Zfra Inhibits the TRAPPC6AΔ-Initiated Pathway of Neurodegeneration
Abstract
:1. Introduction
2. Results
2.1. Protein Aggregation in Knockout Wwox−/− Cells and Transforming Growth Factor Beta (TGF-β) Increased the Aggregation
2.2. Neurotoxin MPP+ Upregulated TPC6AΔ for Aggregation in Neuroblastoma Cells
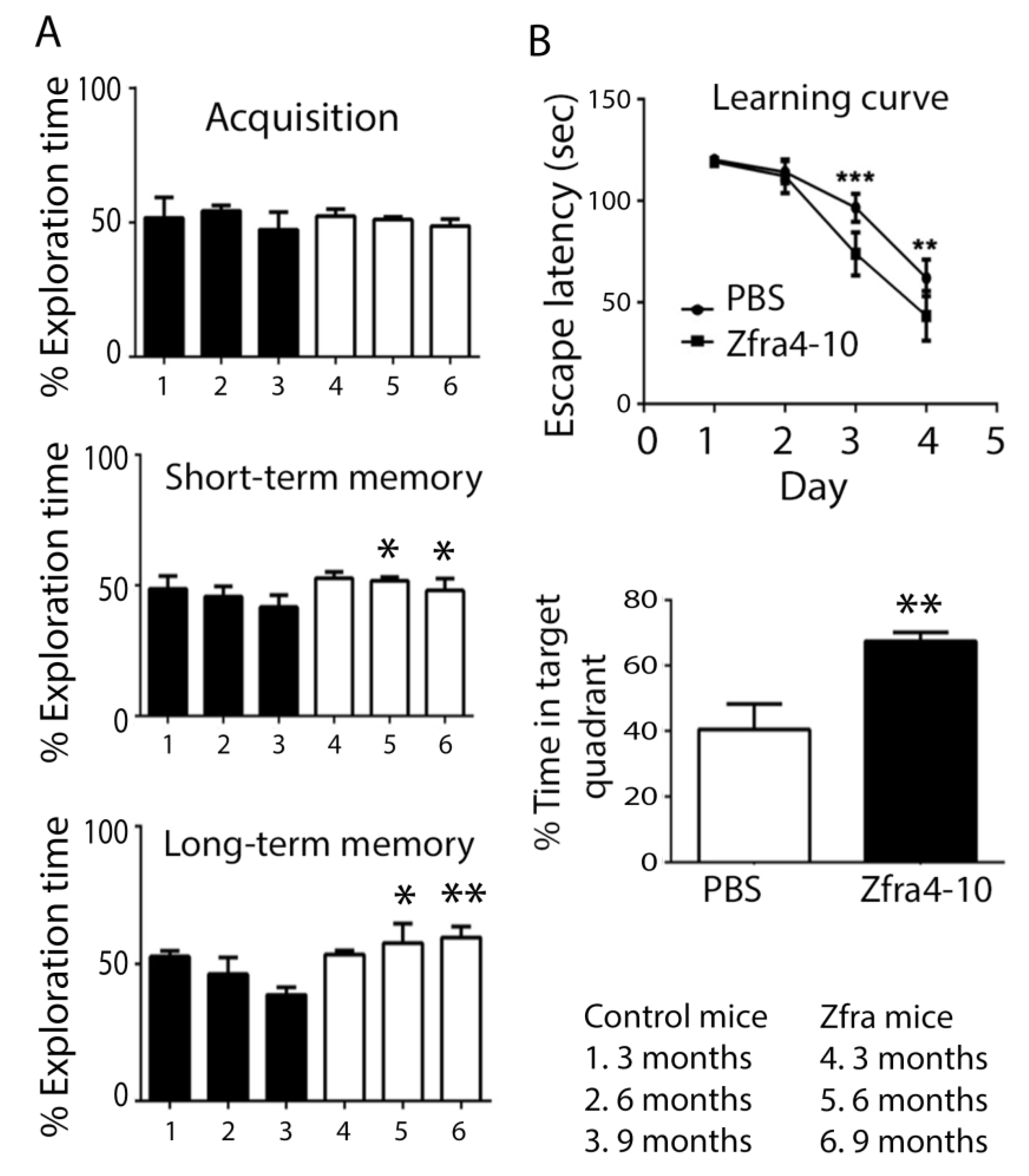
2.3. Zfra Restored the Learning and Memory Capabilities in 10-Month-Old 3xTg AD Mice
2.4. Zfra4-10 Potently Prevented Age-Dependent Memory Loss in 3xTg Mice
2.5. WWOX Deficiency Led to Protein Aggregation and Memory Loss in Mice
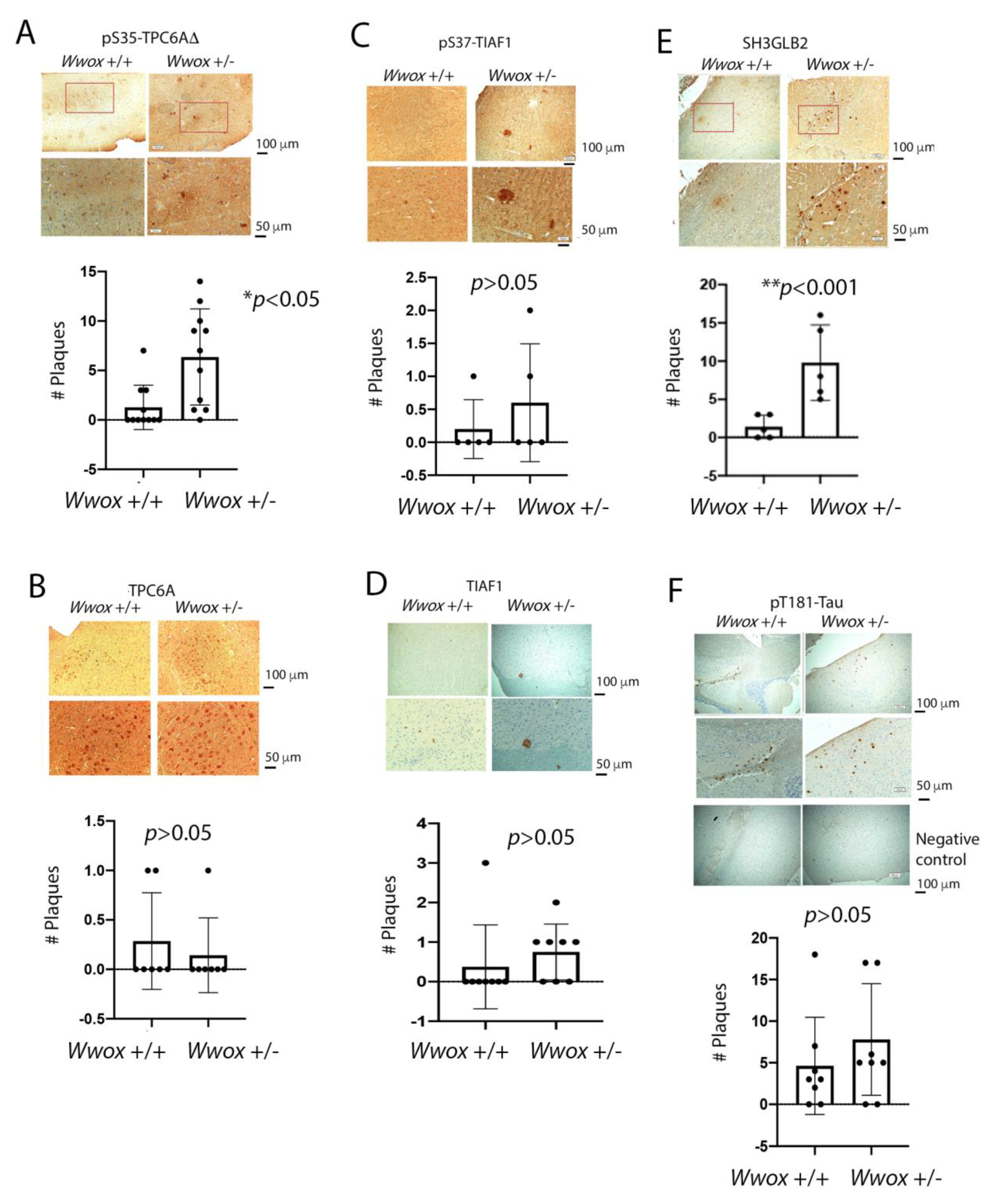
2.6. The Presence of TPC6AΔ, pS35-TPC6AΔ, and SH3GLB2 Aggregates in the Cortex of 11-Month-Old Heterozygous Wwox Mice
2.7. Identification of pT12-WWOX as Protein Aggregates in the Cortex of Old Heterozygous Wwox Mice
3. Discussion
4. Materials and Methods
4.1. Wwox Knockout Mice and Cell Lines
4.2. Synthetic Peptides
4.3. Antibodies and Western Blotting Analysis
4.4. Animals
4.5. Novel Object Recognition Test
4.6. Morris Water Maze Test
4.7. Brain Sections and Immunohistochemistry
4.8. Statistical Analysi
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, J.; Holtzman, D.M. Alzheimer disease: An update on pathobiology and treatment strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.; Perry, G. A multilevel view of the development of Alzheimer’s disease. Neuroscience 2021, 457, 283–293. [Google Scholar] [CrossRef]
- Goedert, M. Neurodegeneration. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 2015, 349, 1255555. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Cruz, L.; Cabrera, D. Transforming growth factor beta type I role in neurodegeneration: Implications for Alzheimers disease. Curr. Protein Pept. Sci. 2018, 19, 1180–1188. [Google Scholar]
- Lee, M.H.; Shih, Y.H.; Lin, S.R.; Chang, J.Y.; Lin, Y.H.; Sze, C.I.; Kuo, Y.M.; Chang, N.S. Zfra Restores Memory Deficits in Alzheimer’s Disease Triple-Transgenic Mice by Blocking Aggregation of TRAPPC6A∆, SH3GLB2, Tau, and Amyloid β, and Inflammatory NF-κB Activation. Alzheimers Dement. 2017, 3, 189–204. [Google Scholar] [CrossRef]
- Chang, N.-S.; Pratt, N.; Heath, J.; Schultz, L.; Sleve, D.; Carey, G.B.; Zevotek, N. Hyaluronidase Induction of a WW Domain- containing Oxidoreductase that Enhances Tumor Necrosis Factor Cytotoxicity. J. Biol. Chem. 2001, 276, 3361–3370. [Google Scholar] [CrossRef] [Green Version]
- Bednarek, A.K.; Laflin, K.J.; Daniel, R.L.; Liao, Q.; Hawkins, K.A.; Aldaz, C.M. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in breast cancer. Cancer Res. 2000, 60, 2140–2145. [Google Scholar]
- Ried, K.; Finnis, M.; Hobson, L.; Mangelsdorf, M.; Dayan, S.; Nancarrow, J.K.; Woollatt, E.; Kremmidiotis, G.; Gardner, A.; Venter, D.; et al. Common chromosomal fragile site FRA16D sequence: Identification of the FOR gene spanning FRA16D and homozygous deletions and translocation breakpoints in cancer cells. Hum. Mol. Genet. 2000, 9, 1651–1663. [Google Scholar] [CrossRef]
- Chang, N.-S.; Hsu, L.-J.; Lin, Y.-S.; Lai, F.-J.; Sheu, H.-M. WW Domain-Containing Oxidoreductase: A Candidate Tumor Suppressor. Trends Mol. Med. 2007, 13, 12–22. [Google Scholar] [CrossRef]
- Lahav, N.; Rotem-Bamberger, S.; Fahoum, J.; Dodson, E.; Kraus, Y.; Mousa, R.; Metanis, N.; Friedler, A.; Schueler-Furman, O. Phosphorylation of the WWOX Protein Regulates Its Interaction with p73. ChemBioChem 2020, 21, 1843–1851. [Google Scholar] [CrossRef]
- Aderca, I.; Moser, C.D.; Veerasamy, M.; Bani-Hani, A.H.; Bonilla-Guerrero, R.; Ahmed, K.; Shire, A.; Cazanave, S.C.; Montoya, D.P.; Mettler, T.A.; et al. The JNK Inhibitor SP600129 Enhances Apoptosis of HCC Cells Induced by the Tumor Suppressor WWOX. J. Hepatol. 2008, 49, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, L.J.; Schultz, L.; Hong, Q.; Van More, K.; Heath, J.; Li, M.Y.; Lai, F.J.; Lin, S.R.; Lee, M.H.; Lo, C.P.; et al. Transforming Growth Factor Beta1 Signaling via Interaction with Cell Surface Hyal-2 and Recruitment of WWOX/WOX1. J. Biol. Chem. 2009, 284, 16049–16059. [Google Scholar] [CrossRef] [PubMed]
- Bouteille, N.; Driouch, K.; Hage, P.E.; Sin, S.; Formstecher, E.; Camonis, J.; Lidereau, R.; Lallemand, F. Inhibition of the Wnt/Beta-catenin Pathway by the WWOX Tumor Suppressor Protein. Oncogene 2009, 28, 2569–2580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Odeh, M.; Salah, Z.; Herbel, C.; Hofmann, T.G.; Aqeilan, R.I. WWOX, the Common Fragile Site FRA16D Gene Product, Regulates ATM Activation and the DNA Damage Response. Proc. Natl. Acad. Sci. USA 2014, 111, E4716–E4725. [Google Scholar] [CrossRef] [Green Version]
- Abu-Remaileh, M.; Joy-Dodson, E.; Schueler-Furman, O.; Aqeilan, R.I. Pleiotropic Functions of Tumor Suppressor WWOX in Normal and Cancer Cells. J. Biol. Chem. 2015, 290, 30728–30735. [Google Scholar] [CrossRef] [Green Version]
- Saigo, C.; Kito, Y.; Takeuchi, T. Cancerous protein network that inhibits the tumor suppressor function of WW domain-containing oxidoreductase (WWOX) by aberrantly expressed molecules. Front. Oncol. 2018, 8, 350. [Google Scholar] [CrossRef]
- Hsu, L.J.; Hong, Q.Y.; Chen, S.T.; Kuo, H.L.; Schultz, L.; Heath, J.; Lin, S.R.; Lee, M.H.; Li, D.Z.; Li, Z.L.; et al. Hyaluronan Activates Hyal-2/WWOX/Smad4 Signaling and Causes Bubbling Cell Death when the Signaling Complex is Overexpressed. Oncotarget 2017, 8, 19137–19155. [Google Scholar] [CrossRef] [Green Version]
- Hsu, L.J.; Chiang, M.F.; Sze, C.I.; Su, W.P.; Yap, Y.V.; Lee, I.T.; Kuo, H.L.; Chang, N.S. HYAL-2-WWOX-SMAD4 Signaling in Cell Death and Anticancer Response. Front. Cell Dev. Biol. 2016, 4, 141. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.S.; Su, W.P.; Lin, H.P.; Kuo, H.L.; Wei, H.L.; Chang, N.S. Role of WW Domain-Containing Oxidoreductase WWOX in Driving T Cell Acute Lymphoblastic Leukemia Maturation. J. Biol. Chem. 2016, 291, 17319–17331. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-S.; Chang, N.-S. Phosphorylation/De-Phosphorylation in Specific Sites of Tumor Suppressor WWOX and Control of Distinct Biological Events. Exp. Biol. Med. 2018, 243, 137–147. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Sie, Y.-D.; Liu, T.-Y.; Kuo, H.-L.; Chou, P.-Y.; Lee, K.-T.; Chen, P.-J.; Chen, S.-T.; Chang, N.-S. Normal Cells Repel WWOX-Negative or -Dysfunctional Cancer Cells via WWOX Cell Surface Epitope 286-299. Commun. Biol. 2021, 4, 753. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.Y.; Lai, F.J.; Chen, Y.A.; Sie, Y.D.; Kuo, H.L.; Su, W.P.; Wu, C.Y.; Liu, T.Y.; Wen, K.Y.; Hsu, L.J.; et al. Strategies by which WWOX-Deficient Metastatic Cancer Cells Utilize to Survive via Dodging, Compromising, and Causing Damage to WWOX-Positive Normal Microenvironment. Cell Death Discov. 2019, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Sze, C.I.; Su, M.; Pugazhenthi, S.; Jambal, P.; Hsu, L.J.; Heath, J.; Schultz, L.; Chang, N.S. Downregulation of WOX1 Induces Tau Phosphorylation In Vitro: A Potential Role in Alzheimer’s Disease. J. Biol. Chem. 2004, 279, 30498–30506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.Y.; Lai, F.J.; Hsu, L.J.; Lo, C.P.; Cheng, C.L.; Lin, S.R.; Lee, M.H.; Chang, J.Y.; Subhan, D.; Tsai, M.S.; et al. Dramatic Coactivation of WWOX/WOX1 with CREB and NF-κB in Delayed Loss of Small Dorsal Root Ganglion Neurons upon Sciatic Nerve Transection in Rats. PLoS ONE 2009, 4, e7820. [Google Scholar] [CrossRef] [Green Version]
- Baryła, I.; Kośla, K.; Bednarek, A.K. WWOX and metabolic regulation in normal and pathological conditions. J. Mol. Med. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Ho, P.-C.; Lee, I.-T.; Chen, Y.-A.; Chu, C.-H.; Teng, C.-C.; Wu, S.-N.; Sze, C.-I.; Chiang, M.-F.; Chang, N.-S. WWOX Phosphorylation, Signaling, and Role in Neurodegeneration. Front. Neurosci. 2018, 12, 563. [Google Scholar] [CrossRef]
- Kośla, K.; Płuciennik, E.; Styczeń-Binkowska, E.; Nowakowska, M.; Orzechowska, M.; Bednarek, A.K. The WWOX Gene Influences Cellular Pathways in the Neuronal Differentiation of Human Neural Progenitor Cells. Front. Cell. Neurosci. 2019, 13, 391. [Google Scholar] [CrossRef] [Green Version]
- Aldaz, C.M.; Hussain, T. WWOX Loss of Function in Neurodevelopmental and Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 8922. [Google Scholar] [CrossRef]
- Carvalho, C.; Correia, S.C.; Seiça, R.; Moreira, P.I. WWOX inhibition by Zfra1-31 restores mitochondrial homeostasis and viability of neuronal cells exposed to high glucose. Cell. Mol. Life Sci. 2022, 79, 487. [Google Scholar] [CrossRef]
- Steinberg, D.J.; Aqeilan, R.I. WWOX-Related Neurodevelopmental Disorders: Models and Future Perspectives. Cells 2021, 10, 3082. [Google Scholar] [CrossRef]
- Chou, P.Y.; Lin, S.R.; Lee, M.H.; Schultz, L.; Sze, C.I.; Chang, N.S. A p53/TIAF1/WWOX Triad Exerts Cancer Suppression but May Cause Brain Protein Aggregation due to p53/WWOX Functional Antagonism. Cell Commun. Signal 2019, 17, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldaz, C.M.; Ferguson, B.W.; Abba, M.C. WWOX at the Crossroads of Cancer, Metabolic Syndrome Related Traits and CNS Pathologies. Biochim. Biophys. Acta Bioenergy 2014, 1846, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-T.; Liu, C.-C.; Chen, S.-T.; Yap, Y.V.; Chang, N.-S.; Sze, C.-I. WW Domain-Containing Oxidoreductase in Neuronal Injury and Neurological Diseases. Oncotarget 2014, 5, 11792–11799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehaideb, S.N.; Al-Bu Ali, M.J.; Al-Obaid, J.J.; Aljassim, K.M.; Alfadhel, M. Novel Homozygous Mutation in the WWOX Gene Causes Seizures and Global Developmental Delay: Report and Review. Transl. Neurosci. 2018, 9, 203–208. [Google Scholar] [CrossRef]
- Sze, C.-I.; Wen, K.-Y.; Chang, N.-S. WWOX is a Risk Factor for Alzheimer’s Disease: How and Why? Proc. Singap. Natl. Acad. Sci. 2020, 14, 31–45. [Google Scholar] [CrossRef]
- Repudi, S.; Steinberg, D.J.; Elazar, N.; Breton, V.L.; Aquilino, M.S.; Saleem, A.; Abu-Swai, S.; Vainshtein, A.; Eshed-Eisenbach, Y.; Vijayaragavan, B.; et al. Neuronal Deletion of Wwox, Associated with WOREE Syndrome, Causes Epilepsy and Myelin Defects. Brain 2021, 144, 3061–3077. [Google Scholar] [CrossRef]
- Banne, E.; Abudiab, B.; Abu-Swai, S.; Repudi, S.; Steinberg, D.; Shatleh, D.; Alshammery, S.; Lisowski, L.; Gold, W.; Carlen, P.; et al. Neurological Disorders Associated with WWOX Germline Mutations—A Comprehensive Overview. Cells 2021, 10, 824. [Google Scholar] [CrossRef]
- Cheng, Y.-Y.; Chou, Y.-T.; Lai, F.-J.; Jan, M.-S.; Chang, T.-H.; Jou, I.-M.; Chen, P.-S.; Lo, J.-Y.; Huang, S.-S.; Chang, N.-S.; et al. Wwox Deficiency Leads to Neurodevelopmental and Degenerative Neuropathies and Glycogen Synthase Kinase 3β-Mediated Epileptic Seizure Activity in Mice. Acta Neuropathol. Commun. 2020, 8, 6. [Google Scholar] [CrossRef]
- Iacomino, M.; Baldassari, S.; Tochigi, Y.; Kośla, K.; Buffelli, F.; Torella, A.; Severino, M.; Paladini, D.; Mandarà, L.; Riva, A.; et al. Loss of Wwox Perturbs Neuronal Migration and Impairs Early Cortical Development. Front. Neurosci. 2020, 14, 644. [Google Scholar] [CrossRef]
- Chang, N.S. Editorial: Downregulation of WWOX and Inhibitory GABAergic Interneurons Correlates with Brain Inflammation during Progression of Alzheimer’s Disease. EC Neurol. 2021, 13, 8–11. [Google Scholar]
- Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; Boland, A.; Vronskaya, M.; Van Der Lee, S.J.; Amlie-Wolf, A.; et al. Genetic Meta-Analysis of Diagnosed Alzheimer’s Disease Identifies New Risk Loci and Implicates Aβ, Tau, Immunity and Lipid Processing. Nat. Genet. 2019, 51, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.H.; Lin, S.R.; Chang, J.Y.; Schultz, L.; Heath, J.; Hsu, L.J.; Kuo, Y.M.; Hong, Q.; Chiang, M.F.; Gong, C.X.; et al. TGF-Beta Induces TIAF1 Self-Aggregation via Type II Receptor-Independent Signaling that Leads to Generation of Amyloid Beta Plaques in Alzheimer’s Disease. Cell Death Dis. 2010, 1, e110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.-Y.; Chiang, M.-F.; Lin, S.-R.; Lee, M.-H.; He, H.; Chou, P.-Y.; Chen, S.-J.; Chen, Y.-A.; Yang, L.-Y.; Lai, F.-J.; et al. TIAF1 Self-Aggregation in Peritumor Capsule Formation, Spontaneous Activation of SMAD-Responsive Promoter in p53-Deficient Environment, and Cell Death. Cell Death Dis. 2012, 3, e302. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-Y.; Lee, M.-H.; Lin, S.-R.; Yang, L.-Y.; Sun, S.; Sze, C.-I.; Hong, Q.; Lin, Y.-S.; Chou, Y.-T.; Hsu, L.-J.; et al. Trafficking Protein Particle Complex 6A Delta (TRAPPC6A∆) is an Extracellular Plaque-Forming Protein in the Brain. Oncotarget 2015, 6, 3578–3589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamoud, H.S.; Ahmed, S.; Jelani, M.; Alrayes, N.; Childs, K.; Vadgama, N.; Almramhi, M.M.; Al-Aama, J.Y.; Goodbourn, S.; Nasir, J. A Missense Mutation in TRAPPC6A Leads to Build-Up of the Protein, in Patients with a Neurodevelopmental Syndrome and Dysmorphic Features. Sci. Rep. 2018, 8, 2053. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.-Y.; Chang, N.-S. WWOX Dysfunction Induces Sequential Aggregation of TRAPPC6A∆, TIAF1, Tau and Amyloid β, and Causes Apoptosis. Cell Death Discov. 2015, 1, 15003. [Google Scholar] [CrossRef] [Green Version]
- Chang, N.S. TRAPPC6AΔ is a Potential Marker for the Progression of Alzheimer’s Disease from Middle Age. EC Neurol. 2021, 13, 23–27. [Google Scholar]
- Hamilton, G.; Harris, S.E.; Davies, G.; Liewald, D.C.; Tenesa, A.; Starr, J.M.; Porteous, D.; Deary, I.J. Alzheimer’s disease genes are associated with measures of cognitive ageing in the lothian birth cohorts of 1921 and 1936. Int. J. Alzheimers Dis. 2011, 2011, 505984. [Google Scholar] [CrossRef] [Green Version]
- Vannier, C.; Pesty, A.; San-Roman, M.J.; Schmidt, A.A. The Bin/amphiphysin/Rvs (BAR) domain protein endophilin B2 interacts with plectin and controls perinuclear cytoskeletal architecture. J. Biol. Chem. 2013, 288, 27619–27637. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Chen, Y.; He, J.; Gou, H.Y.; Zhu, Y.L.; Zhu, Y.M. WGCNA combined with GSVA to explore biomarkers of refractory neocortical epilepsy. IBRO Neurosci. Rep. 2022, 13, 314–321. [Google Scholar] [CrossRef]
- Hong, Q.; Hsu, L.J.; Schultz, L.; Pratt, N.; Mattison, J.; Chang, N.S. Zfra affects TNF-mediated cell death by interacting with death domain protein TRADD and negatively regulates the activation of NF-kappaB, JNK1, p53 and WOX1 during stress response. BMC Mol. Biol. 2007, 8, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.H.; Su, W.P.; Wang, W.J.; Lin, S.R.; Lu, C.Y.; Chen, Y.A.; Chang, J.Y.; Huang, S.S.; Chou, P.Y.; Ye, S.R.; et al. Zfra Activates Memory Hyal-2+CD3-CD19- Spleen Cells to Block Cancer Growth, Stemness, and Metastasis In Vivo. Oncotarget 2015, 6, 3737–3751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, W.-P.; Wang, W.-J.; Chang, J.-Y.; Ho, P.-C.; Liu, T.-Y.; Wen, K.-Y.; Kuo, H.-L.; Chen, Y.-J.; Huang, S.-S.; Subhan, D.; et al. Therapeutic Zfra4-10 or WWOX7-21 Peptide Induces Complex Formation of WWOX with Selective Protein Targets in Organs that Leads to Cancer Suppression and Spleen Cytotoxic Memory Z Cell Activation In Vivo. Cancers 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Ho, P.C.; Nagarajan, G.; Chen, Y.A.; Kuo, H.L.; Subhan, D.; Su, W.P.; Chang, J.Y.; Lu, C.Y.; Chang, K.T.; et al. WWOX Possesses N-Terminal Cell Surface-Exposed Epitopes WWOX7-21 and WWOX7-11 for Signaling Cancer Growth Suppression and Prevention In Vivo. Cancers 2019, 11, 1818. [Google Scholar] [CrossRef] [PubMed]
- Madej, T.; Lanczycki, C.J.; Zhang, D.; Thiessen, P.A.; Geer, R.C.; Marchler-Bauer, A.; Bryant, S.H. MMDB and VAST+: Tracking structural similarities between macromolecular complexes. Nucleic Acids Res. 2014, 42, D297–D303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.Y.; Nagarajan, G.; Chiang, M.F.; Huang, S.S.; Lin, T.C.; Chen, Y.A.; Sze, C.I.; Chang, N.S. WWOX Controls Cell Survival, Immune Response and Disease Progression by pY33 to pS14 Transition to Alternate Signaling Partners. Cells 2022, 11, 2137. [Google Scholar] [CrossRef]
- Narmashiri, A.; Abbaszadeh, M.; Ghazizadeh, A. The effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on the cognitive and motor functions in rodents: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2022, 140, 104792. [Google Scholar] [CrossRef]
- Sonsalla, P.K.; Zeevalk, G.D.; German, D.C. Chronic intraventricular administration of 1-methyl-4-phenylpyridinium as a progressive model of Parkinson’s disease. Park. Relat. Disord. 2008, 14 (Suppl. 2), S116–S118. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Ma, H.; Yv, Q.; Ye, F.; He, Z.; Chen, S.; Keram, A.; Li, W.; Zhu, M. Alpha-synuclein/MPP+ mediated activation of NLRP3 inflammasome through microtubule-driven mitochondrial perinuclear transport. Biochem. Biophys. Res. Commun. 2022, 594, 161–167. [Google Scholar] [CrossRef]
- Wang, H.Y.; Juo, L.I.; Lin, Y.T.; Hsiao, M.; Lin, J.T.; Tsai, C.H.; Tzeng, Y.H.; Chuang, Y.C.; Chang, N.S.; Yang, C.N.; et al. WW Domain-Containing Oxidoreductase Promotes Neuronal Differentiation via Negative Regulation of Glycogen Synthase Kinase 3β. Cell Death Differ. 2012, 19, 1049–1059. [Google Scholar] [CrossRef] [Green Version]
- Lo, C.P.; Hsu, L.J.; Li, M.Y.; Hsu, S.Y.; Chuang, J.I.; Tsai, M.S.; Lin, S.R.; Chang, N.S.; Chen, S.T. MPP+-induced neuronal death in rats involves tyrosine 33 phosphorylation of WW domain-containing oxidoreductase WOX1. Eur. J. Neurosci. 2008, 27, 1634–1646. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.S.; Doherty, J.; Ensign, A. JNK1 physically interacts with WW domain-containing oxidoreductase (WOX1) and inhibits WOX1-mediated apoptosis. J. Biol. Chem. 2003, 278, 9195–9202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Y.Y.; Ko, C.Y.; Wang, W.J.; Wang, S.M.; Gean, P.W.; Kuo, Y.M. Astrocytic CCAAT/enhancer binding protein delta regulates neuronal viability and spatial learning ability via miR-135a. Mol. Neurobiol. 2016, 53, 4173–4188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennison, J.L.; Ricciardi, N.R.; Lohse, I.; Volmar, C.H.; Wahlestedt, C.J. Sexual Dimorphism in the 3xTg-AD Mouse Model and Its Impact on Pre-Clinical Research. Alzheimers Dis. 2021, 80, 41–52. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-H.; Shih, Y.-H.; Yap, Y.V.; Chen, Y.-W.; Kuo, H.-L.; Liu, T.-Y.; Hsu, L.-J.; Kuo, Y.-M.; Chang, N.-S. Zfra Inhibits the TRAPPC6AΔ-Initiated Pathway of Neurodegeneration. Int. J. Mol. Sci. 2022, 23, 14510. https://doi.org/10.3390/ijms232314510
Lin Y-H, Shih Y-H, Yap YV, Chen Y-W, Kuo H-L, Liu T-Y, Hsu L-J, Kuo Y-M, Chang N-S. Zfra Inhibits the TRAPPC6AΔ-Initiated Pathway of Neurodegeneration. International Journal of Molecular Sciences. 2022; 23(23):14510. https://doi.org/10.3390/ijms232314510
Chicago/Turabian StyleLin, Yu-Hao, Yao-Hsiang Shih, Ye Vone Yap, Yen-Wei Chen, Hsiang-Lin Kuo, Tsung-Yun Liu, Li-Jin Hsu, Yu-Min Kuo, and Nan-Shan Chang. 2022. "Zfra Inhibits the TRAPPC6AΔ-Initiated Pathway of Neurodegeneration" International Journal of Molecular Sciences 23, no. 23: 14510. https://doi.org/10.3390/ijms232314510





