Bioadhesion on Textured Interfaces in the Human Oral Cavity—An In Situ Study
Abstract
:1. Introduction
2. Results
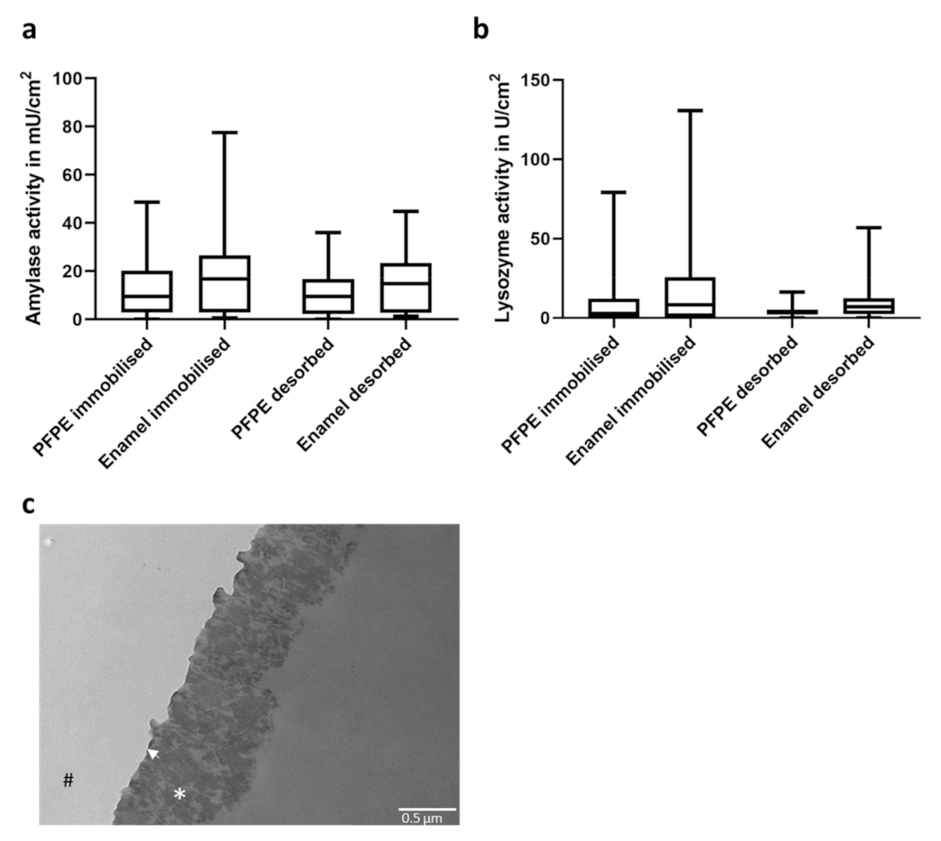
2.1. Pellicle Formation on PFPE
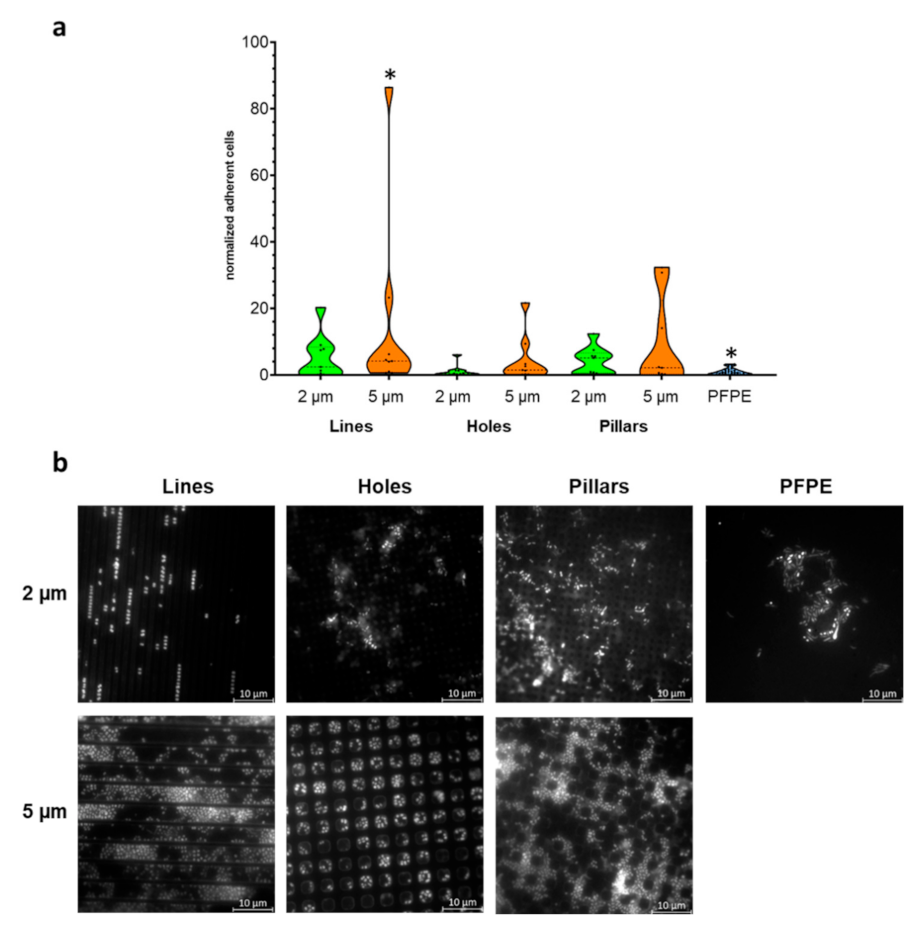
2.2. Larger Textured Surfaces Do Not Influence Biofilm Formation
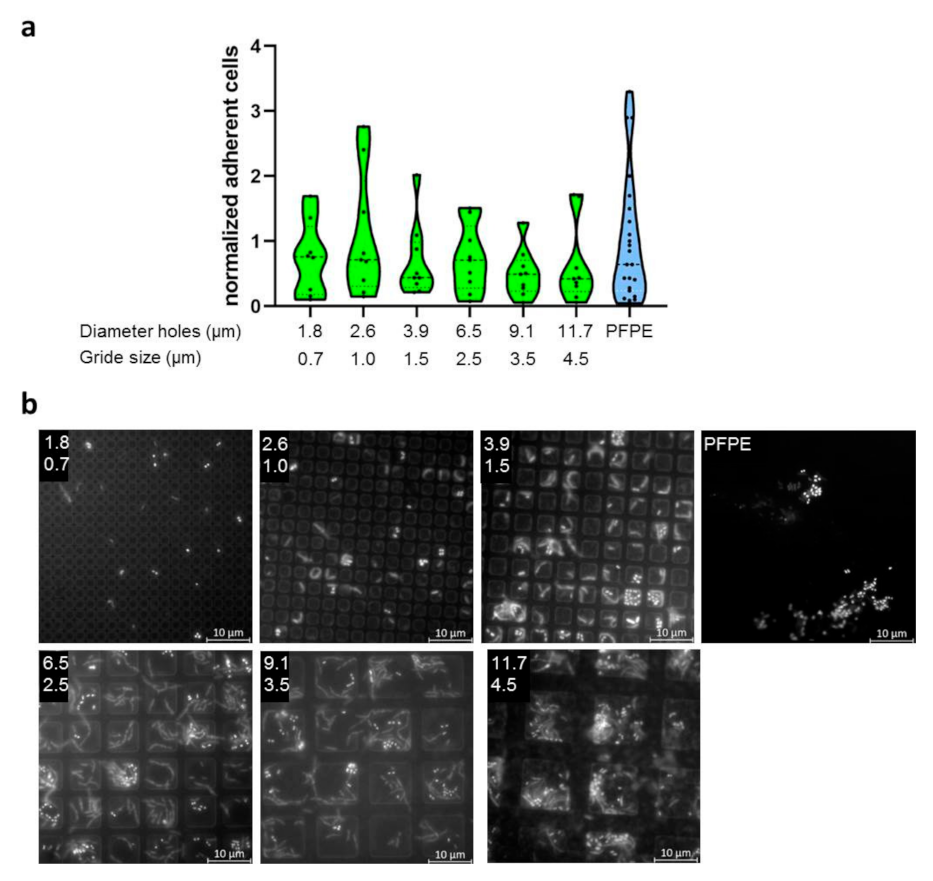
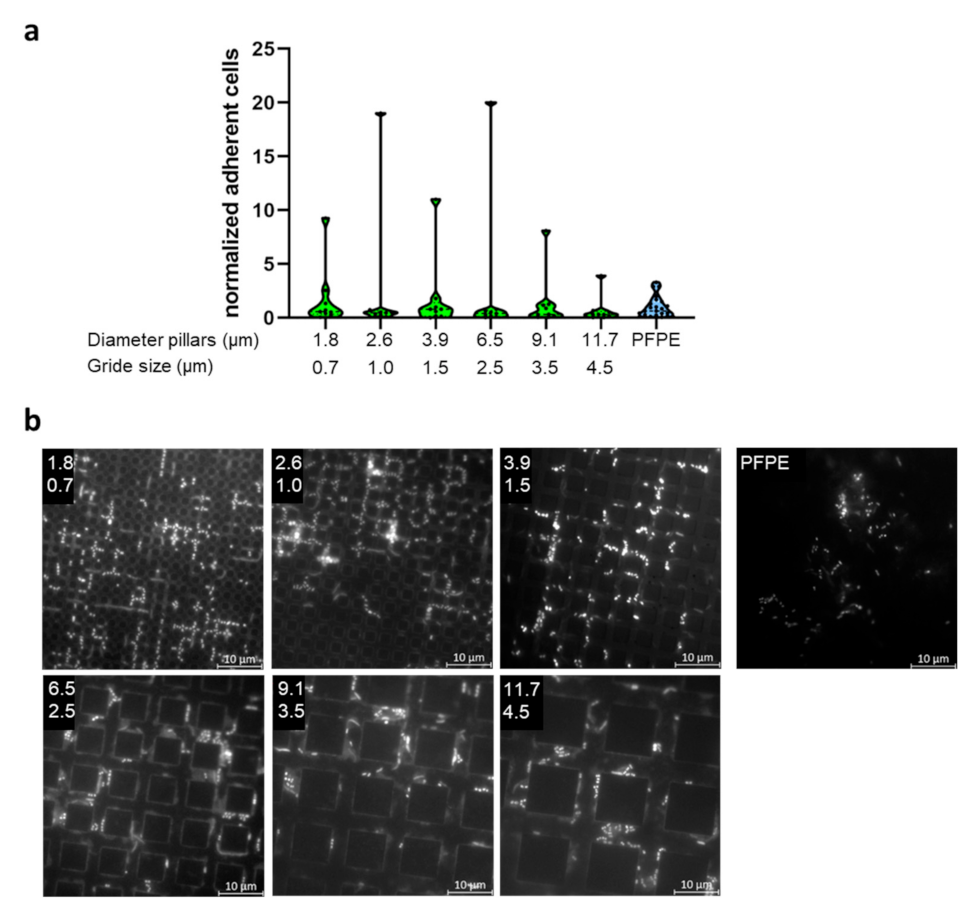
2.3. Evaluation of Bacterial Colonization with Gradient Structures
2.4. Transmission Electron Microscopy of Specimens
3. Discussion
4. Materials and Methods
4.1. Specimen Preparation
4.2. In Situ Experiments
4.3. Enzymatic Assays
4.4. Fluorescence Microscopic Assays
4.5. TEM
4.6. Statistical Analyzes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peres, M.A.; Macpherson, D.L.M.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Keller Celeste, R.; Kearns, C.; Benzian, H.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M. The oral cavity—A key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin. Oral Investig. 2009, 13, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Helbig, R.; Günther, D.; Friedrichs, J.; Rößler, F.; Lasagni, A.; Werner, C. The impact of structure dimensions on initial bacterial adhesion. Biomater. Sci. 2016, 4, 1074–1078. [Google Scholar] [CrossRef]
- Vadillo-Rodríguez, V.; Guerra-García-Mora, A.I.; Perera-Costa, D.; Gónzalez-Martín, M.L.; Fernández-Calderón, M.C. Bacterial response to spatially organized microtopographic surface patterns with nanometer scale roughness. Colloids Surf. B Biointerfaces 2018, 169, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Perera-Costa, D.; Bruque, J.M.; González-Martín, M.L.; Gómez-García, A.C.; Vadillo-Rodríguez, V. Studying the influence of surface topography on bacterial adhesion using spatially organized microtopographic surface patterns. Langmuir 2014, 30, 4633–4641. [Google Scholar] [CrossRef] [PubMed]
- Encinas, N.; Yang, C.Y.; Geyer, F.; Kaltbeitzel, A.; Baumli, P.; Reinholz, J.; Mailänder, V.; Butt, H.J.; Vollmer, D. Submicrometer-Sized Roughness Suppresses Bacteria Adhesion. ACS Appl. Mater. Interfaces 2020, 12, 21192–21200. [Google Scholar] [CrossRef]
- Lagree, K.; Mon, H.H.; Mitchell, A.P.; Ducker, W.A. Impact of surface topography on biofilm formation by Candida albicans. PLoS ONE 2018, 13, e0197925. [Google Scholar] [CrossRef]
- Hannig, M.; Joiner, A. The Structure, Function and Properties of the Acquired Pellicle. Teeth Their Environ. 2006, 19, 29–64. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. The Pellicle and Erosion. Erosive Tooth Wear 2014, 25, 206–214. [Google Scholar] [CrossRef]
- Rehage, M.; Delius, J.; Hofmann, T.; Hannig, M. Oral astringent stimuli alter the enamel pellicle’s ultrastructure as revealed by electron microscopy. J. Dent. 2017, 63, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Hannig, M.; Kensche, A.; Carpenter, G. The mucosal pellicle—An underestimated factor in oral physiology. Arch. Oral Biol. 2017, 80, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, S.; Barghash, A.; Fecher-Trost, C.; Schalkowsky, P.; Hannig, C.; Kirsch, J.; Rupf, S.; Keller, A.; Helms, V.; Hannig, M. Proteomic Analysis of the initial Oral Pellicle in Caries-Active and Caries-Free Individuals. PROTEOMICS—Clin. Appl. 2019, 13, 1800143. [Google Scholar] [CrossRef]
- McConnell, M.D.; Liu, Y.; Nowak, A.P.; Pilch, S.; Masters, J.G.; Composto, R.J. Bacterial plaque retention on oral hard materials: Effect of surface roughness, surface composition, and physisorbed polycarboxylate. J. Biomed. Mater. Res. Part A 2010, 92, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Song, C.W.; Jung, J.H.; Ahn, S.J.; Ferracane, J.L. The effects of surface roughness of composite resin on biofilm formation of Streptococcus mutans in the presence of saliva. Oper. Dent. 2012, 37, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Cheaib, Z.; Rakmathulina, E.; Lussi, A.; Eick, S. Impact of Acquired Pellicle Modification on Adhesion of Early Colonizers. Caries Res. 2015, 49, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, Y.W.; Wilson, M.; Lewis, M.; Williams, D.; Senna, P.M.; Del-Bel-Cury, A.A.; Da Silva, W.J. Salivary pellicles equalise surfaces’ charges and modulate the virulence of Candida albicans biofilm. Arch. Oral Biol. 2016, 66, 129–140. [Google Scholar] [CrossRef]
- Pita, P.P.C.; Rodrigues, J.A.; Ota-Tsuzuki, C.; Miato, T.F.; Zenobio, E.G.; Giro, G.; Figueiredo, L.C.; Gonçalves, C.; Gehrke, S.A.; Cassoni, A.; et al. Oral streptococci biofilm formation on different implant surface topographies. Biomed Res. Int. 2015, 2015, 159625. [Google Scholar] [CrossRef] [PubMed]
- Pacha-Olivenza, M.Á.; Tejero, R.; Fernández-Calderón, M.C.; Anitua, E.; Troya, M.; González-Martín, M.L. Relevance of Topographic Parameters on the Adhesion and Proliferation of Human Gingival Fibroblasts and Oral Bacterial Strains. Biomed Res. Int. 2019, 2019, 8456342. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Helbig, R.; Hilsenbeck, J.; Werner, C.; Hannig, M. Impact of the springtail’s cuticle nanotopography on bioadhesion and biofilm formation in vitro and in the oral cavity. R. Soc. Open Sci. 2018, 5, 171742. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Fackler, A.; Follo, M.; Hellwig, E.; Bächle, M.; Hannig, C.; Han, J.-S.; Wolkewitz, M.; Kohal, R. In vivo study of the initial bacterial adhesion on different implant materials. Arch. Oral Biol. 2013, 58, 1139–1147. [Google Scholar] [CrossRef]
- Zaugg, L.K.; Astasov-Frauenhoffer, M.; Braissant, O.; Hauser-Gerspach, I.; Waltimo, T.; Zitzmann, N.U. Determinants of biofilm formation and cleanability of titanium surfaces. Clin. Oral Implants Res. 2016, 28, 469–475. [Google Scholar] [CrossRef]
- Kirsch, J.; Jung, A.; Hille, K.; König, B.; Hannig, C.; Kölling-Speer, I.; Speer, K.; Hannig, M. Effect of fragaria vesca, hamamelis and tormentil on the initial bacterial colonization in situ. Arch. Oral Biol. 2020, 118, 104853. [Google Scholar] [CrossRef]
- Schulz, A.; Lang, R.; Behr, J.; Hertel, S.; Reich, M.; Kümmerer, K.; Hannig, M.; Hannig, C.; Hofmann, T. Targeted metabolomics of pellicle and saliva in children with different caries activity. Sci. Rep. 2020, 10, 697. [Google Scholar] [CrossRef]
- Kensche, A.; Dürasch, A.; König, B.; Henle, T.; Hannig, C.; Hannig, M. Characterization of the in situ pellicle ultrastructure formed under the influence of bovine milk and milk protein isolates. Arch. Oral Biol. 2019, 104, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M. Transmission electron microscopy of early plaque formation on dental materials in vivo. Eur. J. Oral Sci. 1999, 107, 55–64. [Google Scholar] [CrossRef]
- Hannig, M. Transmission electron microscopic study of in vivo pellicle formation on dental restorative materials. Eur. J. Oral Sci. 1997, 105, 422–433. [Google Scholar] [CrossRef]
- Ghavamian, S.; Hay, I.D.; Habibi, R.; Lithgow, T.; Cadarso, V.J. Three-Dimensional Micropatterning Deters Early Bacterial Adherence and Can Eliminate Colonization. ACS Appl. Mater. Interfaces 2021, 13, 23339–23351. [Google Scholar] [CrossRef]
- Carpenter, G.H. Salivary Factors that Maintain the Normal Oral Commensal Microflora. J. Dent. Res. 2020, 99, 644–649. [Google Scholar] [CrossRef]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef]
- Hannig, C.; Wasser, M.; Becker, K.; Hannig, M.; Huber, K.; Attin, T. Influence of different restorative materials on lysozyme and amylase activity of the salivary pellicle in situ. J. Biomed. Mater. Res. Part A 2006, 79, 963–973. [Google Scholar] [CrossRef]
- Hannig, C.; Huber, K.; Lambrichts, I.; Gräser, J.; D’Haen, J.; Hannig, M. Detection of salivary alpha-amylase and lysozyme exposed on the pellicle formed in situ on different materials. J. Biomed. Mater. Res. Part A 2007, 83, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M. Ultrastructural investigation of pellicle morphogenesis at two different intraoral sites during a 24-h period. Clin. Oral Investig. 1999, 3, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.S.; Han, S.; Lee, J.Y.; Oh, J.E.; Chung, S.; Park, H.D. Hydrodynamic effects on bacterial biofilm development in a microfluidic environment. Lab. Chip 2013, 13, 1846–1849. [Google Scholar] [CrossRef] [PubMed]
- Thomen, P.; Robert, J.; Monmeyran, A.; Bitbol, A.F.; Douarche, C.; Henry, N. Bacterial biofilm under flow: First a physical struggle to stay, then a matter of breathing. PLoS ONE 2017, 12, e0175197. [Google Scholar] [CrossRef] [PubMed]
- Nobbs, A.H.; Jenkinson, H.F.; Jakubovics, N.S. Stick to your gums: Mechanisms of oral microbial adherence. J. Dent. Res. 2011, 90, 1271–1278. [Google Scholar] [CrossRef]
- Trautmann, S.; Künzel, N.; Fecher-Trost, C.; Barghash, A.; Schalkowsky, P.; Dudek, J.; Delius, J.; Helms, V.; Hannig, M. Deep proteomic insights into the individual short-term pellicle formation on enamel—an in situ pilot study. PROTEOMICS—Clin. Appl. 2020, 14, e1900090. [Google Scholar] [CrossRef]
- Hannig, C.; Gaeding, A.; Basche, S.; Richter, G.; Helbig, R.; Hannig, M. Effect of conventional mouthrinses on initial bioadhesion to enamel and dentin in situ. Caries Res. 2013, 47, 150–161. [Google Scholar] [CrossRef]
- Hannig, C.; Attin, T.; Hannig, M.; Henze, E.; Brinkmann, K.; Zech, R. Immobilisation and activity of human α-amylase in the acquired enamel pellicle. Arch. Oral Biol. 2004, 49, 469–475. [Google Scholar] [CrossRef]
- Hannig, C.; Spitzmüller, B.; Hannig, M. Characterisation of lysozyme activity in the in situ pellicle using a fluorimetric assay. Clin. Oral Investig. 2009, 13, 15–21. [Google Scholar] [CrossRef]
- Kirsch, J.; Hannig, M.; Winkel, P.; Basche, S.; Leis, B.; Pütz, N.; Kensche, A.; Hannig, C. Influence of pure fluorides and stannous ions on the initial bacterial colonization in situ. Sci. Rep. 2019, 9, 18499. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Follo, M.; Selzer, A.C.; Hellwig, E.; Hannig, M.; Hannig, C. Bacterial colonization of enamel in situ investigated using fluorescence in situ hybridization. J. Med. Microbiol. 2009, 58, 1359–1366. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C.C.; De With, G. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.X.; Yuan, X.Y.; Bai, S.; Sun, P.C.; Zhao, Y.H.; Zhu, K.Y.; Ren, L.X.; Li, X.H. Antifogging and antibacterial properties of amphiphilic coatings based on zwitterionic copolymers. Sci. China Technol. Sci. 2021, 64, 817–826. [Google Scholar] [CrossRef]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef]
- Youngblood, J.P.; Andruzzi, L.; Ober, C.K.; Hexemer, A.; Kramer, E.J.; Callow, J.A.; Finlay, J.A.; Callow, M.E. Coatings based on side-chain ether-linked poly(ethylene glycol) and fluorocarbon polymers for the control of marine biofouling. Biofouling 2003, 19 (Suppl. 1), 91–98. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. Nanomaterials in preventive dentistry. Nat. Nanotechnol. 2010, 5, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M.; Kriener, L.; Hoth-Hannig, W.; Becker-Willinger, C.; Schmidt, H. Influence of nanocomposite surface coating on biofilm formation in situ. J. Nanosci. Nanotechnol. 2007, 7, 4642–4648. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helbig, R.; Hannig, M.; Basche, S.; Ortgies, J.; Killge, S.; Hannig, C.; Sterzenbach, T. Bioadhesion on Textured Interfaces in the Human Oral Cavity—An In Situ Study. Int. J. Mol. Sci. 2022, 23, 1157. https://doi.org/10.3390/ijms23031157
Helbig R, Hannig M, Basche S, Ortgies J, Killge S, Hannig C, Sterzenbach T. Bioadhesion on Textured Interfaces in the Human Oral Cavity—An In Situ Study. International Journal of Molecular Sciences. 2022; 23(3):1157. https://doi.org/10.3390/ijms23031157
Chicago/Turabian StyleHelbig, Ralf, Matthias Hannig, Sabine Basche, Janis Ortgies, Sebastian Killge, Christian Hannig, and Torsten Sterzenbach. 2022. "Bioadhesion on Textured Interfaces in the Human Oral Cavity—An In Situ Study" International Journal of Molecular Sciences 23, no. 3: 1157. https://doi.org/10.3390/ijms23031157




