Fission Yeast Rho1p-GEFs: From Polarity and Cell Wall Synthesis to Genome Stability
Abstract
:1. Fission Yeast Rho GTPases
2. Structural–Functional Characteristics of Rho1p and Its GEFs Rgf1p, Rgf2p, and Rgf3p
3. Rho1p Functions to Preserve Cell Integrity
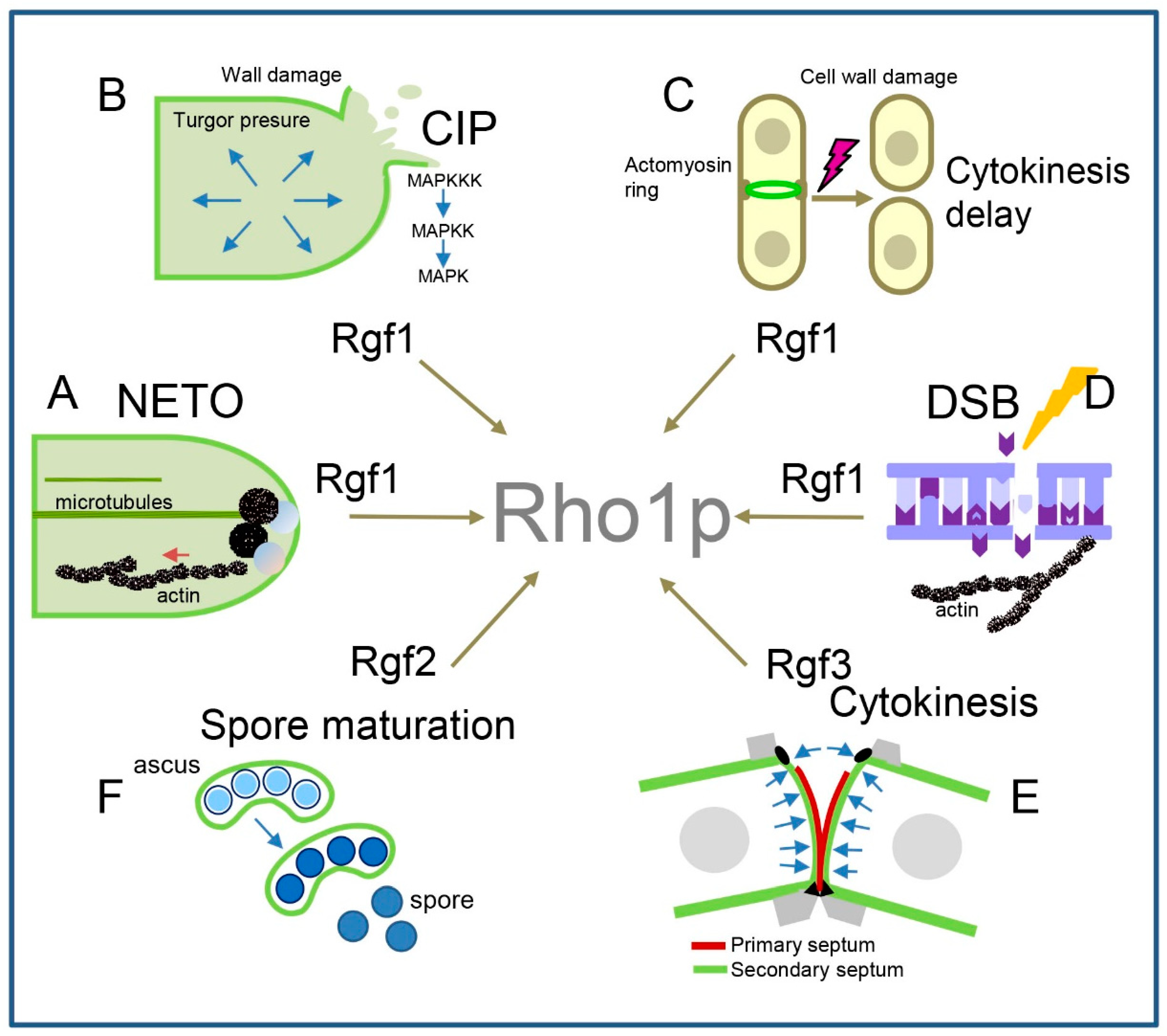
4. Connections between Rho1p and Rho1p GEFs and the Machinery That Determines Polar Growth
5. Role of Rho1p GEFs in Cell Wall Synthesis and Cell Integrity
6. Role of Rho1p GEFs in Cytokinesis
7. Rgf3p Plays an Essential Function in Septum Synthesis
8. Rgf1p Is Involved in a Cytokinesis Checkpoint Together with the Septation Initiation Network (SIN) and the Cell Integrity Pathway (CIP)
9. Role of Rho1p GEFs in Genomic Instability
10. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and Biology. Ann. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef] [Green Version]
- Hodge, R.G.; Ridley, A.J. Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol. 2016, 17, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.D.; Ridley, A.J. Rho GTPase signaling complexes in cell migration and invasion. J. Cell Biol. 2018, 217, 447–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hercyk, B.; Das, M. Rho Family GTPases in Fission Yeast Cytokinesis. Commun. Integr. Biol. 2019, 12, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.G.; Arkowitz, R.A. Cell polarization in budding and fission yeasts. FEMS Microbiol. Rev. 2014, 38, 228–253. [Google Scholar] [CrossRef] [Green Version]
- Vicente-Soler, J.; Soto, T.; Franco, A.; Cansado, J.; Madrid, M. The Multiple Functions of Rho GTPases in Fission Yeasts. Cells 2021, 10, 1422. [Google Scholar] [CrossRef] [PubMed]
- Manjon, E.; Edreira, T.; Munoz, S.; Sanchez, Y. Rgf1p (Rho1p GEF) is required for double-strand break repair in fission yeast. Nucleic Acids Res. 2017, 45, 5269–5284. [Google Scholar] [CrossRef] [Green Version]
- Perez, P.; Cortes, J.C.G.; Cansado, J.; Ribas, J.C. Fission yeast cell wall biosynthesis and cell integrity signalling. Cell Surf. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Kita, A.; Li, C.; Yu, Y.; Umeda, N.; Doi, A.; Yasuda, M.; Ishiwata, S.; Taga, A.; Horiuchi, Y.; Sugiura, R. Role of the Small GTPase Rho3 in Golgi/Endosome trafficking through functional interaction with adaptin in Fission Yeast. PLoS ONE 2011, 6, e16842. [Google Scholar] [CrossRef] [Green Version]
- Perez, P.; Portales, E.; Santos, B. Rho4 interaction with exocyst and septins regulates cell separation in fission yeast. Microbiology 2015, 161, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, X.; Balasubramanian, M.K. Rho3p regulates cell separation by modulating exocyst function in Schizosaccharomyces pombe. Genetics 2003, 164, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Rincon, S.A.; Santos, B.; Perez, P. Fission yeast Rho5p GTPase is a functional paralogue of Rho1p that plays a role in survival of spores and stationary-phase cells. Eukaryot. Cell 2006, 5, 435–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaiswal, M.; Dvorsky, R.; Ahmadian, M.R. Deciphering the molecular and functional basis of Dbl family proteins: A novel systematic approach toward classification of selective activation of the Rho family proteins. J. Biol. Chem. 2013, 288, 4486–4500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bos, J.L.; Rehmann, H.; Wittinghofer, A. GEFs and GAPs: Critical elements in the control of small G proteins. Cell 2007, 129, 865–877. [Google Scholar] [CrossRef] [Green Version]
- Cherfils, J.; Zeghouf, M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 2013, 93, 269–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittinghofer, A.; Vetter, I.R. Structure-function relationships of the G domain, a canonical switch motif. Annu. Rev. Biochem. 2011, 80, 943–971. [Google Scholar] [CrossRef]
- Nakano, K.; Arai, R.; Mabuchi, I. The small GTP-binding protein Rho1 is a multifunctional protein that regulates actin localization, cell polarity, and septum formation in the fission yeast Schizosaccharomyces pombe. Genes Cells 1997, 2, 679–694. [Google Scholar] [CrossRef]
- Garcia, P.; Garcia, I.; Marcos, F.; de Garibay, G.R.; Sanchez, Y. Fission yeast rgf2p is a rho1p guanine nucleotide exchange factor required for spore wall maturation and for the maintenance of cell integrity in the absence of rgf1p. Genetics 2009, 181, 1321–1334. [Google Scholar] [CrossRef] [Green Version]
- Garcia, P.; Tajadura, V.; Garcia, I.; Sanchez, Y. Rgf1p is a specific Rho1-GEF that coordinates cell polarization with cell wall biogenesis in fission yeast. Mol. Biol. Cell 2006, 17, 1620–1631. [Google Scholar] [CrossRef] [Green Version]
- Morrell-Falvey, J.L.; Ren, L.; Feoktistova, A.; Haese, G.D.; Gould, K.L. Cell wall remodeling at the fission yeast cell division site requires the Rho-GEF Rgf3p. J. Cell Sci. 2005, 118, 5563–5573. [Google Scholar] [CrossRef]
- Mutoh, T.; Nakano, K.; Mabuchi, I. Rho1-GEFs Rgf1 and Rgf2 are involved in formation of cell wall and septum, while Rgf3 is involved in cytokinesis in fission yeast. Genes Cells 2005, 10, 1189–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajadura, V.; Garcia, B.; Garcia, I.; Garcia, P.; Sanchez, Y. Schizosaccharomyces pombe Rgf3p is a specific Rho1 GEF that regulates cell wall beta-glucan biosynthesis through the GTPase Rho1p. J. Cell Sci. 2004, 117, 6163–6174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calonge, T.M.; Arellano, M.; Coll, P.M.; Perez, P. Rga5p is a specific Rho1p GTPase-activating protein that regulates cell integrity in Schizosaccharomyces pombe. Mol. Microbiol. 2003, 47, 507–518. [Google Scholar] [CrossRef] [Green Version]
- Nakano, K.; Mutoh, T.; Mabuchi, I. Characterization of GTPase-activating proteins for the function of the Rho-family small GTPases in the fission yeast Schizosaccharomyces pombe. Genes Cells 2001, 6, 1031–1042. [Google Scholar] [CrossRef]
- Harris, M.A.; Rutherford, K.M.; Hayles, J.; Lock, A.; Bahler, J.; Oliver, S.G.; Mata, J.; Wood, V. Fission stories: Using PomBase to understand Schizosaccharomyces pombe biology. Genetics 2022, 220, iyab222. [Google Scholar] [CrossRef]
- Krause, S.A.; Cundell, M.J.; Poon, P.P.; McGhie, J.; Johnston, G.C.; Price, C.; Gray, J.V. Functional specialisation of yeast Rho1 GTP exchange factors. J. Cell Sci. 2012, 125, 2721–2731. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.; Bickle, M.; Beck, T.; Hall, M.N. The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell 1997, 88, 531–542. [Google Scholar] [CrossRef] [Green Version]
- Bartual, S.G.; Wei, W.; Zhou, Y.; Pravata, V.M.; Fang, W.; Yan, K.; Ferenbach, A.T.; Lockhart, D.E.A.; van Aalten, D.M.F. The citron homology domain as a scaffold for Rho1 signaling. Proc. Natl. Acad. Sci. USA 2021, 118, e2110298118. [Google Scholar] [CrossRef]
- Cook, D.R.; Rossman, K.L.; Der, C.J. Rho guanine nucleotide exchange factors: Regulators of Rho GTPase activity in development and disease. Oncogene 2014, 33, 4021–4035. [Google Scholar] [CrossRef] [Green Version]
- Buchsbaum, R.J. Rho activation at a glance. J. Cell Sci. 2007, 120, 1149–1152. [Google Scholar] [CrossRef]
- Mosaddeghzadeh, N.; Ahmadian, M.R. The RHO Family GTPases: Mechanisms of Regulation and Signaling. Cells 2021, 10, 1831. [Google Scholar] [CrossRef] [PubMed]
- Dvorsky, R.; Ahmadian, M.R. Always look on the bright site of Rho: Structural implications for a conserved intermolecular interface. EMBO Rep. 2004, 5, 1130–1136. [Google Scholar] [CrossRef] [Green Version]
- Rossman, K.L.; Channing, J.D.; Sondek, J. GEF means go: Turning on Rho GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 2005, 6, 167–180. [Google Scholar] [CrossRef]
- Singh, N.; Reyes-Ordonez, A.; Compagnone, M.A.; Moreno, J.F.; Leslie, B.J.; Ha, T.; Chen, J. Redefining the specificity of phosphoinositide-binding by human PH domain-containing proteins. Nat. Commun. 2021, 12, 4339. [Google Scholar] [CrossRef]
- Viaud, J.; Gaits-Iacovoni, F.; Payrastre, B. Regulation of the DH-PH tandem of guanine nucleotide exchange factor for Rho GTPases by phosphoinositides. Adv. Biol. Regul. 2012, 52, 303–314. [Google Scholar] [CrossRef]
- Yu, J.W.; Mendrola, J.M.; Audhya, A.; Singh, S.; Keleti, D.; DeWald, D.B.; Murray, D.; Emr, S.D.; Lemmon, M.A. Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol. Cell 2004, 13, 677–688. [Google Scholar] [CrossRef]
- Snider, C.E.; Willet, A.H.; Chen, J.S.; Arpag, G.; Zanic, M.; Gould, K.L. Phosphoinositide-mediated ring anchoring resists perpendicular forces to promote medial cytokinesis. J. Cell Biol. 2017, 216, 3041–3050. [Google Scholar] [CrossRef] [Green Version]
- Snider, C.E.; Willet, A.H.; Brown, H.T.; Gould, K.L. Analysis of the contribution of phosphoinositides to medial septation in fission yeast highlights the importance of PI(4,5)P2 for medial contractile ring anchoring. Mol. Biol. Cell 2018, 29, 2148–2155. [Google Scholar] [CrossRef]
- Munoz, S.; Manjon, E.; Garcia, P.; Sunnerhagen, P.; Sanchez, Y. The checkpoint-dependent nuclear accumulation of Rho1p exchange factor Rgf1p is important for tolerance to chronic replication stress. Mol. Biol. Cell 2014, 25, 1137–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Consonni, S.V.; Maurice, M.M.; Bos, J.L. DEP domains: Structurally similar but functionally different. Nat. Rev. Mol. Cell Biol. 2014, 15, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Gammons, M.V.; Renko, M.; Johnson, C.M.; Rutherford, T.J.; Bienz, M. Wnt Signalosome Assembly by DEP Domain Swapping of Dishevelled. Mol. Cell 2016, 64, 92–104. [Google Scholar] [CrossRef] [Green Version]
- Ravala, S.K.; Hopkins, J.B.; Plescia, C.B.; Allgood, S.R.; Kane, M.A.; Cash, J.N.; Stahelin, R.V.; Tesmer, J.J.G. The first DEP domain of the RhoGEF P-Rex1 autoinhibits activity and contributes to membrane binding. J. Biol. Chem. 2020, 295, 12635–12647. [Google Scholar] [CrossRef] [PubMed]
- Ballon, D.R.; Flanary, P.; Gladue, P.D.; Konopka, J.; Dohlman, H.G.; Thorner, J. DEP-domain-mediated regulation of GPCR signaling responses. Cell 2006, 126, 1079–1093. [Google Scholar] [CrossRef] [Green Version]
- D’Avino, P.P. Citron kinase—Renaissance of a neglected mitotic kinase. J. Cell Sci. 2017, 130, 1701–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Reiner, D.J. A signalling cascade for Ral. Small GTPases 2021. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Chan, N.L.; Wang, A.H. The many blades of the beta-propeller proteins: Conserved but versatile. Trends Biochem. Sci. 2011, 36, 553–561. [Google Scholar] [CrossRef]
- Chang, F.; Martin, S.G. Shaping fission yeast with microtubules. Cold Spring Harb. Perspect. Biol. 2009, 1, a001347. [Google Scholar] [CrossRef] [Green Version]
- Arellano, M.; Duran, A.; Perez, P. Localisation of the Schizosaccharomyces pombe rho1p GTPase and its involvement in the organisation of the actin cytoskeleton. J. Cell Sci. 1997, 110, 2547–2555. [Google Scholar] [CrossRef]
- Haupt, A.; Minc, N. Gradients of phosphatidylserine contribute to plasma membrane charge localization and cell polarity in fission yeast. Mol. Biol. Cell 2017, 28, 210–220. [Google Scholar] [CrossRef]
- Behrens, R.; Nurse, P. Roles of fission yeast tea1p in the localization of polarity factors and in organizing the microtubular cytoskeleton. J. Cell Biol. 2002, 157, 783–793. [Google Scholar] [CrossRef]
- Feierbach, B.; Verde, F.; Chang, F. Regulation of a formin complex by the microtubule plus end protein tea1p. J. Cell Biol. 2004, 165, 697–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, S.G.; McDonald, W.H.; Yates, J.R., 3rd; Chang, F. Tea4p links microtubule plus ends with the formin for3p in the establishment of cell polarity. Dev. Cell 2005, 8, 479–491. [Google Scholar] [CrossRef] [Green Version]
- Mata, J.; Nurse, P. tea1 and the microtubular cytoskeleton are important for generating global spatial order within the fission yeast cell. Cell 1997, 89, 939–949. [Google Scholar] [CrossRef] [Green Version]
- Tatebe, H.; Shimada, K.; Uzawa, S.; Morigasaki, S.; Shiozaki, K. Wsh3/Tea4 is a novel cell-end factor essential for bipolar distribution of Tea1 and protects cell polarity under environmental stress in S. pombe. Curr. Biol. 2005, 15, 1006–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bicho, C.C.; Kelly, D.A.; Snaith, H.A.; Goryachev, A.B.; Sawin, K.E. A catalytic role for Mod5 in the formation of the Tea1 cell polarity landmark. Curr. Biol. 2010, 20, 1752–1757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snaith, H.A.; Sawin, K.E. Fission yeast mod5p regulates polarized growth through anchoring of tea1p at cell tips. Nature 2003, 423, 647–651. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.G. Microtubule-dependent cell morphogenesis in the fission yeast. Trends Cell Biol. 2009, 19, 447–454. [Google Scholar] [CrossRef]
- Feierbach, B.; Chang, F. Roles of the fission yeast formin for3p in cell polarity, actin cable formation and symmetric cell division. Curr. Biol. 2001, 11, 1656–1665. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, R.; Suo, J.; Young, E.; Chang, E.C. Schizosaccharomyces pombe Arc3 is a conserved subunit of the Arp2/3 complex required for polarity, actin organization, and endocytosis. Yeast 2011, 28, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Castagnetti, S.; Behrens, R.; Nurse, P. End4/Sla2 is involved in establishment of a new growth zone in Schizosaccharomyces pombe. J. Cell Sci. 2005, 118, 1843–1850. [Google Scholar] [CrossRef]
- Rincon, S.; Coll, P.M.; Perez, P. Spatial regulation of Cdc42 during cytokinesis. Cell Cycle 2007, 6, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D. Actin and Actin-Binding Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, P.; Tajadura, V.; Sanchez, Y. The Rho1p exchange factor Rgf1p signals upstream from the Pmk1 mitogen-activated protein kinase pathway in fission yeast. Mol. Biol. Cell 2009, 20, 721–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osumi, M.; Yamada, N.; Kobori, H.; Taki, A.; Naito, N.; Baba, M.; Nagatani, T. Cell wall formation in regenerating protoplast of Schizosaccharomyces pombe: Study by high resolution, low voltage scanning electron microscopy. J. Electron Microsc. 1989, 38, 457–468. [Google Scholar]
- Sugawara, T.; Sato, M.; Takagi, T.; Kamasaki, T.; Ohno, N.; Osumi, M. In situ localization of cell wall alpha-1,3-glucan in the fission yeast Schizosaccharomyces pombe. J. Electron Microsc. 2003, 52, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Ribas, J.C.; Carnero, E.; Duran, A.; Sanchez, Y. bgs2+, a sporulation-specific glucan synthase homologue is required for proper ascospore wall maturation in fission yeast. Mol. Microbiol. 2000, 38, 308–321. [Google Scholar] [CrossRef] [Green Version]
- Osumi, M.; Sato, M.; Ishijima, S.A.; Konomi, M.; Tanagi, T.; Yaguchi, H. Dynamics of cell wall formation in fission yeast, Schizosaccharomyces pombe. Fungal Genet. Biol. 1998, 24, 178–206. [Google Scholar] [CrossRef]
- Humbel, B.M.; Konomi, M.; Takagi, T.; Kamasawa, N.; Ishijima, S.A.; Osumi, M. In situ localization of beta-glucans in the cell wall of Schizosaccharomyces pombe. Yeast 2001, 18, 433–444. [Google Scholar] [CrossRef] [Green Version]
- Manners, D.J.; Meyer, M.T. The molecular structures of some glucans from the cell wall of S. pombe. Carbohyd. Res. 1977, 57, 189–203. [Google Scholar] [CrossRef]
- Cabib, E.; Drgonova, J.; Drgon, T. Role of small G proteins in yeast cell polarization and wall biosynthesis. Annu. Rev. Biochem. 1998, 67, 307–333. [Google Scholar] [CrossRef]
- Cortes, J.C.; Carnero, E.; Ishiguro, J.; Sanchez, Y.; Duran, A.; Ribas, J.C. The novel fission yeast (1,3)beta-D-glucan synthase catalytic subunit Bgs4p is essential during both cytokinesis and polarized growth. J. Cell Sci. 2005, 118, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.C.; Ishiguro, J.; Duran, A.; Ribas, J.C. Localization of the (1,3)beta-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J. Cell Sci. 2002, 115, 4081–4096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wang, H.; Balasubramanian, M.K. A checkpoint that monitors cytokinesis in Schizosaccharomyces pombe. J. Cell Sci. 2000, 113 Pt 7, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; McCollum, D.; Balasubramanian, M.K. Drc1p/Cps1p, a 1,3-beta-glucan synthase subunit, is essential for division septum assembly in Schizosaccharomyces pombe. Genetics 1999, 153, 1193–1203. [Google Scholar] [CrossRef]
- Martin, V.; Garcia, B.; Carnero, E.; Duran, A.; Sanchez, Y. Bgs3p, a putative 1,3-beta-glucan synthase subunit, is required for cell wall assembly in Schizosaccharomyces pombe. Eukaryot. Cell 2003, 2, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Arellano, M.; Duran, A.; Perez, P. Rho 1 GTPase activates the (1-3)beta-D-glucan synthase and is involved in Schizosaccharomyces pombe morphogenesis. EMBO J. 1996, 15, 4584–4591. [Google Scholar] [CrossRef]
- Iwaki, N.; Karatsu, K.; Miyamoto, M. Role of guanine nucleotide exchange factors for Rho family GTPases in the regulation of cell morphology and actin cytoskeleton in fission yeast. Biochem. Biophys. Res. Commun. 2003, 312, 414–420. [Google Scholar] [CrossRef]
- Arellano, M.; Valdivieso, M.H.; Calonge, T.M.; Coll, P.M.; Duran, A.; Perez, P. Schizosaccharomyces pombe protein kinase C homologues, pck1p and pck2p, are targets of rho1p and rho2p and differentially regulate cell integrity. J. Cell Sci. 1999, 112 Pt 20, 3569–3578. [Google Scholar] [CrossRef]
- Martins, I.M.; Cortes, J.C.; Munoz, J.; Moreno, M.B.; Ramos, M.; Clemente-Ramos, J.A.; Duran, A.; Ribas, J.C. Differential activities of three families of specific beta(1,3)glucan synthase inhibitors in wild-type and resistant strains of fission yeast. J. Biol. Chem. 2011, 286, 3484–3496. [Google Scholar] [CrossRef] [Green Version]
- Davi, V.; Tanimoto, H.; Ershov, D.; Haupt, A.; De Belly, H.; Le Borgne, R.; Couturier, E.; Boudaoud, A.; Minc, N. Mechanosensation Dynamically Coordinates Polar Growth and Cell Wall Assembly to Promote Cell Survival. Dev. Cell 2018, 45, 170–182.e7. [Google Scholar] [CrossRef] [Green Version]
- Garcia, P.; Tajadura, V.; Garcia, I.; Sanchez, Y. Role of Rho GTPases and Rho-GEFs in the regulation of cell shape and integrity in fission yeast. Yeast 2006, 23, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.D.; Mata, J. The translational landscape of fission-yeast meiosis and sporulation. Nat. Struct. Mol. Biol. 2014, 21, 641–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davi, V.; Minc, N. Mechanics and morphogenesis of fission yeast cells. Curr. Opin. Microbiol. 2015, 28, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.; Munoz, S.; Manjon, E.; Garcia, P.; Sanchez, Y. The fission yeast cell wall stress sensor-like proteins Mtl2 and Wsc1 act by turning on the GTPase Rho1p but act independently of the cell wall integrity pathway. Microbiologyopen 2013, 2, 778–794. [Google Scholar] [CrossRef] [Green Version]
- Neeli-Venkata, R.; Diaz, C.M.; Celador, R.; Sanchez, Y.; Minc, N. Detection of surface forces by the cell-wall mechanosensor Wsc1 in yeast. Dev. Cell 2021, 56, 2856–2870.e2857. [Google Scholar] [CrossRef]
- Barba, G.; Soto, T.; Madrid, M.; Nunez, A.; Vicente, J.; Gacto, M.; Cansado, J.; Yeast Physiology, G. Activation of the cell integrity pathway is channelled through diverse signalling elements in fission yeast. Cell. Signal. 2008, 20, 748–757. [Google Scholar] [CrossRef]
- Ma, Y.; Kuno, T.; Kita, A.; Asayama, Y.; Sugiura, R. Rho2 is a target of the farnesiltransferase Cpp1 and acts upstream of Pmk1 mitogen-activated protein kinase signaling in fission yeast. Mol. Biol. Cell 2006, 17, 5028–5037. [Google Scholar] [CrossRef]
- Madrid, M.; Jimenez, R.; Sanchez-Mir, L.; Soto, T.; Franco, A.; Vicente-Soler, J.; Gacto, M.; Perez, P.; Cansado, J. Multiple layers of regulation influence cell integrity control by the PKC ortholog Pck2 in fission yeast. J. Cell Sci. 2015, 128, 266–280. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Mir, L.; Soto, T.; Franco, A.; Madrid, M.; Viana, R.A.; Vicente, J.; Gacto, M.; Perez, P.; Cansado, J. Rho1 GTPase and PKC ortholog Pck1 are upstream activators of the cell integrity MAPK pathway in fission yeast. PLoS ONE 2014, 9, e88020. [Google Scholar] [CrossRef] [Green Version]
- Madrid, M.; Soto, T.; Khong, H.K.; Franco, A.; Vicente, J.; Perez, P.; Gacto, M.; Cansado, J. Stress-induced response, localization, and regulation of the Pmk1 cell integrity pathway in Schizosaccharomyces pombe. J. Biol. Chem. 2006, 281, 2033–2043. [Google Scholar] [CrossRef] [Green Version]
- Roncero, C.; Celador, R.; Sanchez, N.; Garcia, P.; Sanchez, Y. The Role of the Cell Integrity Pathway in Septum Assembly in Yeast. J. Fungi 2021, 7, 729. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.A.; Pinar, M.; Soto, T.; Coll, P.M.; Cansado, J.; Perez, P. Negative functional interaction between cell integrity MAPK pathway and Rho1 GTPase in fission yeast. Genetics 2013, 195, 421–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magliozzi, J.O.; Moseley, J.B. Connecting cell polarity signals to the cytokinetic machinery in yeast and metazoan cells. Cell Cycle 2021, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; O’Shaughnessy, B. Molecular Mechanism of Cytokinesis. Annu. Rev. Biochem. 2019, 88, 661–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rincon, S.A.; Paoletti, A. Molecular control of fission yeast cytokinesis. Semin. Cell Dev. Biol. 2016, 53, 28–38. [Google Scholar] [CrossRef]
- Akamatsu, M.; Berro, J.; Pu, K.M.; Tebbs, I.R.; Pollard, T.D. Cytokinetic nodes in fission yeast arise from two distinct types of nodes that merge during interphase. J. Cell Biol. 2014, 204, 977–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moseley, J.B.; Mayeux, A.; Paoletti, A.; Nurse, P. A spatial gradient coordinates cell size and mitotic entry in fission yeast. Nature 2009, 459, 857–860. [Google Scholar] [CrossRef]
- Laporte, D.; Coffman, V.C.; Lee, I.J.; Wu, J.Q. Assembly and architecture of precursor nodes during fission yeast cytokinesis. J. Cell Biol. 2011, 192, 1005–1021. [Google Scholar] [CrossRef] [Green Version]
- Willet, A.H.; McDonald, N.A.; Gould, K.L. Regulation of contractile ring formation and septation in Schizosaccharomyces pombe. Curr. Opin. Microbiol. 2015, 28, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Garcia Cortes, J.C.; Ramos, M.; Osumi, M.; Perez, P.; Ribas, J.C. The Cell Biology of Fission Yeast Septation. Microbiol. Mol. Biol. Rev. 2016, 80, 779–791. [Google Scholar] [CrossRef] [Green Version]
- Perez, P.; Cortes, J.C.; Martin-Garcia, R.; Ribas, J.C. Overview of fission yeast septation. Cell Microbiol. 2016, 18, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- McDonald, N.A.; Lind, A.L.; Smith, S.E.; Li, R.; Gould, K.L. Nanoscale architecture of the Schizosaccharomyces pombe contractile ring. eLife 2017, 6, e28865. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Willet, A.H.; Roberts-Galbraith, R.H.; McDonald, N.A.; Feoktistova, A.; Chen, J.S.; Huang, H.; Guillen, R.; Boone, C.; Sidhu, S.S.; et al. The Cdc15 and Imp2 SH3 domains cooperatively scaffold a network of proteins that redundantly ensure efficient cell division in fission yeast. Mol. Biol. Cell 2015, 26, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Roberts-Galbraith, R.H.; Chen, J.S.; Wang, J.; Gould, K.L. The SH3 domains of two PCH family members cooperate in assembly of the Schizosaccharomyces pombe contractile ring. J. Cell Biol. 2009, 184, 113–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts-Galbraith, R.H.; Ohi, M.D.; Ballif, B.A.; Chen, J.S.; McLeod, I.; McDonald, W.H.; Gygi, S.P.; Yates, J.R., 3rd; Gould, K.L. Dephosphorylation of F-BAR protein Cdc15 modulates its conformation and stimulates its scaffolding activity at the cell division site. Mol. Cell 2010, 39, 86–99. [Google Scholar] [CrossRef] [Green Version]
- Davidson, R.; Laporte, D.; Wu, J.Q. Regulation of Rho-GEF Rgf3 by the arrestin Art1 in fission yeast cytokinesis. Mol. Biol. Cell 2015, 26, 453–466. [Google Scholar] [CrossRef]
- Edreira, T.; Celador, R.; Manjon, E.; Sanchez, Y. A novel checkpoint pathway controls actomyosin ring constriction trigger in fission yeast. eLife 2020, 9, e59333. [Google Scholar] [CrossRef]
- Alonso-Nunez, M.L.; An, H.; Martin-Cuadrado, A.B.; Mehta, S.; Petit, C.; Sipiczki, M.; del Rey, F.; Gould, K.L.; de Aldana, C.R. Ace2p controls the expression of genes required for cell separation in Schizosaccharomyces pombe. Mol. Biol. Cell 2005, 16, 2003–2017. [Google Scholar] [CrossRef] [Green Version]
- Arasada, R.; Pollard, T.D. Contractile ring stability in S. pombe depends on F-BAR protein Cdc15p and Bgs1p transport from the Golgi complex. Cell Rep. 2014, 8, 1533–1544. [Google Scholar] [CrossRef] [Green Version]
- Arasada, R.; Pollard, T.D. A role for F-BAR protein Rga7p during cytokinesis in S. pombe. J. Cell Sci. 2015, 128, 2259–2268. [Google Scholar] [CrossRef] [Green Version]
- Martin-Cuadrado, A.B.; Duenas, E.; Sipiczki, M.; Vazquez de Aldana, C.R.; del Rey, F. The endo-beta-1,3-glucanase eng1p is required for dissolution of the primary septum during cell separation in Schizosaccharomyces pombe. J. Cell Sci. 2003, 116, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Dekker, N.; Speijer, D.; Grun, C.H.; van den Berg, M.; de Haan, A.; Hochstenbach, F. Role of the alpha-glucanase Agn1p in fission-yeast cell separation. Mol. Biol. Cell 2004, 15, 3903–3914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, I.; Jimenez, D.; Martin, V.; Duran, A.; Sanchez, Y. The alpha-glucanase Agn1p is required for cell separation in Schizosaccharomyces pombe. Biol. Cell 2005, 97, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.E.; McCollum, D.; Gould, K.L. Polar opposites: Fine-tuning cytokinesis through SIN asymmetry. Cytoskeleton 2012, 69, 686–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simanis, V. Pombe’s thirteen—Control of fission yeast cell division by the septation initiation network. J. Cell Sci. 2015, 128, 1465–1474. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Li, W.; Liu, Y.; Amon, A. Cross-compartment signal propagation in the mitotic exit network. eLife 2021, 10, e63645. [Google Scholar] [CrossRef]
- Sparks, C.A.; Morphew, M.; McCollum, D. Sid2p, a spindle pole body kinase that regulates the onset of cytokinesis. J. Cell Biol. 1999, 146, 777–790. [Google Scholar] [CrossRef] [Green Version]
- Bohnert, K.A.; Grzegorzewska, A.P.; Willet, A.H.; Vander Kooi, C.W.; Kovar, D.R.; Gould, K.L. SIN-dependent phosphoinhibition of formin multimerization controls fission yeast cytokinesis. Genes Dev. 2013, 27, 2164–2177. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Mana-Capelli, S.; McLean, J.R.; Chen, C.T.; Ray, S.; Gould, K.L.; McCollum, D. Identification of SIN pathway targets reveals mechanisms of crosstalk between NDR kinase pathways. Curr. Biol. 2013, 23, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Mana-Capelli, S.; McLean, J.R.; Chen, C.T.; Gould, K.L.; McCollum, D. The kinesin-14 Klp2 is negatively regulated by the SIN for proper spindle elongation and telophase nuclear positioning. Mol. Biol. Cell 2012, 23, 4592–4600. [Google Scholar] [CrossRef]
- Rincon, S.A.; Estravis, M.; Dingli, F.; Loew, D.; Tran, P.T.; Paoletti, A. SIN-Dependent Dissociation of the SAD Kinase Cdr2 from the Cell Cortex Resets the Division Plane. Curr. Biol. 2017, 27, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Willet, A.H.; DeWitt, A.K.; Beckley, J.R.; Clifford, D.M.; Gould, K.L. NDR Kinase Sid2 Drives Anillin-like Mid1 from the Membrane to Promote Cytokinesis and Medial Division Site Placement. Curr. Biol. 2019, 29, 1055–1063.e1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hachet, O.; Simanis, V. Mid1p/anillin and the septation initiation network orchestrate contractile ring assembly for cytokinesis. Genes Dev. 2008, 22, 3205–3216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Q.W.; Zhou, M.; Bimbo, A.; Balasubramanian, M.K.; McCollum, D. A role for the septation initiation network in septum assembly revealed by genetic analysis of sid2-250 suppressors. Genetics 2006, 172, 2101–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcaide-Gavilan, M.; Lahoz, A.; Daga, R.R.; Jimenez, J. Feedback regulation of SIN by Etd1 and Rho1 in fission yeast. Genetics 2014, 196, 455–470. [Google Scholar] [CrossRef] [Green Version]
- Sengar, A.S.; Markley, N.A.; Marini, N.J.; Young, D. Mkh1, a MEK kinase required for cell wall integrity and proper response to osmotic and temperature stress in Schizosaccharomyces pombe. Mol. Cell Biol. 1997, 17, 3508–3519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaitsevskaya-Carter, T.; Cooper, J.A. Spm1, a stress-activated MAP kinase that regulates morphogenesis in S. pombe. EMBO J. 1997, 16, 1318–1331. [Google Scholar] [CrossRef] [Green Version]
- Bohnert, K.A.; Gould, K.L. Cytokinesis-based constraints on polarized cell growth in fission yeast. PLoS Genet. 2012, 8, e1003004. [Google Scholar] [CrossRef] [Green Version]
- Bohnert, K.A.; Rossi, A.M.; Jin, Q.W.; Chen, J.S.; Gould, K.L. Phosphoregulation of the cytokinetic protein Fic1 contributes to fission yeast growth polarity establishment. J. Cell Sci. 2020, 133, jcs244392. [Google Scholar] [CrossRef]
- Rajakyla, E.K.; Vartiainen, M.K. Rho, nuclear actin, and actin-binding proteins in the regulation of transcription and gene expression. Small GTPases 2014, 5, e27539. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, J.A.; Vartiainen, M.K. Diverse functions for different forms of nuclear actin. Curr. Opin. Cell Biol. 2017, 46, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.; Chen, M.; Winkler, D.D.; Luger, K.; Shen, X. Evidence for monomeric actin function in INO80 chromatin remodeling. Nat. Struct. Mol. Biol. 2013, 20, 426–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belin, B.J.; Lee, T.; Mullins, R.D. DNA damage induces nuclear actin filament assembly by Formin -2 and Spire-(1/2) that promotes efficient DNA repair. [corrected]. eLife 2015, 4, e07735. [Google Scholar] [CrossRef]
- Caridi, C.P.; Plessner, M.; Grosse, R.; Chiolo, I. Nuclear actin filaments in DNA repair dynamics. Nat. Cell Biol. 2019, 21, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Hurst, V.; Shimada, K.; Gasser, S.M. Nuclear Actin and Actin-Binding Proteins in DNA Repair. Trends Cell Biol. 2019, 29, 462–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGowan, C.H.; Russell, P. The DNA damage response: Sensing and signaling. Curr. Opin. Cell Biol. 2004, 16, 629–633. [Google Scholar] [CrossRef]
- Kume, K. Control of cellular organization and its coordination with the cell cycle. Biosci. Biotechnol. Biochem. 2020, 84, 869–875. [Google Scholar] [CrossRef]
- Meister, P.; Poidevin, M.; Francesconi, S.; Tratner, I.; Zarzov, P.; Baldacci, G. Nuclear factories for signalling and repairing DNA double strand breaks in living fission yeast. Nucleic Acids Res. 2003, 31, 5064–5073. [Google Scholar] [CrossRef]
- Raji, H.; Hartsuiker, E. Double-strand break repair and homologous recombination in Schizosaccharomyces pombe. Yeast 2006, 23, 963–976. [Google Scholar] [CrossRef]
- Schrank, B.R.; Aparicio, T.; Li, Y.; Chang, W.; Chait, B.T.; Gundersen, G.G.; Gottesman, M.E.; Gautier, J. Nuclear ARP2/3 drives DNA break clustering for homology-directed repair. Nature 2018, 559, 61–66. [Google Scholar] [CrossRef]
- Swaffer, M.P.; Jones, A.W.; Flynn, H.R.; Snijders, A.P.; Nurse, P. Quantitative Phosphoproteomics Reveals the Signaling Dynamics of Cell-Cycle Kinases in the Fission Yeast Schizosaccharomyces pombe. Cell Rep. 2018, 24, 503–514. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, P.; Celador, R.; Pérez-Parrilla, J.; Sánchez, Y. Fission Yeast Rho1p-GEFs: From Polarity and Cell Wall Synthesis to Genome Stability. Int. J. Mol. Sci. 2022, 23, 13888. https://doi.org/10.3390/ijms232213888
García P, Celador R, Pérez-Parrilla J, Sánchez Y. Fission Yeast Rho1p-GEFs: From Polarity and Cell Wall Synthesis to Genome Stability. International Journal of Molecular Sciences. 2022; 23(22):13888. https://doi.org/10.3390/ijms232213888
Chicago/Turabian StyleGarcía, Patricia, Rubén Celador, Jorge Pérez-Parrilla, and Yolanda Sánchez. 2022. "Fission Yeast Rho1p-GEFs: From Polarity and Cell Wall Synthesis to Genome Stability" International Journal of Molecular Sciences 23, no. 22: 13888. https://doi.org/10.3390/ijms232213888




