Development of Chitosan/Gelatin-Based Hydrogels Incorporated with Albumin Particles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Protein Particles
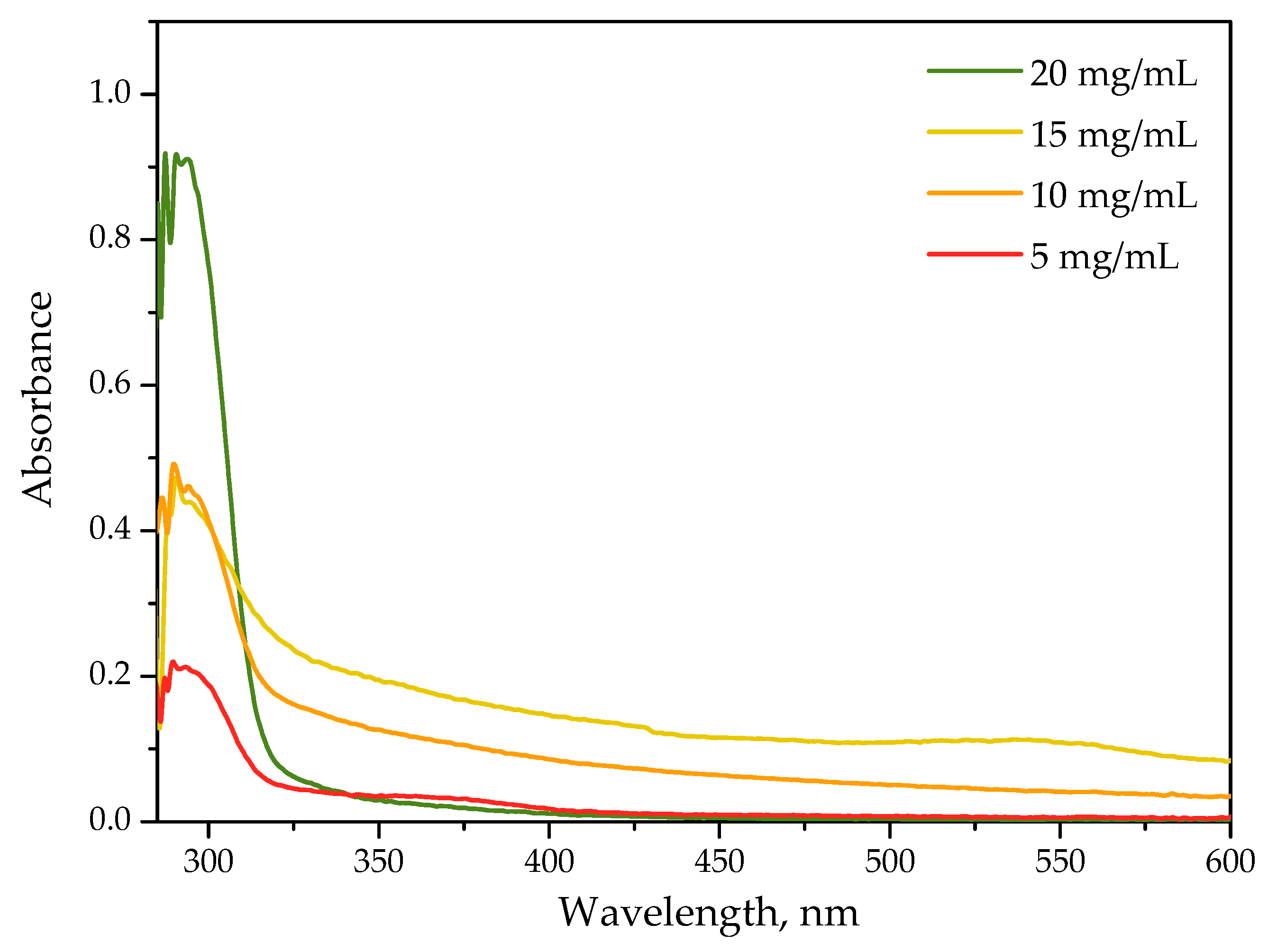
2.1.1. Optical Properties of Protein Particles Verified via the UV-Vis Spectroscopy
2.1.2. Results of Spectroscopic Analysis of the Protein Particles
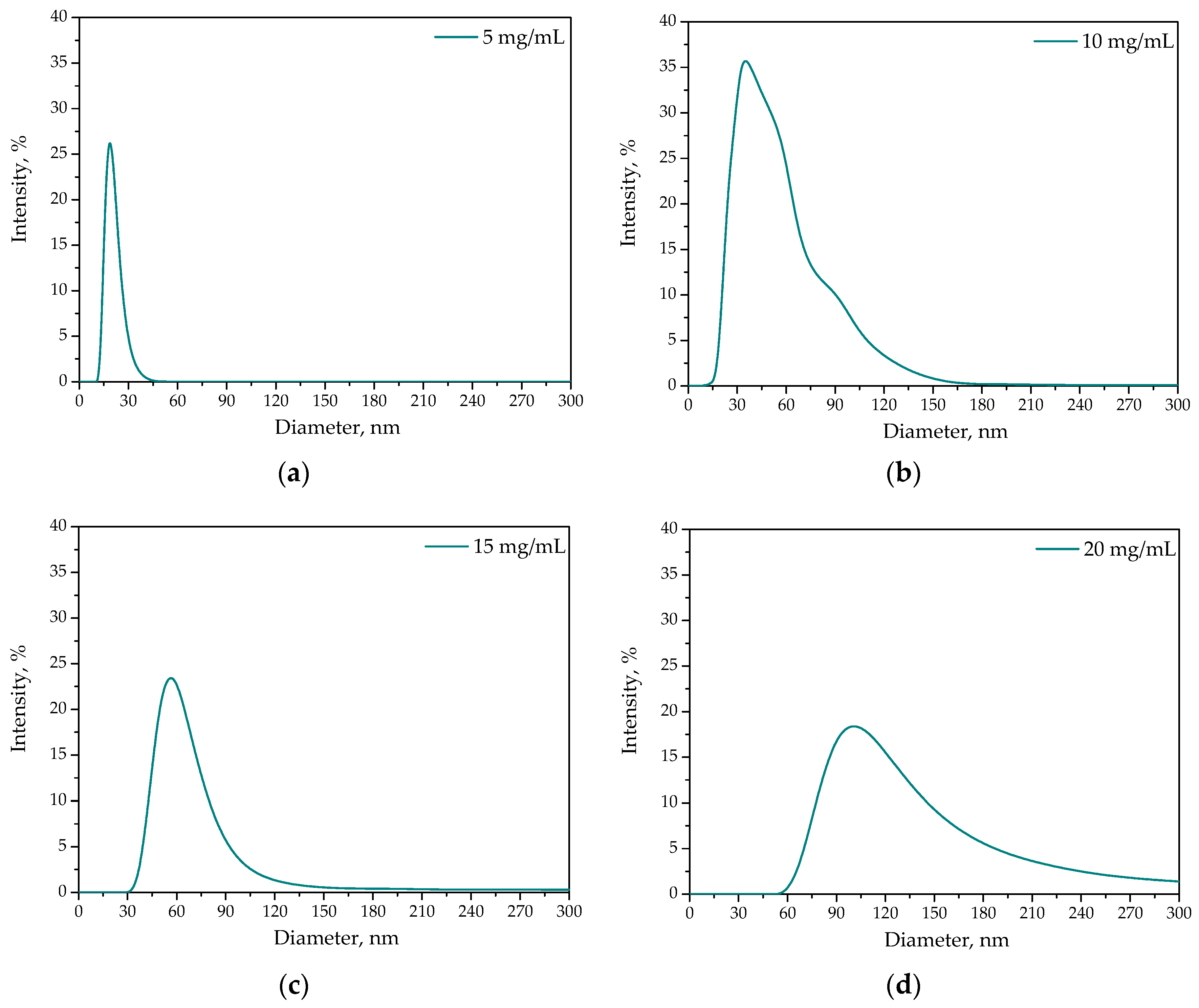
2.1.3. Results of DLS Analysis
2.2. Characterization of Chitosan/Gelatin Based Hydrogels Incorporated with Protein Particles
2.2.1. Analysis of Hydrogels’ Swelling Capability
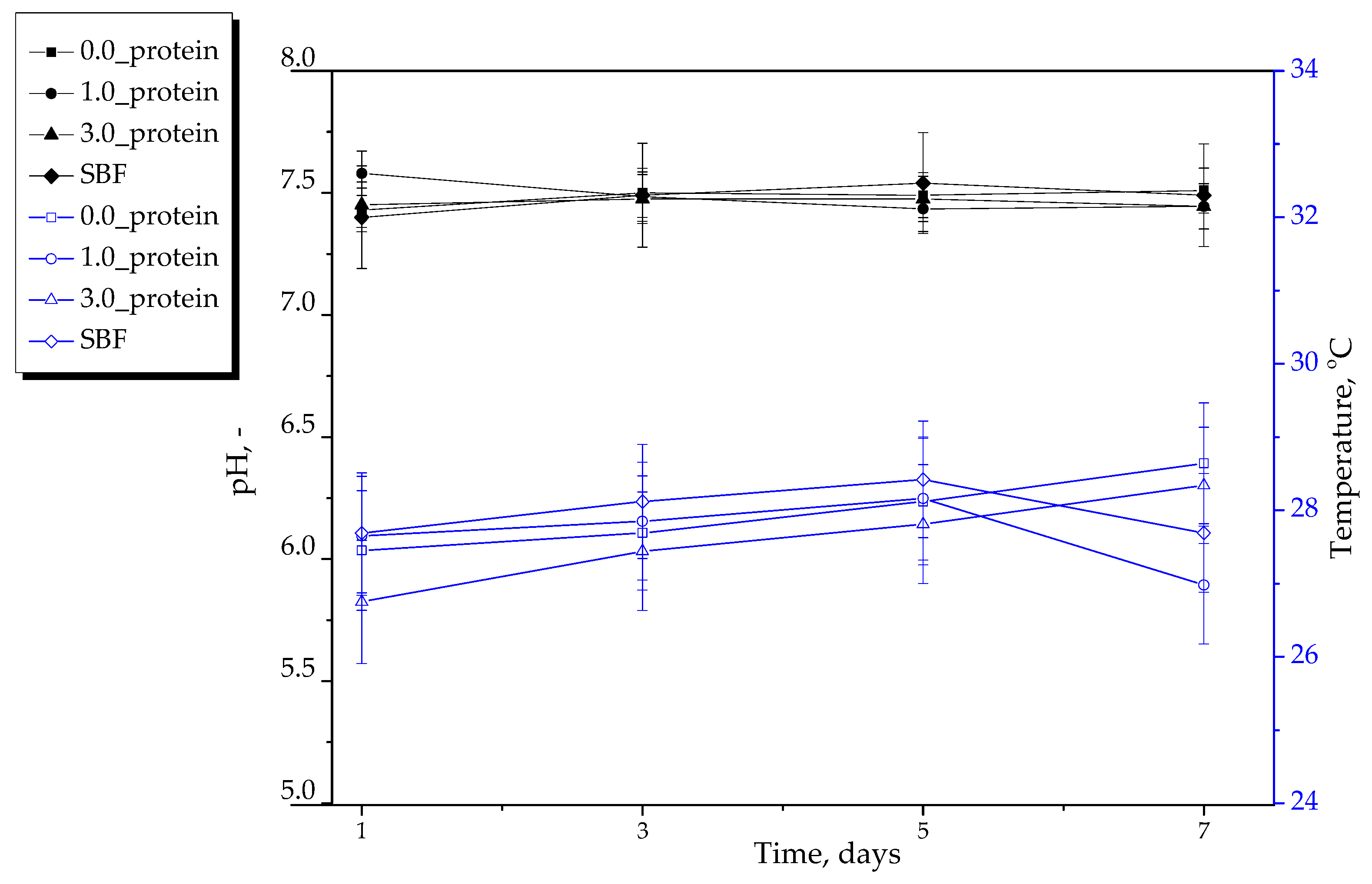
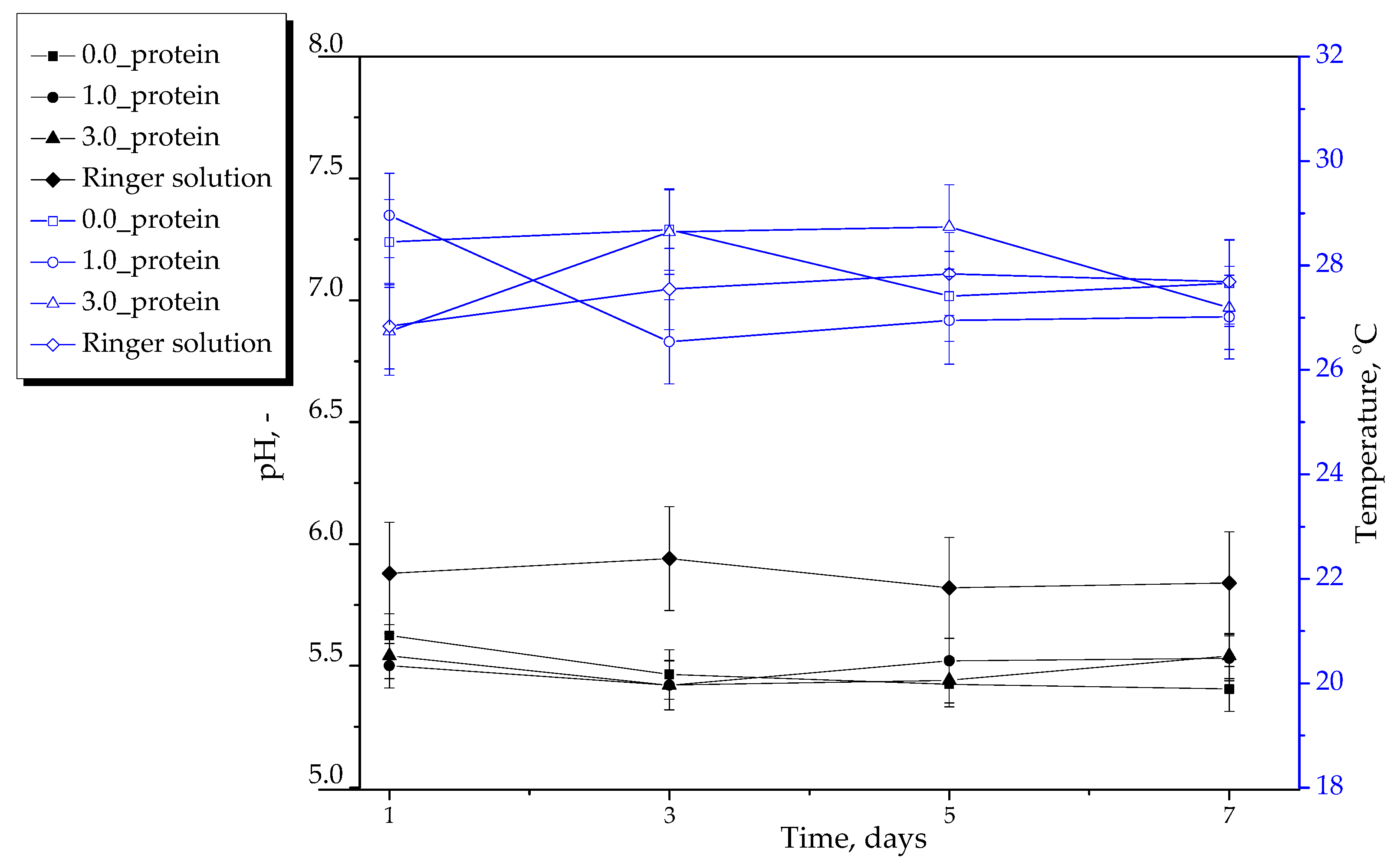
2.2.2. Results of Incubation of Hydrogels in Selected Liquids
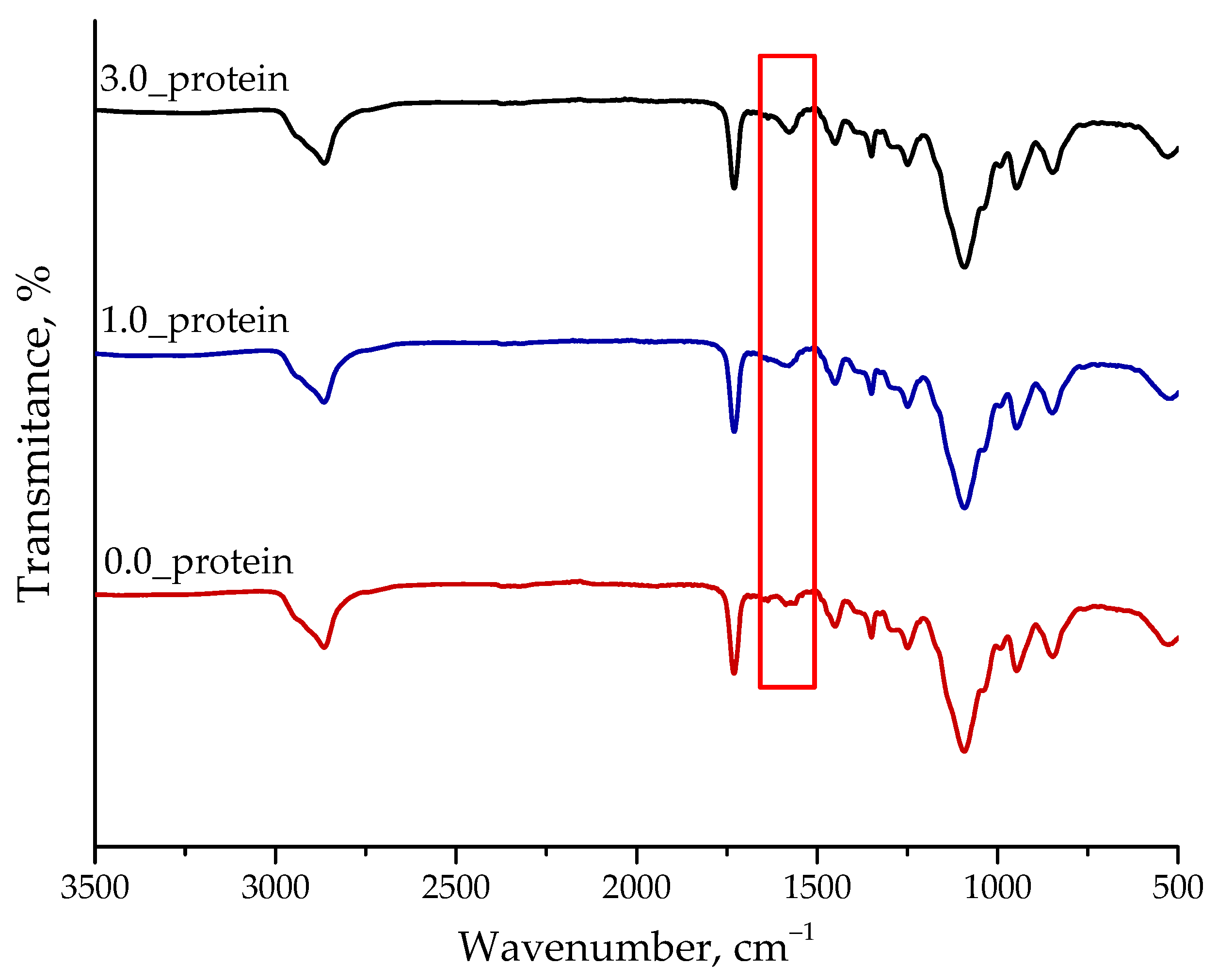
2.2.3. Analysis of the Chemical Structure of Hydrogels Using FT-IR Spectroscopy
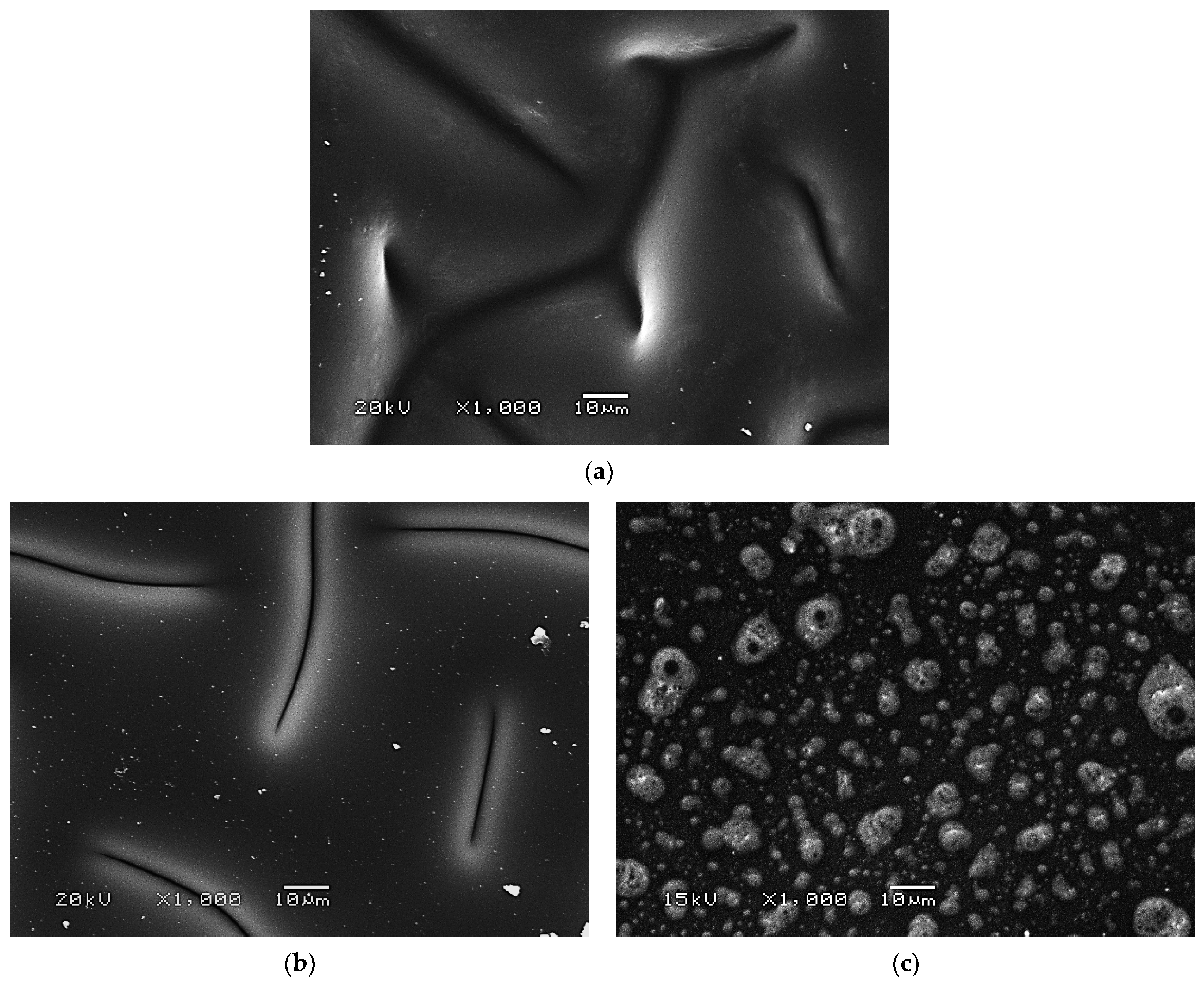
2.2.4. Results of SEM Imaging of Hydrogels
2.2.5. Studies on the Wettability of Hydrogels
2.2.6. Mechanical Characteristics of Hydrogels including Determining Their Tensile Strength and Percentage Elongation
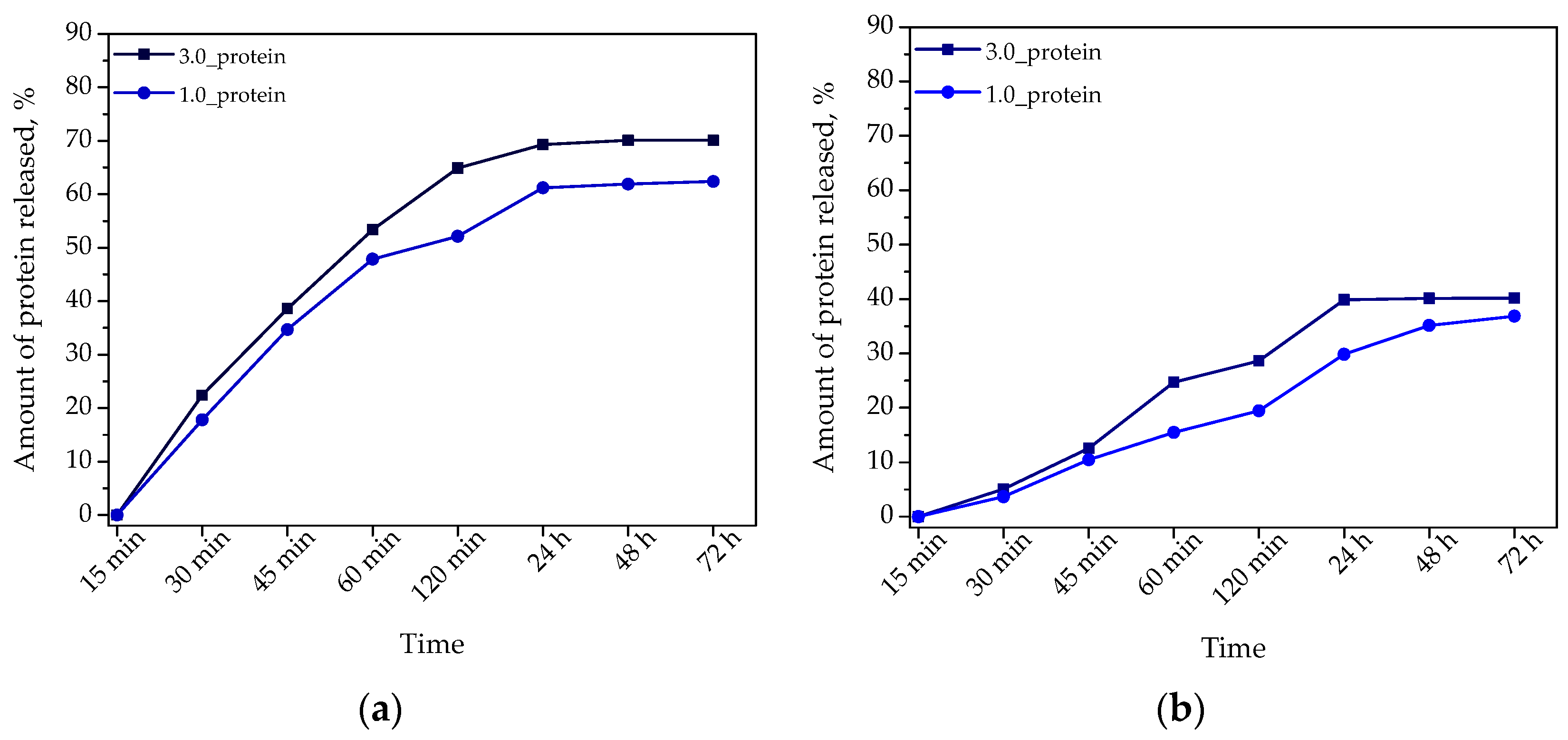
2.2.7. Studies on Determining the Release Profile of Albumin from Hydrogel Materials
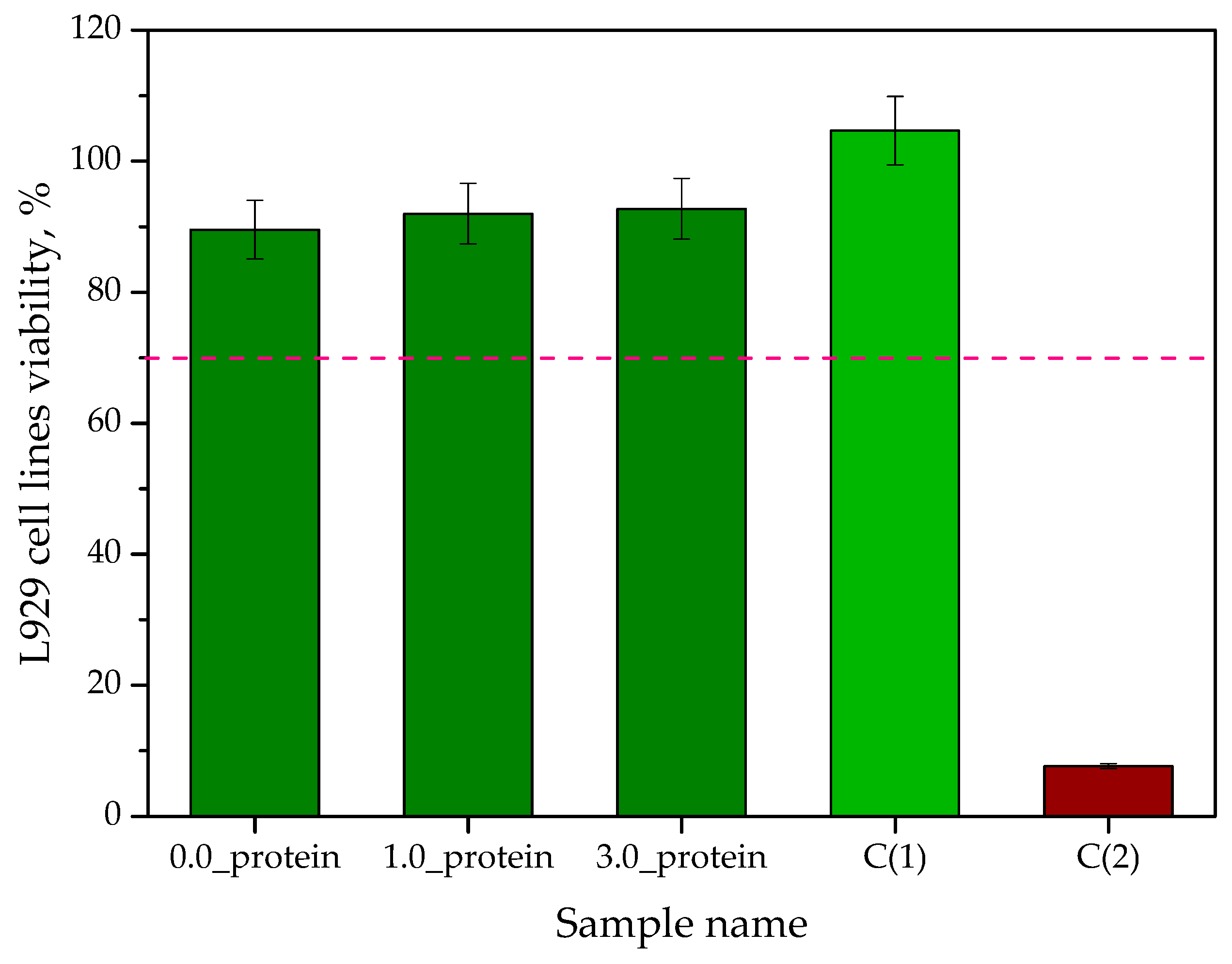
2.2.8. In Vitro Biological Studies on Hydrogels via the MTT Reduction Assay
2.2.9. Analysis of the Pro-Inflammatory Activity of the Hydrogels
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Albumin Particles via the Salt-Induced Precipitation Process
3.3. Synthesis of Chitosan/Gelatin-Based Hydrogels Incorporated with Albumin Particles by the Photopolymerization Process
3.4. Analysis of the Size of the Albumin Particles via DLS Technique
3.5. Characterization of Albumin Particles Using UV-Vis Spectroscopy
3.6. Determining the Chemical Structure of Both Protein Particles and Hydrogels by FT-IR Spectroscopy
3.7. Swelling Capability of Hydrogels
3.8. Incubation of Hydrogels in Simulated Body Liquids
3.9. Analysis of the Surface Morphology of Hydrogels via SEM Technique
3.10. Studies on Hydrogels’ Mechanical Properties
3.11. Wettability of Hydrogels
3.12. Determining the Ability of Hydrogels to Release Albumin
3.13. In Vitro Biological Studies on Hydrogels via the MTT Reduction Assay
3.14. Analysis of the Pro-Inflammatory Activity of the Hydrogels
4. Conclusions
- The applied synthesis methodology of protein carriers allowed to prepare albumin particles with nanometer sizes (less than 40 nm) showing low polydispersity.
- Both spectroscopic analyses performed—FT-IR and UV-Vis—confirmed the occurrence of the adsorption bands characteristic for the protein used during the carriers’ synthesis.
- Obtained protein carriers may be successfully applied as modifying agents of hydrogel materials based on such natural polymers as chitosan and gelatin.
- Hydrogels modified with protein particles showed higher swelling ability, higher elasticity and more hydrophilic surface compared to unmodified hydrogels.
- Both unmodified hydrogels and hydrogels incorporated with protein particles demonstrated stability during 7-day incubation in simulated physiological liquids.
- FT-IR spectroscopy confirmed the occurrence of additional adsorption bands characteristic for albumin on the FT-IR spectra of protein-modified hydrogels. However, SEM analysis indicated that in the case of sample containing 3.0 mg albumin/1.0 g hydrogel, the agglomeration of the particles may take place.
- Presented results indicated the possibilities of the potential application of developed materials as dressings supporting wound healing processes and delivering drugs via the protein carriers. It was demonstrated that the release of protein particles from hydrogels is more effective in an acidic environment—in these conditions approximately 70% protein was released wherein the release profile is prolonged.
- Considering the potential application of developed hydrogels as materials supporting the skin cancer treatment, their ability of effective releasing albumin in an acidic environment constitutes an advantage of these materials because this will enable or release this protein (combined with a chemotherapeutic drug) near the neoplastic tissue where the local acidification of the environment is observed.
- The results of MTT reduction assay confirmed the lack of cytotoxic activity of developed hydrogels towards L929 murine fibroblasts. The viability of tested cell lines was 89.54% for unmodified hydrogel while in the case of hydrogels containing albumin particles the values of this parameter was 91.98% (1.0_protein) and 92.73% (3.0_protein), respectively.
- Based on the analysis of pro-inflammatory activity, the negative immune response of THP-1XBlueCells™ cells incubated in the presence of the obtained hydrogels was excluded.
- Developed materials constitute an interesting solution with a potential for further research. Obtained results confirmed appropriately selected research direction.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, S.; Cui, Y.; Guo, X.; Chen, S.; Sun, H.; Wang, L.; Wang, J.; Zhao, Y.; Liu, Z. Biocompatible bovine serum albumin stabilized platinum nanoparticles for the oxidation of morin. New J. Chem. 2019, 43, 8774–8780. [Google Scholar] [CrossRef]
- Sun, X.; Ma, X.; Kumar, C.V.; Lei, Y. Protein-based sensitive, selective and rapid fluorescence detection of picric acid in aqueous media. Anal. Methods 2014, 6, 8464–8468. [Google Scholar] [CrossRef]
- Ma, X.; Sun, X.; Hargrove, D.; Chen, J.; Song, D.; Dong, Q.; Lu, X.; Fan, T.H.; Fu, Y.; Lei, Y. A Biocompatible and Biodegradable Protein Hydrogel with Green and Red Autofluorescence: Preparation, Characterization and In Vivo Biodegradation Tracking and Modeling. Sci. Rep. 2016, 6, 19370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Trimmel, B.; Gyulai-Gaál, S.; Kivovics, M.; Jákob, N.P.; Hegedűs, C.; Szabó, B.T.; Dobó-Nagy, C.; Szabó, G. Evaluation of the Histomorphometric and Micromorphometric Performance of a Serum Albumin-Coated Bone Allograft Combined with A-PRF for Early and Conventional Healing Protocols after Maxillary Sinus Augmentation: A Randomized Clinical Trial. Materials 2021, 14, 1810. [Google Scholar] [CrossRef]
- Li, P.S.; Liang Lee, I.; Yu, W.L.; Sun, J.S.; Jane, W.N.; Shen, H.H. A Novel Albumin-Based Tissue Scaffold for Autogenic Tissue Engineering Applications. Sci. Rep. 2014, 4, 5600. [Google Scholar] [CrossRef] [Green Version]
- Ferracci, G.; Zhu, M.; Ibrahim, M.S.; Ma, G.; Fan, T.F.; Lee, B.H.; Cho, N.-J. Photocurable Albumin Methacryloyl Hydrogels as a Versatile Platform for Tissue Engineering. ACS Appl. Bio Mater. 2020, 3, 920–934. [Google Scholar] [CrossRef]
- Hierro-Oliva, M.; Gallardo-Moreno, A.M.; González-Martín, M.L. Surface Characterisation of Human Serum Albumin Layers on Activated Ti6Al4V. Materials 2021, 14, 7416. [Google Scholar] [CrossRef]
- Klok, O.; Igual Munoz, A.; Mischler, S. An Overview of Serum Albumin Interactions with Biomedical Alloys. Materials 2020, 13, 4858. [Google Scholar] [CrossRef]
- Mishra, V.; Heath, R.J. Structural and Biochemical Features of Human Serum Albumin Essential for Eukaryotic Cell Culture. Int. J. Mol. Sci. 2021, 22, 8411. [Google Scholar] [CrossRef]
- Siemiradzka, W.; Bułaś, L.; Dolińska, B. Permeation of albumin through the skin depending on its concentration and the substrate used in simulated conditions in vivo. Biomed. Pharmacother. 2022, 155, 113722. [Google Scholar] [CrossRef] [PubMed]
- Chubarov, A.; Spitsyna, A.; Krumkacheva, O.; Mitin, D.; Suvorov, D.; Tormyshev, V.; Fedin, M.; Bowman, M.K.; Bagryanskaya, E. Reversible Dimerization of Human Serum Albumin. Molecules 2021, 26, 108. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell Ther. 2016, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Oliva, R.; Banerjee, S.; Cinar, H.; Ehrt, C.; Winter, R. Alteration of Protein Binding Affinities by Aqueous Two-Phase Systems Revealed by Pressure Perturbation. Sci. Rep. 2020, 10, 8074. [Google Scholar] [CrossRef]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol. Pharmaceutics 2021, 18, 1862–1894. [Google Scholar] [CrossRef] [PubMed]
- Rejinold, N.S.; Choi, G.; Piao, H.; Choy, J.-H. Bovine Serum Albumin-Coated Niclosamide-Zein Nanoparticles as Potential Injectable Medicine against COVID-19. Materials 2021, 14, 3792. [Google Scholar] [CrossRef]
- Deng, L.; Xia, T.; Cheng, W.; Yang, M.; Zhu, W.; Chen, X. Injectable redox albumin-based hydrogel with in-situ loaded dihydromyricetin. Colloids Surf. B Biointerfaces 2022, 220, 112871. [Google Scholar] [CrossRef]
- Van de Sande, L.; Cosyns, S.; Willaert, W.; Ceelen, W. Albumin-based cancer therapeutics for intraperitoneal drug delivery: A review. Drug Deliv. 2020, 27, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Guo, Z.; Cao, Z.; Zhou, W.; Zhang, Y.; Chen, Q.; Lu, Y.; Chen, X.; Guo, Q.; Li, C.; et al. Endogenous albumin-mediated delivery of redox responsive paclitaxel-loaded micelles for targeted cancer therapy. Biomaterials 2018, 183, 243–257. [Google Scholar] [CrossRef]
- Iqbal, H.; Razzaq, A.; Khan, N.U.; Rehman, S.U.; Webster, T.J.; Xiao, R.; Menaa, F. pH-responsive albumin-coated biopolymeric nanoparticles with lapatinab for targeted breast cancer therapy. Mater. Sci. Eng. C 2022, 139, 213039. [Google Scholar] [CrossRef]
- Hassanin, I.; Elzoghby, A. Albumin-based nanoparticles: A promising strategy to overcome cancer drug resistance. Cancer Drug Resist. 2020, 3, 930–946. [Google Scholar] [CrossRef] [PubMed]
- Zayed, D.G.; Ebrahim, S.M.; Helmy, M.W.; Khattab, S.N.; Bahey-El-Din, M.; Fang, J.-Y.; Elkhodairy, K.A.; Elzoghby, A.O. Combining hydrophilic chemotherapy and hydrophobic phytotherapy via tumor-targeted albumin–QDs nano-hybrids: Covalent coupling and phospholipid complexation approaches. J. Nanobiotechnol. 2019, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Pentak, D.; Ploch-Jankowska, A.; Zięba, A.; Kozik, V. The Advances and Challenges of Liposome-Assisted Drug Release in the Presence of Serum Albumin Molecules: The Influence of Surrounding pH. Materials 2022, 15, 1586. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.N.; Varga, N.; Gombár, G.; Hornok, V.; Csapo, E. Novel feasibilities for preparation of serum albumin-based core-shell nanoparticles in flow conditions. J. Flow Chem. 2020, 10, 497–505. [Google Scholar] [CrossRef]
- Hornok, V. Serum Albumin Nanoparticles: Problems and Prospects. Polymers 2021, 13, 3759. [Google Scholar] [CrossRef]
- Meng, R.; Zhu, H.; Wang, Z.; Hao, S.; Wang, B. Preparation of Drug-Loaded Albumin Nanoparticles and Its Application in Cancer Therapy. J. Nanomater. 2022, 2022, 3052175. [Google Scholar] [CrossRef]
- Kudłacik-Kramarczyk, S.; Drabczyk, A.; Głąb, M.; Gajda, P.; Czopek, A.; Zagórska, A.; Jaromin, A.; Gubernator, J.; Makara, A.; Tyliszczak, B. The Development of the Innovative Synthesis Methodology of Albumin Nanoparticles Supported by Their Physicochemical, Cytotoxic and Hemolytic Evaluation. Materials 2021, 14, 4386. [Google Scholar] [CrossRef]
- Li, J.; Jin, H.; Razzak, A.; Kim, E.J.; Choi, S.S. Crosslinker-free Bovine Serum Albumin-loaded Chitosan/alginate Nanocomplex for pH-responsive Bursting Release of Oral-administered Protein. Biotechnol. Bioprocess Eng. 2022, 27, 40–50. [Google Scholar] [CrossRef]
- Kim, G.O.; Kim, N.; Kim, D.Y.; Kwon, J.S.; Min, B.-H. An electrostatically crosslinked chitosan hydrogel as a drug carrier. Molecules 2012, 17, 13704–13711. [Google Scholar] [CrossRef] [Green Version]
- Kudłacik-Kramarczyk, S.; Głąb, M.; Drabczyk, A.; Kordyka, A.; Godzierz, M.; Wróbel, P.S.; Krzan, M.; Uthayakumar, M.; Kędzierska, M.; Tyliszczak, B. Physicochemical Characteristics of Chitosan-Based Hydrogels Containing Albumin Particles and Aloe vera Juice as Transdermal Systems Functionalized in the Viewpoint of Potential Biomedical Applications. Materials 2021, 14, 5832. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O. A short review on chitosan and gelatin-based hydrogel composite polymers for wound healing. J. Biomater. Sci. Polym. Ed. 2022, 33, 1595–1622. [Google Scholar] [CrossRef] [PubMed]
- Magli, S.; Rossi, G.B.; Risi, G.; Bertimi, S.; Cosentino, C.; Crippa, L.; Ballarini, E.; Cavaletti, G.; Piazza, L.; Masseroni, E.; et al. Design and Synthesis of Chitosan—Gelatin Hybrid Hydrogels for 3D Printable in vitro Models. Front. Chem. 2020, 8, 524. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.A.M.; Januário, A.P.; Félix, R.; Alves, N.; Lemos, M.F.L.; Dias, J.R. Multifunctional Gelatin/Chitosan Electrospun Wound Dressing Dopped with Undaria pinnatifida Phlorotannin-Enriched Extract for Skin Regeneration. Pharmaceutics 2021, 13, 2152. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Jokar, S.; Pourjavadi, A.; Adeli, M. Albumin–graphene oxide conjugates; carriers for anticancer drugs. RSC Adv. 2014, 4, 33001. [Google Scholar] [CrossRef]
- Bonnier, F.; Brachet, G.; Duong, R.; Sojinrin, T.; Respaud, R.; Aubrey, N.; Baker, M.J.; Byrne, H.J.; Chourpa, I. Screening the low molecular weight fraction of human serum using ATR-IR spectroscopy. J. Biophotonics 2016, 9, 1085–1097. [Google Scholar] [CrossRef] [Green Version]
- Alhazmi, H.A. FT-IR Spectroscopy for the Identification of Binding Sites and Measurements of the Binding Interactions of Important Metal Ions with Bovine Serum Albumin. Sci. Pharm. 2019, 87, 5. [Google Scholar] [CrossRef] [Green Version]
- Oudemans, T.F.M.; Boon, J.J.; Botto, R.E. FTIR and solid-state 13C CP/MAS NMR spectroscopy of charred and non-charred solid organic residues preserved in Roman Iron Age vessels from the Netherlands. Archaeometry 2007, 49, 571–594. [Google Scholar] [CrossRef]
- Schiebel, J.; Gaspari, R.; Wulsdorf, T.; Ngo, K.; Sohn, C.; Schrander, T.E.; Cavalli, A.; Ostermann, A.; Heine, A.; Klebe, G. Intriguing role of water in protein-ligand binding studied by neutron crystallography on trypsin complexes. Nat. Commun. 2018, 9, 3559. [Google Scholar] [CrossRef] [Green Version]
- Raschke, T. Water structure and interactions with protein surfaces. Curr. Opin. Struct. Biol. 2006, 16, 152–159. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.S.; Yan, K.; Qi, Y.; Wang, G.F.; Cui, Y.L. Preparation, characterization, and evaluation of genipin crosslinked chitosan/gelatin three-dimensional scaffolds for liver tissue engineering applications. J. Biomed. Mater. Res. A 2016, 104, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Hajiabbas, M.; Mashayekhan, S.; Nazaripouya, A.; Naji, M.; Hunkeler, D.; Zeleti, S.R.; Sharifiaghdas, F. Chitosan-gelatin sheets as scaffolds for muscle tissue engineering. Artif. Cells Nanomed. Biotechnol. 2015, 43, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Foggia, M.; Taddei, P.; Torreggiani, A.; Dettin, M.; Tinti, A. Self-Assembling Peptides for Biomedical Applications: Ir And Raman Spectroscopies For The Study Of Secondary Structure. Proteom. Res. J. 2011, 2, 231–272. [Google Scholar]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Al-Azzam, N.; Alazzam, A. Micropatterning of cells via adjusting surface wettability using plasma treatment and graphene oxide deposition. PLoS ONE 2022, 17, e0269914. [Google Scholar] [CrossRef]
- Wei, J.; Yoshinari, M.; Takemoto, S.; Hattori, M.; Kawada, E.; Liu, B.; Oda, Y. Adhesion of Mouse Fibroblasts on Hexamethyldisiloxane Surfaces with Wide Range of Wettability. J. Biomed. Mater. Res. 2007, 81, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Kuten Pella, O.; Hornyák, I.; Horváthy, D.; Fodor, E.; Nehrer, S.; Lacza, Z. Albumin as a Biomaterial and Therapeutic Agent in Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 10557. [Google Scholar] [CrossRef]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing Albumin as a Carrier for Cancer Therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2014, 73, 1524–1535. [Google Scholar] [CrossRef] [Green Version]
- ISO-10993-5-2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Technical Committee: ISO/TC 194 Biological and Clinical Evaluation of Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2009.
- Gonzales Porto, S.A.; Domenech, N.; Gonzalez Rodriguez, A.; Oviedo, E.M.A.; Blanco, F.J.; Gonda, M.C.A.; Jorge, A.A.; Ibazen, J.S.; Vazquez, E.R. The addition of albumin improves Schwann cells viability in nerve cryopreservation. Cell Tissue Bank. 2018, 19, 507–517. [Google Scholar] [CrossRef]
- Ashman, N.; Harwood, S.M.; Kieswich, J.; Allen, D.A.; Roberts, N.B.; Mendes0Ribeiro, C.; Yaqoob, M.M. Albumin stimulates cell growth, L-arginine transport, and metabolism to polyamines in human proximal tubular cells. Kidney Int. 2005, 67, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Dąbek, J.; Kułach, A.; Gąsior, Z. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB): A new potential therapeutic target in atherosclerosis? Pharmacol. Rep. 2010, 62, 778–783. [Google Scholar] [CrossRef]
- Kavaliauskaitė, J.; Kazlauskaitė, A.; Lazutka, J.R.; Mozolevskis, G.; Stirkė, A. Pulsed Electric Fields Alter Expression of NF-κB Promoter-Controlled Gene. Int. J. Mol. Sci. 2022, 23, 451. [Google Scholar] [CrossRef] [PubMed]
- Drabczyk, A.; Kudłacik-Kramarczyk, S.; Głąb, M.; Kędzierska, M.; Jaromin, A.; Mierzwiński, D.; Tyliszczak, B. Physicochemical Investigations of Chitosan-Based Hydrogels Containing Aloe vera Designed for Biomedical Use. Materials 2020, 13, 3073. [Google Scholar] [CrossRef] [PubMed]
- Kudłacik-Kramarczyk, S.; Drabczyk, A.; Głąb, M.; Alves-Lima, D.; Lin, H.; Douglas, T.; Kuciel, S.; Zagórska, A.; Tyliszczak, B. Investigations on the impact of the introduction of the Aloe vera into the hydrogel matrix on cytotoxic and hydrophilic properties of these systems considered as potential wound dressings. Mater. Sci. Eng. C 2021, 123, 111977. [Google Scholar] [CrossRef]

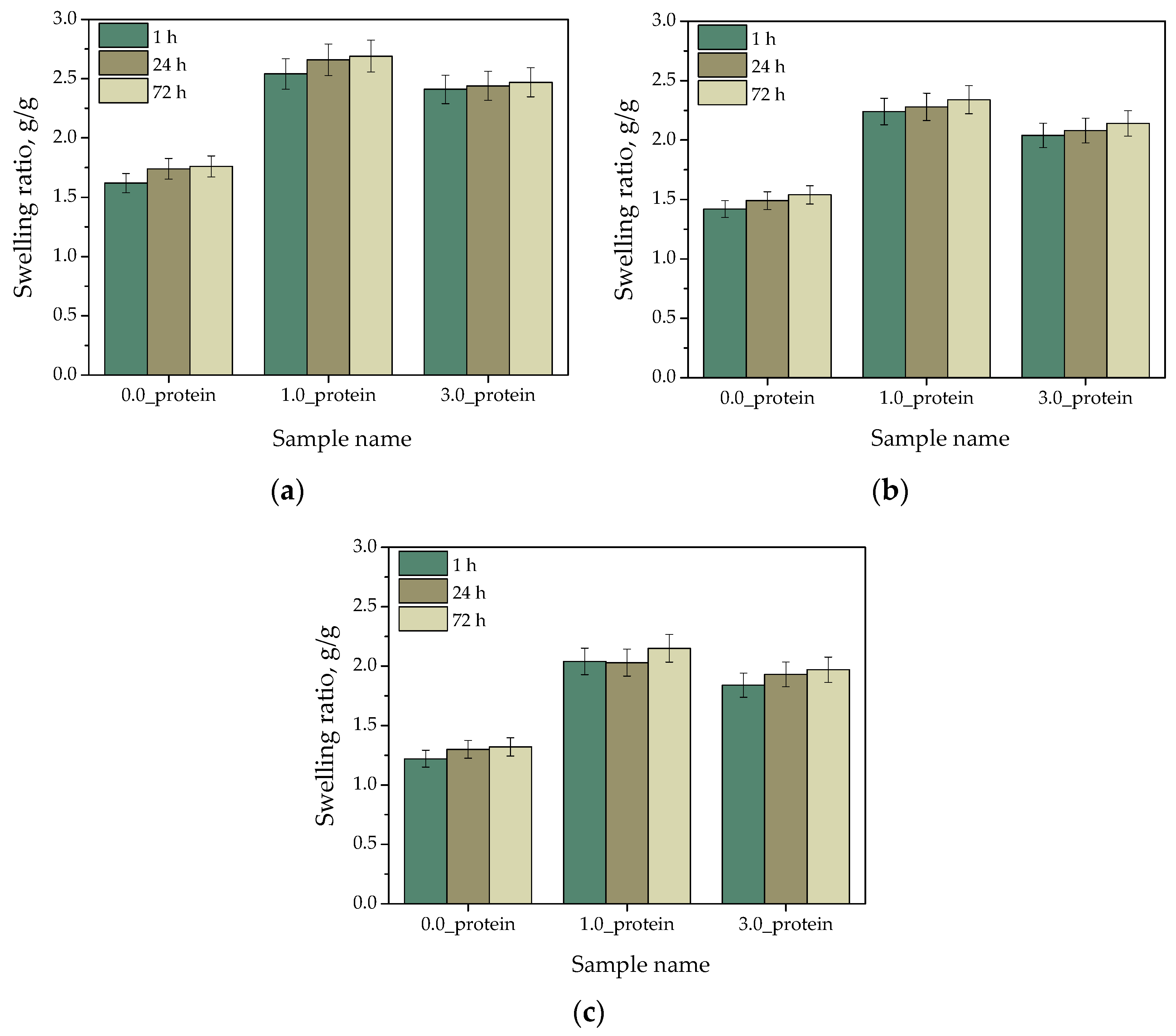



| Sample Name | Total Surface Free Energy, mJ/m2 | Contact Angle, ° | Image of Hydrogel during Its First Contact with Water |
|---|---|---|---|
| 0.0_protein | 50.72 | 68.75 ± 0.79 |  |
| 1.0_protein | 49.16 | 64.51 ± 1.05 |  |
| 3.0_protein | 48.96 | 62.28 ± 0.66 |  |
| Concentration of K3PO4, M | Concentration of Albumin, mg/mL |
|---|---|
| 2.5 | 5 |
| 10 | |
| 15 | |
| 20 |
| Base Solution, mL | Photoinitiator *, mL | Crosslinking Agent **, mL | Content of Albumin in Hydrogel Sample, mg Albumin/g Hydrogel | Sample Name |
|---|---|---|---|---|
| 30 | 0.15 | 5.0 | - | 0.0_protein |
| 1.0 | 1.0_protein | |||
| 3.0 | 3.0_protein |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bańkosz, M. Development of Chitosan/Gelatin-Based Hydrogels Incorporated with Albumin Particles. Int. J. Mol. Sci. 2022, 23, 14136. https://doi.org/10.3390/ijms232214136
Bańkosz M. Development of Chitosan/Gelatin-Based Hydrogels Incorporated with Albumin Particles. International Journal of Molecular Sciences. 2022; 23(22):14136. https://doi.org/10.3390/ijms232214136
Chicago/Turabian StyleBańkosz, Magdalena. 2022. "Development of Chitosan/Gelatin-Based Hydrogels Incorporated with Albumin Particles" International Journal of Molecular Sciences 23, no. 22: 14136. https://doi.org/10.3390/ijms232214136
APA StyleBańkosz, M. (2022). Development of Chitosan/Gelatin-Based Hydrogels Incorporated with Albumin Particles. International Journal of Molecular Sciences, 23(22), 14136. https://doi.org/10.3390/ijms232214136





