How Functional Lipids Affect the Structure and Gating of Mechanosensitive MscS-like Channels
Abstract
:1. Introduction
2. MscS and MscS-like Channels
2.1. Architecture of MscS
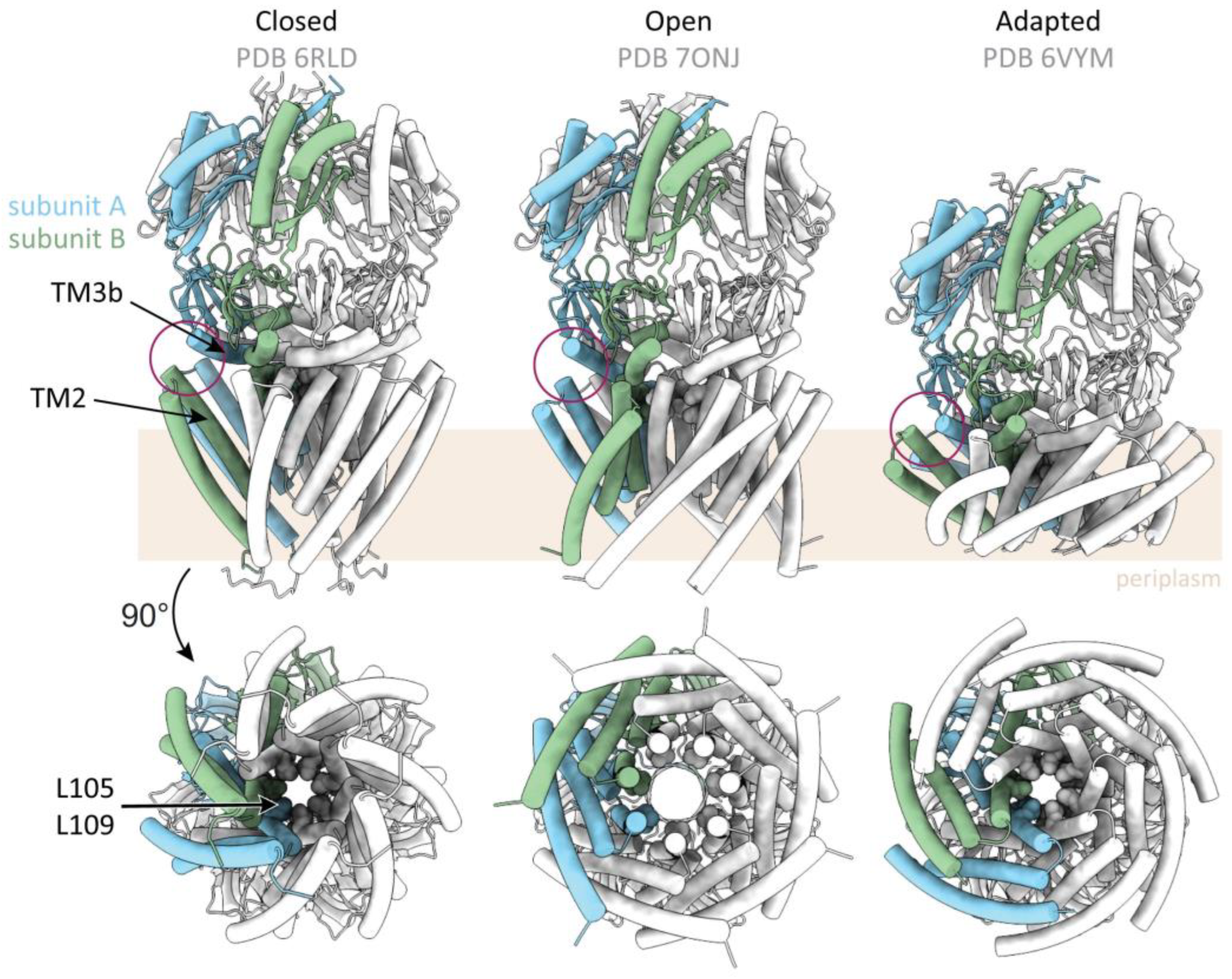
2.2. Structure of YnaI and Further Larger MscS-like Homologs
| Channel | Organism | # TM Helices/SU | # Amino Acids/SU | Conductance (nS) | PDB | EMDB | Reconstitution | Putative State | Observed Lipids | Mutant? | Struct. Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MscS | Helicobacter pylori | 3 | 274 | n.r. | 4HW9 | - | DDM | Closed | - | - | [33] |
| MscS | Thermoanaerobacter tencongensis | 3 | 282 | 0.05–0.13 [29,52] | 3T9N | - | Triton-X100 | Closed | - | - | [29] |
| MscS | Escherichia coli | 3 | 286 | 1.25 [28,39] | 2OAU | - | Fos-choline 14 | Closed | - | - | [26,27] |
| 2VV5 | - | Fos-choline 14 | Open | - | A106V | [37] | |||||
| 4AGF | - | DDM | Open | - | L124C | [53] | |||||
| 4HWA | - | DDM | Open | - | - | [33] | |||||
| 5AJI | - | DDM | Open | Aliphatic chains | D67C | [32] | |||||
| 6RLD | 4919 | MSP1E3D1 + Azolecin | Closed | 3 per SU + aliphatic chains | - | [16] | |||||
| 6PWN | 20508 | MSP1E3D1 + PC:PG (4:1) | Closed | 2 per SU + aliphatic chains | - | [17] | |||||
| 6UZH | 20959 | Peptidiscs | Closed | - | - | [54] | |||||
| 6VYK | 2146 | MSP1E3D1 + PC-18:1 | Closed | 1 per SU + aliphatic chains | - | [19] | |||||
| 6VYL | 21463 | MSP1E3D1 + PC-10 | Subconduct. | - | - | [19] | |||||
| 6VYM | 21464 | MSP1E3D1 + PC-18:1 + βCD | Adapted (inactivated) | - | - | [19] | |||||
| 7OO0 | 13003 | DDM | Open | 1 per SU + DDM | - | [18] | |||||
| 7OO6 | 13006 | DDM + Azolectin | Closed | 1 per SU + DDM | - | [18] | |||||
| 7ONJ | 12996 | LMNG | Open | 1 per SU + DDM + LMNG | - | [20] | |||||
| YnaI | Escherichia coli | 5 | 343 | 0.1 [53] | 5Y4O | 6805 | Amphipols A8-35 | Closed | - | [56] | |
| 6ZYD | 11557 | DIBMA | Closed | 1 per SU | - | [23] | |||||
| 6ZYE | 11560 | DIBMA + LPC | Subcond. /unknown | - | - | [23] | |||||
| 6URT | 20862 | LMNG | Closed | 1 per SU | - | [24] | |||||
| 7N4T | 24177 | SMA2000 | Closed | 1 per SU | - | [25] | |||||
| MSL1 | Arabidopsis thaliana | 5 | 497 | 1.2 [64] | 6LYP | 30017 | digitonin | Closed | - | - | [26] |
| 6VXM | 21444 | glyco-diosgenin | Closed | n.m. | - | [27] | |||||
| 6VXN | 21445 | glyco-diosgenin | Open | n.m. | A320V | [27] | |||||
| 6VXP | 21447 | MSP2NS + soybean polar lipid extract | Closed | n.m. | - | [27] | |||||
| FLYC1 | Dionaea muscipula | 6 | 753 | 0.16–0.27 [28,57] | 7N5D | 24186 | glyco-diosgenin | (not fully) open | n.m. | - | [28] |
| 7N5F | 24188 | glyco-diosgenin | “down” | - | - | [28] | |||||
| 7N5G | 24189 | glyco-diosgenin | “up” | - | - | [28] |
3. The Force-From-Lipids Principle
4. Coordination of Lipids in MscS and YnaI
4.1. Pocket Lipids
4.2. The ‘Hook’ Lipid
4.3. Pore Lipids
5. State-of-the-Art Models for Lipid-Driven Gating of MscS
5.1. The Hook Lipid as Gating Initiator
5.2. Gating Initiation by Pocket Lipid Removal
6. Modulation of Membrane Properties
6.1. Bilayer Thickness
6.2. Polyunsaturated Fatty Acids
6.3. Cardiolipin
6.4. Cholesterol and Membrane Stiffness
6.5. Lysophosphatidylcholine and Other Amphipathic Compounds
7. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinac, B. Mechanosensitive ion channels: An evolutionary and scientific tour de force in mechanobiology. Channels 2012, 6, 211–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murthy, S.; Dubin, A.E.; Patapoutian, A. Piezos thrive under pressure: Mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Brohawn, S.G.; Campbell, E.B.; MacKinnon, R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature 2014, 516, 126–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, W.; Valdebenito, B.; Caballero, J.; Riadi, G.; Riedelsberger, J.; Martínez, G.; Ramírez, D.; Zúñiga, L.; Sepúlveda, F.V.; Dreyer, I.; et al. K2P channels in plants and animals. Pflügers Arch.-Eur. J. Physiol. 2014, 467, 1091–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef]
- Berrier, C.; Coulombe, A.; Houssin, C.; Ghazi, A. A patch-clamp study of ion channels of inner and outer membranes and of contact zones of E. coli, fused into giant liposomes. Pressure-activated channels are localized in the inner membrane. FEBS Lett. 1989, 259, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Delcour, A.; Martinac, B.; Adler, J.; Kung, C. Modified reconstitution method used in patch-clamp studies of Escherichia coli ion channels. Biophys. J. 1989, 56, 631–636. [Google Scholar] [CrossRef] [Green Version]
- Martinac, B.; Buechner, M.; Delcour, A.H.; Adler, J.; Kung, C. Pressure-sensitive ion channel in Escherichia coli. Proc. Natl. Acad. Sci. USA 1987, 84, 2297–2301. [Google Scholar] [CrossRef] [Green Version]
- Sukharev, S.; Martinac, B.; Arshavsky, V.; Kung, C. Two types of mechanosensitive channels in the Escherichia coli cell envelope: Solubilization and functional reconstitution. Biophys. J. 1993, 65, 177–183. [Google Scholar] [CrossRef]
- Booth, I.R.; Blount, P. The MscS and MscL Families of Mechanosensitive Channels Act as Microbial Emergency Release Valves. J. Bacteriol. 2012, 194, 4802–4809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, Y.; Yoshimura, K.; Iida, H. Organellar mechanosensitive channels in fission yeast regulate the hypo-osmotic shock response. Nat. Commun. 2012, 3, 1020. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, Y.; Hirata, A.; Iida, H. Mechanosensitive channels Msy1 and Msy2 are required for maintaining organelle integrity upon hypoosmotic shock in Schizosaccharomyces pombe. FEMS Yeast Res. 2014, 14, 992–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, C.D.; Bavi, N.; Martinac, B. Bacterial Mechanosensors. Annu. Rev. Physiol. 2018, 80, 71–93. [Google Scholar] [CrossRef] [PubMed]
- Haswell, E.S.; Phillips, R.; Rees, D.C. Mechanosensitive Channels: What Can They Do and How Do They Do It? Structure 2011, 19, 1356–1369. [Google Scholar] [CrossRef] [Green Version]
- Sidarta, M.; Baruah, L.; Wenzel, M. Roles of Bacterial Mechanosensitive Channels in Infection and Antibiotic Susceptibility. Pharmaceuticals 2022, 15, 770. [Google Scholar] [CrossRef]
- Kakuda, T.; Koide, Y.; Sakamoto, A.; Takai, S. Characterization of two putative mechanosensitive channel proteins of Campylobacter jejuni involved in protection against osmotic downshock. Vet. Microbiol. 2012, 160, 53–60. [Google Scholar] [CrossRef]
- Williamson, D.R.; Dewan, K.K.; Patel, T.; Wastella, C.M.; Ning, G.; Kirimanjeswara, G.S. A Single Mechanosensitive Channel Protects Francisella tularensis subsp. holarctica from Hypoosmotic Shock and Promotes Survival in the Aquatic Environment. Appl. Environ. Microbiol. 2018, 84, e02203-17. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, T.; Flegler, V.J.; Rasmussen, A.; Böttcher, B. Structure of the Mechanosensitive Channel MscS Embedded in the Membrane Bilayer. J. Mol. Biol. 2019, 431, 3081–3090. [Google Scholar] [CrossRef]
- Reddy, B.; Bavi, N.; Lu, A.; Park, Y.; Perozo, E. Molecular basis of force-from-lipids gating in the mechanosensitive channel MscS. eLife 2019, 8, e50486. [Google Scholar] [CrossRef]
- Flegler, V.J.; Rasmussen, A.; Borbil, K.; Boten, L.; Chen, H.-A.; Deinlein, H.; Halang, J.; Hellmanzik, K.; Löffler, J.; Schmidt, V.; et al. Mechanosensitive channel gating by delipidation. Proc. Natl. Acad. Sci. USA 2021, 118, e2107095118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Daday, C.; Gu, R.-X.; Cox, C.D.; Martinac, B.; de Groot, B.L.; Walz, T. Visualization of the mechanosensitive ion channel MscS under membrane tension. Nature 2021, 590, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Flegler, V.J.; Rasmussen, A.; Rao, S.; Wu, N.; Zenobi, R.; Sansom, M.S.P.; Hedrich, R.; Rasmussen, T.; Böttcher, B. The MscS-like channel YnaI has a gating mechanism based on flexible pore helices. Proc. Natl. Acad. Sci. USA 2020, 117, 28754–28762. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Zheng, H. Mechanosensitive channel YnaI has lipid-bound extended sensor paddles. Commun. Biol. 2021, 4, 602. [Google Scholar] [CrossRef] [PubMed]
- Catalano, C.; Ben-Hail, D.; Qiu, W.; Blount, P.; Georges, A.D.; Guo, Y. Cryo-EM Structure of Mechanosensitive Channel YnaI Using SMA2000: Challenges and Opportunities. Membranes 2021, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Wang, J.; Liu, X.; Zhang, W.; Sun, L. Structural Insights into a Plant Mechanosensitive Ion Channel MSL1. Cell Rep. 2020, 30, 4518–4527.e3. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Maksaev, G.; Schlegel, A.M.; Zhang, J.; Rau, M.; Fitzpatrick, J.A.J.; Haswell, E.S.; Yuan, P. Structural mechanism for gating of a eukaryotic mechanosensitive channel of small conductance. Nat. Commun. 2020, 11, 3690. [Google Scholar] [CrossRef]
- Jojoa-Cruz, S.; Saotome, K.; Tsui, C.C.A.; Lee, W.-H.; Sansom, M.S.P.; Murthy, S.E.; Patapoutian, A.; Ward, A.B. Structural insights into the Venus flytrap mechanosensitive ion channel Flycatcher1. Nat. Commun. 2022, 13, 850. [Google Scholar] [CrossRef]
- Bass, R.B.; Strop, P.; Barclay, M.; Rees, D.C. Crystal Structure of Escherichia coli MscS, a Voltage-Modulated and Mechanosensitive Channel. Science 2002, 298, 1582–1587. [Google Scholar] [CrossRef] [Green Version]
- Steinbacher, S.; Bass, R.; Strop, P.; Rees, D.C. Structures of the Prokaryotic Mechanosensitive Channels MscL and MscS. Curr. Top. Membr. 2007, 58, 1–24. [Google Scholar] [CrossRef]
- Sukharev, S. Purification of the Small Mechanosensitive Channel of Escherichia coli (MscS): The Subunit Structure, Conduction, and Gating Characteristicsin Liposomes. Biophys. J. 2002, 83, 290–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, J.; Feng, Y.; Ge, J.; Li, W.; Sun, W.; Iscla, I.; Yu, J.; Blount, P.; Li, Y.; et al. Structure and molecular mechanism of an anion-selective mechanosensitive channel of small conductance. Proc. Natl. Acad. Sci. USA 2012, 109, 18180–18185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, C.D.; Nomura, T.; Ziegler, C.S.; Campbell, A.; Wann, K.; Martinac, B. Selectivity mechanism of the mechanosensitive channel MscS revealed by probing channel subconducting states. Nat. Commun. 2013, 4, 2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumann, U.; Edwards, M.D.; Li, C.; Booth, I.R. The conserved carboxy-terminus of the MscS mechanosensitive channel is not essential but increases stability and activity. FEBS Lett. 2004, 572, 233–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pliotas, C.; Dahl, A.C.E.; Rasmussen, T.; Mahendran, K.R.; Smith, T.K.; Marius, P.; Gault, J.; Banda, T.; Rasmussen, A.; Miller, S.; et al. The role of lipids in mechanosensation. Nat. Struct. Mol. Biol. 2015, 22, 991–998. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.Y.; Poon, Y.S.; Kaiser, J.T.; Rees, D.C. Open and shut: Crystal structures of the dodecylmaltoside solubilized mechanosensitive channel of small conductance from Escherichia coli and Helicobacter pylori at 4.4 Å and 4.1 Å resolutions. Protein Sci. 2013, 22, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Sotomayor, M.; van der Straaten, T.A.; Ravaioli, U.; Schulten, K. Electrostatic Properties of the Mechanosensitive Channel of Small Conductance MscS. Biophys. J. 2006, 90, 3496–3510. [Google Scholar] [CrossRef] [Green Version]
- Sotomayor, M.; Schulten, K. Molecular Dynamics Study of Gating in the Mechanosensitive Channel of Small Conductance MscS. Biophys. J. 2004, 87, 3050–3065. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, T.; Rasmussen, A.; Yang, L.; Kaul, C.; Black, S.; Galbiati, H.; Conway, S.J.; Miller, S.; Blount, P.; Booth, I.R. Interaction of the Mechanosensitive Channel, MscS, with the Membrane Bilayer through Lipid Intercalation into Grooves and Pockets. J. Mol. Biol. 2019, 431, 3339–3352. [Google Scholar] [CrossRef]
- Wang, W.; Black, S.S.; Edwards, M.D.; Miller, S.; Morrison, E.L.; Bartlett, W.; Dong, C.; Naismith, J.H.; Booth, I.R. The Structure of an Open Form of an E. coli Mechanosensitive Channel at 3.45 A Resolution. Science 2008, 321, 1179–1183. [Google Scholar] [CrossRef]
- Koprowski, P.; Kubalski, A. Voltage-independent Adaptation of Mechanosensitive Channels in Escherichia coli Protoplasts. J. Membr. Biol. 1998, 164, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Levina, N.; Totemeyer, S.; Stokes, N.R.; Louis, P.; Jones, M.; Booth, I.R. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: Identification of genes required for MscS activity. EMBO J. 1999, 18, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Akitake, B.; Anishkin, A.; Liu, N.; Sukharev, S. Straightening and sequential buckling of the pore-lining helices define the gating cycle of MscS. Nat. Struct. Mol. Biol. 2007, 14, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Grinkova, Y.V.; Denisov, I.; Sligar, S.G. Engineering extended membrane scaffold proteins for self-assembly of soluble nanoscale lipid bilayers. Protein Eng. Des. Sel. 2010, 23, 843–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayburt, T.H.; Sligar, S.G. Membrane protein assembly into Nanodiscs. FEBS Lett. 2009, 584, 1721–1727. [Google Scholar] [CrossRef] [Green Version]
- Bayburt, T.H.; Grinkova, Y.V.; Sligar, S.G. Self-Assembly of Discoidal Phospholipid Bilayer Nanoparticles with Membrane Scaffold Proteins. Nano Lett. 2002, 2, 853–856. [Google Scholar] [CrossRef]
- Bayburt, T.H.; Carlson, J.W.; Sligar, S.G. Reconstitution and Imaging of a Membrane Protein in a Nanometer-Size Phospholipid Bilayer. J. Struct. Biol. 1998, 123, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Sukharev, S.I.; Blount, P.; Martinac, B.; Kung, A.C. Mechanosensitive channels of Escherichia coli: The MscL Gene, Protein, and Activities. Annu. Rev. Physiol. 1997, 59, 633–657. [Google Scholar] [CrossRef]
- Booth, I.R. Bacterial mechanosensitive channels: Progress towards an understanding of their roles in cell physiology. Curr. Opin. Microbiol. 2014, 18, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, T.; Rasmussen, A. Bacterial Mechanosensitive Channels. In Membrane Protein Complexes: Structure and Function; Springer: Singapore, 2018; Volume 87, pp. 83–116. [Google Scholar] [CrossRef]
- Bialecka-Fornal, M.; Lee, H.J.; Phillips, R. The Rate of Osmotic Downshock Determines the Survival Probability of Bacterial Mechanosensitive Channel Mutants. J. Bacteriol. 2015, 197, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Bialecka-Fornal, M.; Lee, H.J.; DeBerg, H.A.; Gandhi, C.S.; Phillips, R. Single-Cell Census of Mechanosensitive Channels in Living Bacteria. PLoS ONE 2012, 7, e33077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, M.D.; Black, S.; Rasmussen, T.; Rasmussen, A.; Stokes, N.R.; Stephen, T.-L.; Miller, S.; Booth, I.R. Characterization of three novel mechanosensitive channel activities in Escherichia coli. Channels 2012, 6, 272–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumann, U.; Edwards, M.D.; Rasmussen, T.; Bartlett, W.; van West, P.; Booth, I.R. YbdG in Escherichia coli is a threshold-setting mechanosensitive channel with MscM activity. Proc. Natl. Acad. Sci. USA 2010, 107, 12664–12669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Moe, P.C.; Chandrasekaran, S.; Booth, I.R.; Blount, P. Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J. 2002, 21, 5323–5330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Zhang, B.; Zhang, Y.; Xu, C.-Q.; Zhuo, W.; Ge, J.; Li, J.; Gao, N.; Li, Y.; Yang, M. A binding-block ion selective mechanism revealed by a Na/K selective channel. Protein Cell 2017, 9, 629–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Procko, C.; Murthy, S.; Keenan, W.T.; Mousavi, S.A.R.; Dabi, T.; Coombs, A.; Procko, E.; Baird, L.; Patapoutian, A.; Chory, J. Stretch-activated ion channels identified in the touch-sensitive structures of carnivorous Droseraceae plants. eLife 2021, 10, e64250. [Google Scholar] [CrossRef]
- Iosip, A.L.; Böhm, J.; Scherzer, S.; Al-Rasheid, K.A.S.; Dreyer, I.; Schultz, J.; Becker, D.; Kreuzer, I.; Hedrich, R. The Venus flytrap trigger hair–specific potassium channel KDM1 can reestablish the K+ gradient required for hapto-electric signaling. PLoS Biol. 2020, 18, e3000964. [Google Scholar] [CrossRef]
- Perozo, E.; Kloda, A.; Cortes, D.M.; Martinac, B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 2002, 9, 696–703. [Google Scholar] [CrossRef]
- Perozo, E.; Cortes, D.M.; Sompornpisut, P.; Kloda, A.; Martinac, B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature 2002, 418, 942–948. [Google Scholar] [CrossRef]
- Corry, B.; Martinac, B. Bacterial mechanosensitive channels: Experiment and theory. Biochim. Biophys. Acta-Biomembr. 2008, 1778, 1859–1870. [Google Scholar] [CrossRef] [Green Version]
- Nomura, T.; Cranfield, C.G.; Deplazes, E.; Owen, D.M.; Macmillan, A.; Battle, A.R.; Constantine, M.; Sokabe, M.; Martinac, B. Differential effects of lipids and lyso-lipids on the mechanosensitivity of the mechanosensitive channels MscL and MscS. Proc. Natl. Acad. Sci. USA 2012, 109, 8770–8775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maingret, F.; Patel, A.J.; Lesage, F.; Lazdunski, M.; Honore, E. Lysophospholipids Open the Two-pore Domain Mechano-gated K+ Channels TREK-1 and TRAAK. J. Biol. Chem. 2000, 275, 10128–10133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.P.; Maksaev, G.; Jensen, G.S.; Murcha, M.W.; Wilson, M.E.; Fricker, M.; Hell, R.; Haswell, E.S.; Millar, A.H.; Sweetlove, L.J. MSL1 is a mechanosensitive ion channel that dissipates mitochondrial membrane potential and maintains redox homeostasis in mitochondria during abiotic stress. Plant J. 2016, 88, 809–825. [Google Scholar] [CrossRef] [Green Version]
- Sokabe, M.; Sachs, F.; Jing, Z. Quantitative video microscopy of patch clamped membranes stress, strain, capacitance, and stretch channel activation. Biophys. J. 1991, 59, 722–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moe, P.; Blount, P. Assessment of Potential Stimuli for Mechano-Dependent Gating of MscL: Effects of Pressure, Tension, and Lipid Headgroups. Biochemistry 2005, 44, 12239–12244. [Google Scholar] [CrossRef]
- Martinac, B.; Adler, J.; Kung, C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature 1990, 348, 261–263. [Google Scholar] [CrossRef]
- Cantor, R.S. Lateral Pressures in Cell Membranes: A Mechanism for Modulation of Protein Function. J. Phys. Chem. B 1997, 101, 1723–1725. [Google Scholar] [CrossRef]
- Gullingsrud, J.; Schulten, K. Lipid Bilayer Pressure Profiles and Mechanosensitive Channel Gating. Biophys. J. 2004, 86, 3496–3509. [Google Scholar] [CrossRef] [Green Version]
- Ridone, P.; Grage, S.L.; Patkunarajah, A.; Battle, A.R.; Ulrich, A.S.; Martinac, B. “Force-from-lipids” gating of mechanosensitive channels modulated by PUFAs. J. Mech. Behav. Biomed. Mater. 2018, 79, 158–167. [Google Scholar] [CrossRef]
- Cantor, R.S. The lateral pressure profile in membranes: A physical mechanism of general anesthesia. Toxicol. Lett. 1998, 100–101, 451–458. [Google Scholar] [CrossRef]
- Kung, C. A possible unifying principle for mechanosensation. Nature 2005, 436, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Bavi, O.; Cox, C.D.; Vossoughi, M.; Naghdabadi, R.; Jamali, Y.; Martinac, B. Influence of Global and Local Membrane Curvature on Mechanosensitive Ion Channels: A Finite Element Approach. Membranes 2016, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamill, O.P.; Martinac, B. Molecular Basis of Mechanotransduction in Living Cells. Physiol. Rev. 2001, 81, 685–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, F.; Cox, C.D.; Bavi, N.; Rohde, P.R.; Nakayama, Y.; Martinac, B. Membrane stiffness is one of the key determinants of E. coli MscS channel mechanosensitivity. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183203. [Google Scholar] [CrossRef] [PubMed]
- Bavi, N.; Cox, C.D.; Perozo, E.; Martinac, B. Toward a structural blueprint for bilayer-mediated channel mechanosensitivity. Channels 2016, 11, 91–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bavi, N.; Cortes, D.M.; Cox, C.D.; Rohde, P.R.; Liu, W.; Deitmer, J.W.; Bavi, O.; Strop, P.; Hill, A.P.; Rees, D.; et al. The role of MscL amphipathic N terminus indicates a blueprint for bilayer-mediated gating of mechanosensitive channels. Nat. Commun. 2016, 7, 11984. [Google Scholar] [CrossRef] [Green Version]
- Iscla, I.; Wray, R.; Blount, P. On the Structure of the N-Terminal Domain of the MscL Channel: Helical Bundle or Membrane Interface. Biophys. J. 2008, 95, 2283–2291. [Google Scholar] [CrossRef] [Green Version]
- Martinac, B.; Cox, C.D. Mechanosensory Transduction: Focus on Ion Channels. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017; p. 9780128096338082000. ISBN 978-0-12-809633-8. [Google Scholar]
- Yoo, J.; Cui, Q. Curvature Generation and Pressure Profile Modulation in Membrane by Lysolipids: Insights from Coarse-Grained Simulations. Biophys. J. 2009, 97, 2267–2276. [Google Scholar] [CrossRef] [Green Version]
- Phillips, R.; Ursell, T.; Wiggins, P.; Sens, P. Emerging roles for lipids in shaping membrane-protein function. Nature 2009, 459, 379–385. [Google Scholar] [CrossRef]
- Carney, J.; Powl, A.; Marius, P.; East, J.; Lee, A. Lipid interactions with bacterial channels: Fluorescence studies. Biochem. Soc. Trans. 2005, 33, 905–909. [Google Scholar] [CrossRef]
- Carney, J.; East, J.M.; Mall, S.; Marius, P.; Powl, A.M.; Wright, J.N.; Lee, A.G. Fluorescence Quenching Methods to Study Lipid-Protein Interactions. Curr. Protoc. Protein Sci. 2006, 45, 19.12.1–19.12.17. [Google Scholar] [CrossRef] [PubMed]
- Bolen, E.J.; Holloway, P.W. Quenching of tryptophan fluorescence by brominated phospholipid. Biochemistry 1990, 29, 9638–9643. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.; Edwards, M.D.; Black, S.S.; Rasmussen, A.; Miller, S.; Booth, I.R. Tryptophan in the Pore of the Mechanosensitive Channel MscS: Assessment of pore conformations by fluorescence spectroscopy. J. Biol. Chem. 2010, 285, 5377–5384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böttcher, B.; Prazak, V.; Rasmussen, A.; Black, S.S.; Rasmussen, T. The Structure of YnaI Implies Structural and Mechanistic Conservation in the MscS Family of Mechanosensitive Channels. Structure 2015, 23, 1705–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, T. How do mechanosensitive channels sense membrane tension? Biochem. Soc. Trans. 2016, 44, 1019–1025. [Google Scholar] [CrossRef]
- Zhang, X.C.; Liu, Z.; Li, J. From membrane tension to channel gating: A principal energy transfer mechanism for mechanosensitive channels. Protein Sci. 2016, 25, 1954–1964. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, T.; Rasmussen, A.; Singh, S.; Galbiati, H.; Edwards, M.D.; Miller, S.; Booth, I.R. Properties of the Mechanosensitive Channel MscS Pore Revealed by Tryptophan Scanning Mutagenesis. Biochemistry 2015, 54, 4519–4530. [Google Scholar] [CrossRef] [Green Version]
- Vásquez, V.; Sotomayor, M.; Cordero-Morales, J.; Schulten, K.; Perozo, E. A Structural Mechanism for MscS Gating in Lipid Bilayers. Science 2008, 321, 1210–1214. [Google Scholar] [CrossRef] [Green Version]
- Akitake, B.; Anishkin, A.; Sukharev, S. The “Dashpot” Mechanism of Stretch-dependent Gating in MscS. J. Gen. Physiol. 2005, 125, 143–154. [Google Scholar] [CrossRef]
- Malcolm, H.R.; Heo, Y.-Y.; Elmore, D.E.; Maurer, J.A. Defining the Role of the Tension Sensor in the Mechanosensitive Channel of Small Conductance. Biophys. J. 2011, 101, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Malcolm, H.R.; Blount, P.; Maurer, J.A. The mechanosensitive channel of small conductance (MscS) functions as a Jack-In-The Box. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Anishkin, A.; Akitake, B.; Sukharev, S. Characterization of the Resting MscS: Modeling and Analysis of the Closed Bacterial Mechanosensitive Channel of Small Conductance. Biophys. J. 2008, 94, 1252–1266. [Google Scholar] [CrossRef] [Green Version]
- Belyy, V.; Anishkin, A.; Kamaraju, K.; Liu, N.; Sukharev, S. The tension-transmitting ‘clutch’ in the mechanosensitive channel MscS. Nat. Struct. Mol. Biol. 2010, 17, 451–458. [Google Scholar] [CrossRef]
- Kamaraju, K.; Belyy, V.; Rowe, I.; Anishkin, A.; Sukharev, S. The pathway and spatial scale for MscS inactivation. J. Gen. Physiol. 2011, 138, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Edwards, M.D.; Bartlett, W.; Booth, I.R. Pore Mutations of the Escherichia coli MscS Channel Affect Desensitization but Not Ionic Preference. Biophys. J. 2008, 94, 3003–3013. [Google Scholar] [CrossRef] [Green Version]
- Martinac, B.; Bavi, N.; Ridone, P.; Nikolaev, Y.A.; Martinac, A.D.; Nakayama, Y.; Rohde, P.R.; Bavi, O. Tuning ion channel mechanosensitivity by asymmetry of the transbilayer pressure profile. Biophys. Rev. 2018, 10, 1377–1384. [Google Scholar] [CrossRef]
- Cantor, R.S. Lipid Composition and the Lateral Pressure Profile in Bilayers. Biophys. J. 1999, 76, 2625–2639. [Google Scholar] [CrossRef] [Green Version]
- Corradi, V.; Mendez-Villuendas, E.; Ingólfsson, H.I.; Gu, R.-X.; Siuda, I.; Melo, M.N.; Moussatova, A.; DeGagné, L.J.; Sejdiu, B.I.; Singh, G.; et al. Lipid–Protein Interactions Are Unique Fingerprints for Membrane Proteins. ACS Central Sci. 2018, 4, 709–717. [Google Scholar] [CrossRef]
- Lee, A.G. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta Biomembr. 2004, 1666, 62–87. [Google Scholar] [CrossRef]
- Lewis, B.A.; Engelman, D.M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J. Mol. Biol. 1983, 166, 211–217. [Google Scholar] [CrossRef]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [CrossRef] [Green Version]
- Rawicz, W.; Olbrich, K.; McIntosh, T.; Needham, D.; Evans, E. Effect of Chain Length and Unsaturation on Elasticity of Lipid Bilayers. Biophys. J. 2000, 79, 328–339. [Google Scholar] [CrossRef] [Green Version]
- Morein, S.; Andersson, A.-S.; Rilfors, L.; Lindblom, G. Wild-type Escherichia coli Cells Regulate the Membrane Lipid Composition in a “Window” between Gel and Non-lamellar Structures. J. Biol. Chem. 1996, 271, 6801–6809. [Google Scholar] [CrossRef] [Green Version]
- Romantsov, T.; Guan, Z.; Wood, J.M. Cardiolipin and the osmotic stress responses of bacteria. Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1788, 2092–2100. [Google Scholar] [CrossRef] [Green Version]
- McGarrity, J.T.; Armstrong, J.B. The effect of salt on phospholipid fatty acid composition in Escherichia coli K-12. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1975, 398, 258–264. [Google Scholar] [CrossRef]
- Romantsov, T.; Helbig, S.; Culham, D.E.; Gill, C.; Stalker, L.; Wood, J.M. Cardiolipin promotes polar localization of osmosensory transporter ProP in Escherichia coli. Mol. Microbiol. 2007, 64, 1455–1465. [Google Scholar] [CrossRef]
- Romantsov, T.; Battle, A.R.; Hendel, J.L.; Martinac, B.; Wood, J.M. Protein Localization in Escherichia coli Cells: Comparison of the Cytoplasmic Membrane Proteins ProP, LacY, ProW, AqpZ, MscS, and MscL. J. Bacteriol. 2010, 192, 912–924. [Google Scholar] [CrossRef] [Green Version]
- Berg, J.V.D.; Galbiati, H.; Rasmussen, A.; Miller, S.; Poolman, B. On the mobility, membrane location and functionality of mechanosensitive channels in Escherichia coli. Sci. Rep. 2016, 6, 32709:1–32709:11. [Google Scholar] [CrossRef] [Green Version]
- Powl, A.; East, J.M.; Lee, A.G. Anionic Phospholipids Affect the Rate and Extent of Flux through the Mechanosensitive Channel of Large Conductance MscL. Biochemistry 2008, 47, 4317–4328. [Google Scholar] [CrossRef]
- Powl, A.M.; East, J.M.; Lee, A.G. Heterogeneity in the Binding of Lipid Molecules to the Surface of a Membrane Protein: Hot Spots for Anionic Lipids on the Mechanosensitive Channel of Large Conductance MscL and Effects on Conformation. Biochemistry 2005, 44, 5873–5883. [Google Scholar] [CrossRef]
- Powl, A.M.; East, A.J.M.; Lee, A.G. Lipid–Protein Interactions Studied by Introduction of a Tryptophan Residue: The Mechanosensitive Channel MscL. Biochemistry 2003, 42, 14306–14317. [Google Scholar] [CrossRef]
- Ridone, P.; Nakayama, Y.; Martinac, B.; Battle, A.R. Patch clamp characterization of the effect of cardiolipin on MscS of E. coli. Eur. Biophys. J. 2015, 44, 567–576. [Google Scholar] [CrossRef]
- Martens, J.R.; O’Connell, K.; Tamkun, M. Targeting of ion channels to membrane microdomains: Localization of KV channels to lipid rafts. Trends Pharmacol. Sci. 2004, 25, 16–21. [Google Scholar] [CrossRef]
- Chen, Z.; Rand, R. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys. J. 1997, 73, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Saeedimasine, M.; Montanino, A.; Kleiven, S.; Villa, A. Role of lipid composition on the structural and mechanical features of axonal membranes: A molecular simulation study. Sci. Rep. 2019, 9, 8000:1–8000:12. [Google Scholar] [CrossRef] [Green Version]
- Nezil, F.; Bloom, M. Combined influence of cholesterol and synthetic amphiphillic peptides upon bilayer thickness in model membranes. Biophys. J. 1992, 61, 1176–1183. [Google Scholar] [CrossRef] [Green Version]
- Smondyrev, A.M.; Berkowitz, M.L. Structure of Dipalmitoylphosphatidylcholine/Cholesterol Bilayer at Low and High Cholesterol Concentrations: Molecular Dynamics Simulation. Biophys. J. 1999, 77, 2075–2089. [Google Scholar] [CrossRef] [Green Version]
- Needham, D.; Nunn, R. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys. J. 1990, 58, 997–1009. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Tristram-Nagle, S.; Nagle, J. Effect of cholesterol on structural and mechanical properties of membranes depends on lipid chain saturation. Phys. Rev. E 2009, 80, 21931:1–21931:12. [Google Scholar] [CrossRef]
- Dawaliby, R.; Trubbia, C.; Delporte, C.; Noyon, C.; Ruysschaert, J.-M.; Van Antwerpen, P.; Govaerts, C. Phosphatidylethanolamine Is a Key Regulator of Membrane Fluidity in Eukaryotic Cells. J. Biol. Chem. 2016, 291, 3658–3667. [Google Scholar] [CrossRef] [Green Version]
- Belyy, V.; Kamaraju, K.; Akitake, B.; Anishkin, A.; Sukharev, S. Adaptive behavior of bacterial mechanosensitive channels is coupled to membrane mechanics. J. Gen. Physiol. 2010, 135, 641–652. [Google Scholar] [CrossRef]
- Cui, C.; Smith, D.O.; Adler, J. Characterization of mechanosensitive channels in Escherichia coli cytoplasmic membrane by whole-cell patch clamp recording. J. Membr. Biol. 1995, 144, 31–42. [Google Scholar] [CrossRef]
- Corry, B.; Hurst, A.C.; Pal, P.; Nomura, T.; Rigby, P.; Martinac, B. An improved open-channel structure of MscL determined from FRET confocal microscopy and simulation. J. Gen. Physiol. 2010, 136, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, N.; Jose, M.D.; Birkner, J.P.; Walko, M.; Ingólfsson, H.I.; Dimitrova, A.; Arnarez, C.; Marrink, S.J.; Koçer, A. The activation mode of the mechanosensitive ion channel, MscL, by lysophosphatidylcholine differs from tension-induced gating. FASEB J. 2014, 28, 4292–4302. [Google Scholar] [CrossRef] [Green Version]
- Bavi, N.; Martinac, A.D.; Cortes, D.M.; Bavi, O.; Ridone, P.; Nomura, T.; Hill, A.P.; Martinac, B.; Perozo, E. Structural Dynamics of the MscL C-terminal Domain. Sci. Rep. 2017, 7, 17229:1–17229:11. [Google Scholar] [CrossRef] [Green Version]
- Strutt, R.; Hindley, J.W.; Gregg, J.; Booth, P.J.; Harling, J.D.; Law, R.V.; Friddin, M.S.; Ces, O. Activating mechanosensitive channels embedded in droplet interface bilayers using membrane asymmetry. Chem. Sci. 2021, 12, 2138–2145. [Google Scholar] [CrossRef]
- Corry, B.; Rigby, P.; Liu, Z.-W.; Martinac, B. Conformational Changes Involved in MscL Channel Gating Measured using FRET Spectroscopy. Biophys. J. 2005, 89, L49–L51. [Google Scholar] [CrossRef] [Green Version]
- Benfield, A.H.; Henriques, S.T. Mode-of-Action of Antimicrobial Peptides: Membrane Disruption vs. Intracellular Mechanisms. Front. Med. Technol. 2020, 2, 610997:1–610997:10. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Cetuk, H.; Maramba, J.; Britt, M.; Scott, A.J.; Ernst, R.K.; Mihailescu, M.; Cotten, M.L.; Sukharev, S. Differential Interactions of Piscidins with Phospholipids and Lipopolysaccharides at Membrane Interfaces. Langmuir 2020, 36, 5065–5077. [Google Scholar] [CrossRef]
- Comert, F.; Greenwood, A.; Maramba, J.; Acevedo, R.; Lucas, L.; Kulasinghe, T.; Cairns, L.S.; Wen, Y.; Fu, R.; Hammer, J.; et al. The host-defense peptide piscidin P1 reorganizes lipid domains in membranes and decreases activation energies in mechanosensitive ion channels. J. Biol. Chem. 2019, 294, 18557–18570. [Google Scholar] [CrossRef]
- Nguyen, T.; Clare, B.; Guo, W.; Martinac, B. The effects of parabens on the mechanosensitive channels of E. coli. Eur. Biophys. J. 2005, 34, 389–395. [Google Scholar] [CrossRef]
- Kamaraju, K.; Sukharev, S. The Membrane Lateral Pressure-Perturbing Capacity of Parabens and Their Effects on the Mechanosensitive Channel Directly Correlate with Hydrophobicity. Biochemistry 2008, 47, 10540–10550. [Google Scholar] [CrossRef]
- López, D.; Kolter, R. Functional microdomains in bacterial membranes. Genes Dev. 2010, 24, 1893–1902. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Kusaka, J.; Nishibori, A.; Hara, H. Lipid domains in bacterial membranes. Mol. Microbiol. 2006, 61, 1110–1117. [Google Scholar] [CrossRef]
- Nickels, J.D.; Hogg, J.; Cordner, D.; Katsaras, J. Lipid Rafts in Bacteria: Structure and Function. In Health Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids; Goldfine, H., Ed.; Springer: Cham, Switzerland, 2020; pp. 3–32. ISBN 978-3-030-15146-1. [Google Scholar] [CrossRef]
- Sáenz, J.P.; Sezgin, E.; Schwille, P.; Simons, K. Functional convergence of hopanoids and sterols in membrane ordering. Proc. Natl. Acad. Sci. USA 2012, 109, 14236–14240. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flegler, V.J.; Rasmussen, T.; Böttcher, B. How Functional Lipids Affect the Structure and Gating of Mechanosensitive MscS-like Channels. Int. J. Mol. Sci. 2022, 23, 15071. https://doi.org/10.3390/ijms232315071
Flegler VJ, Rasmussen T, Böttcher B. How Functional Lipids Affect the Structure and Gating of Mechanosensitive MscS-like Channels. International Journal of Molecular Sciences. 2022; 23(23):15071. https://doi.org/10.3390/ijms232315071
Chicago/Turabian StyleFlegler, Vanessa Judith, Tim Rasmussen, and Bettina Böttcher. 2022. "How Functional Lipids Affect the Structure and Gating of Mechanosensitive MscS-like Channels" International Journal of Molecular Sciences 23, no. 23: 15071. https://doi.org/10.3390/ijms232315071






