Micro-chamber Array (HMCA)-based Plates. J. Vis. Exp. 2018, e58359. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Kim, K.; Kang, H. Matrigel uses in cell biology and for the identification of thymosin β4, a mediator of tissue regeneration. Appl. Biol. Chem. 2018, 61, 703–708. [Google Scholar] [CrossRef] [Green Version]
- Albini, A.; Iwamoto, Y.; Kleinman, H.K.; Martin, G.R.; A Aaronson, S.; Kozlowski, J.M.; McEwan, R.N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987, 47, 7. [Google Scholar]
- Rama-Esendagli, D.; Esendagli, G.; Yilmaz, G.; Guc, D. Spheroid formation and invasion capacity are differentially influenced by co-cultures of fibroblast and macrophage cells in breast cancer. Mol. Biol. Rep. 2014, 41, 2885–2892. [Google Scholar] [CrossRef] [PubMed]
- Ehrbar, M.; Sala, A.; Lienemann, P.; Ranga, A.; Mosiewicz, K.; Bittermann, A.; Rizzi, S.; Weber, F.; Lutolf, M. Elucidating the Role of Matrix Stiffness in 3D Cell Migration and Remodeling. Biophys. J. 2011, 100, 284–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balion, Z.; Sipailaite, E.; Stasyte, G.; Vailionyte, A.; Mazetyte-Godiene, A.; Seskeviciute, I.; Bernotiene, R.; Phopase, J.; Jekabsone, A. Investigation of Cancer Cell Migration and Proliferation on Synthetic Extracellular Matrix Peptide Hydrogels. Front. Bioeng. Biotechnol. 2020, 8, 773. [Google Scholar] [CrossRef]
- Bradshaw, M.; Ho, D.; Fear, M.; Gelain, F.; Wood, F.M.; Iyer, K.S. Designer self-assembling hydrogel scaffolds can impact skin cell proliferation and migration. Sci. Rep. 2014, 4, 6903. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, C.F.; Santos, S.; Custódio, C.A.; Mano, J.F. Human Platelet Lysates-Based Hydrogels: A Novel Personalized 3D Platform for Spheroid Invasion Assessment. Adv. Sci. 2020, 7, 1902398. [Google Scholar] [CrossRef]
- Oharazawa, H.; Ibaraki, N.; Ohara, K.; Reddy, V.N. Inhibitory Effects of Arg-Gly-Asp (RGD) Peptide on Cell Attachment and Migration in a Human Lens Epithelial Cell Line. Ophthalmic Res. 2005, 37, 191–196. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, K.-L.; Huang, C.-F.; Lan, W.-C.; Huang, B.-H.; Pan, H.-A.; Shen, Y.-K.; Saito, T.; Tsai, H.-Y.; Cho, Y.-C.; Hung, K.-S.; et al. An Innovative Customized Biomimetic Hydrogel for Drug Screening Application Potential: Biocompatibility and Cell Invasion Ability. Int. J. Mol. Sci. 2022, 23, 1488. https://doi.org/10.3390/ijms23031488
Ou K-L, Huang C-F, Lan W-C, Huang B-H, Pan H-A, Shen Y-K, Saito T, Tsai H-Y, Cho Y-C, Hung K-S, et al. An Innovative Customized Biomimetic Hydrogel for Drug Screening Application Potential: Biocompatibility and Cell Invasion Ability. International Journal of Molecular Sciences. 2022; 23(3):1488. https://doi.org/10.3390/ijms23031488
Chicago/Turabian StyleOu, Keng-Liang, Chiung-Fang Huang, Wen-Chien Lan, Bai-Hung Huang, Hsu-An Pan, Yung-Kang Shen, Takashi Saito, Hsin-Yu Tsai, Yung-Chieh Cho, Kuo-Sheng Hung, and et al. 2022. "An Innovative Customized Biomimetic Hydrogel for Drug Screening Application Potential: Biocompatibility and Cell Invasion Ability" International Journal of Molecular Sciences 23, no. 3: 1488. https://doi.org/10.3390/ijms23031488