Intraoperative Tumor Detection Using Pafolacianine
Abstract
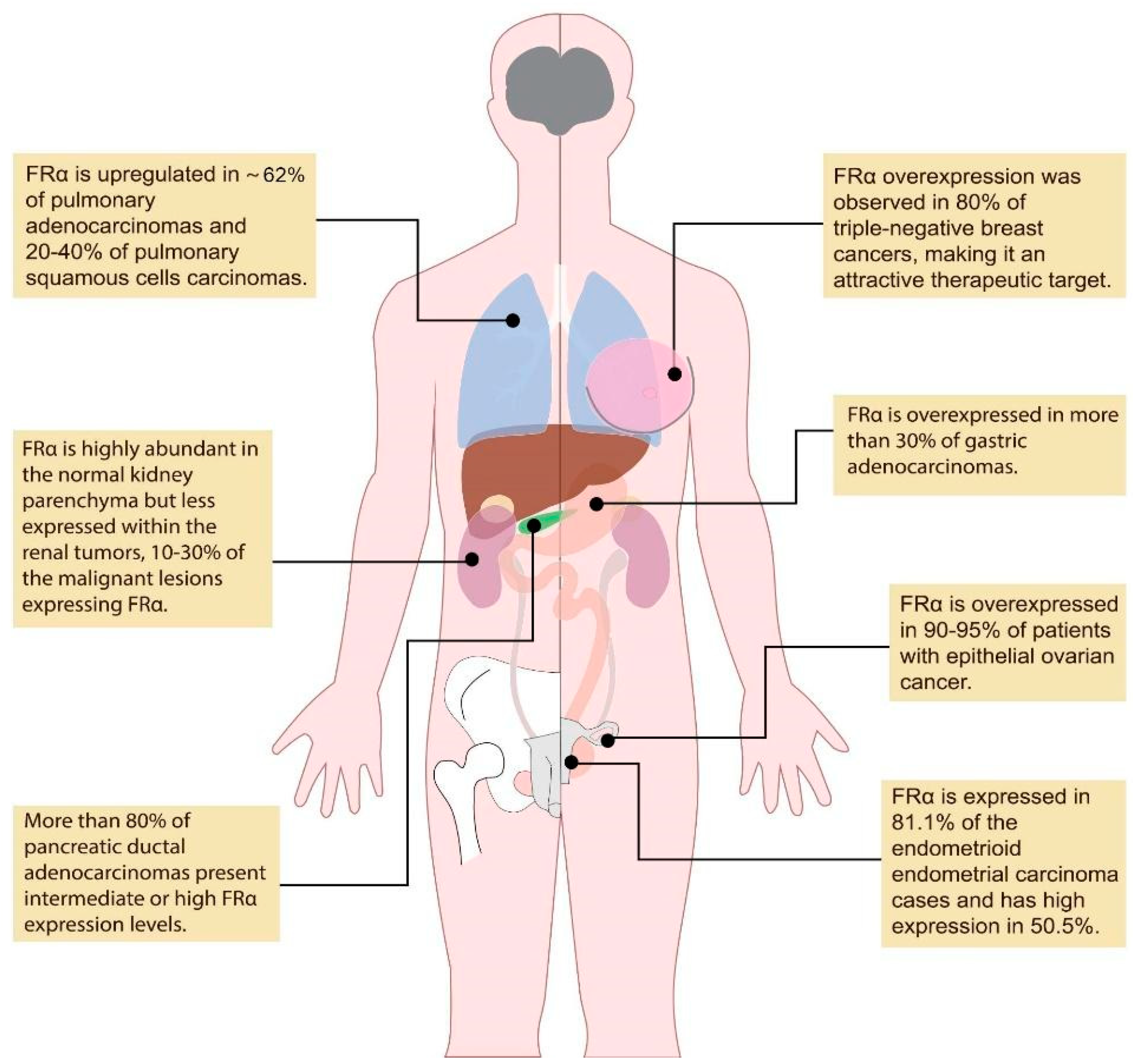
:1. Introduction
2. FDA-Approved Pafolacianine for Intraoperative Ovarian Cancer Detection
3. Pafolacianine for the Detection of a Wide Variety of Malignancies during Surgery
3.1. Lung Cancer
3.2. Renal Cancer
3.3. Pituitary Tumors
3.4. Gastric Cancer
4. Pafolacianine in Comparison with Other Similar Agents Used for Intraoperative Molecular Imaging
5. Benefits and Limitations of Using Pafolacianine for Cancer Detection during Surgery
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 25 August 2022).
- National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/understanding/statistics (accessed on 25 August 2022).
- Canadian Cancer Society. Available online: https://cancer.ca/en/treatments/treatment-types/surgery (accessed on 25 August 2022).
- Hoogstins, C.E.; Tummers, Q.R.; Gaarenstroom, K.N.; De Kroon, C.D.; Trimbos, J.B.; Bosse, T. A novel tumor-specific agent for intraoperative near-infrared fluorescence imaging: A translational study in healthy volunteers and patients with ovarian cancer. Clin. Cancer Res. 2016, 22, 2929–2938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Network. Available online: https://www.cancernetwork.com/view/a-look-behind-development-of-pafolacianine-for-tumor-detection-during-ovarian-cancer-surgery (accessed on 25 August 2022).
- U.S. Food and Drug Administration (FDA). Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pafolacianine-identifying-malignant-ovarian-cancer-lesions (accessed on 25 August 2022).
- National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Pafolacianine (accessed on 25 August 2022).
- Pafolacianine. Drugbank. Available online: https://go.drugbank.com/drugs/DB15413 (accessed on 25 August 2022).
- Zhang, R.R.; Schroeder, A.B.; Grudzinski, J.J.; Rosenthal, E.L.; Warram, J.M.; Pinchuk, A.N. Beyond the margins: Real-time detection of cancer using targeted fluorophores. Nat. Rev. Clin. Oncol. 2017, 14, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, W.; Low, P.S. Folate-targeted therapies for cancer. J. Med. Chem. 2010, 53, 6811–6824. [Google Scholar] [CrossRef] [PubMed]
- O’Shannessy, D.J.; Yu, G.; Smale, R.; Fu, Y.S.; Singhal, S.; Thiel, R.P. Folate receptor alpha expression in lung cancer: Diagnostic and prognostic significance. Oncotarget 2012, 3, 414–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boogerd, L.S.; Boonstra, M.C.; Beck, A.J.; Charehbili, A.; Hoogstins, C.E.; Prevoo, H.A. Concordance of folate receptor-α expression between biopsy, primary tumor and metastasis in breast cancer and lung cancer patients. Oncotarget 2016, 7, 17442–17454. [Google Scholar] [CrossRef] [Green Version]
- Predina, J.D.; Newton, A.D.; Connolly, C.; Dunbar, A.; Baldassari, M.; Deshpande, C. Identification of a Folate Receptor-Targeted Near-Infrared Molecular Contrast Agent to Localize Pulmonary Adenocarcinomas. Mol. Ther. 2018, 26, 390–403. [Google Scholar] [CrossRef] [Green Version]
- Kalli, K.R.; Oberg, A.L.; Keeney, G.L.; Christianson, T.J.; Low, P.S.; Knutson, K.L. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol. Oncol. 2008, 108, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Markert, S.; Lassmann, S.; Gabriel, B.; Klar, M.; Werner, M.; Gitsch, G. Alpha-folate receptor expression in epithelial ovarian carcinoma and non-neoplastic ovarian tissue. Anticancer Res. 2008, 28, 3567–3572. [Google Scholar]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef]
- Ross, J.F.; Chaudhuri, P.K.; Ratnam, M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer 1994, 73, 2432–2443. [Google Scholar] [CrossRef]
- Sulek, J.E.; Steward, J.E.; Bahler, C.D.; Jacobsen, M.H.; Sundaram, A.; Shum, C.F. Folate-targeted intraoperative fluorescence, OTL38, in robotic-assisted laparoscopic partial nephrectomy. Scand. J. Urol. 2021, 55, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Michelakos, T.; Ferrone, C.R.; Zhang, L.; Deshpande, V.; Shen, Q. Expression status of folate receptor alpha is a predictor of survival in pancreatic ductal adenocarcinoma. Oncotarget 2017, 8, 37646–37656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senol, S.; Ceyran, A.B.; Aydin, A.; Zemheri, E.; Ozkanli, S.; Kösemetin, D. Folate receptor α expression and significance in endometrioid endometrium carcinoma and endometrial hyperplasia. Int. J. Clin. Exp. Pathol. 2015, 8, 5633–5641. [Google Scholar] [PubMed]
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016, 7, 52553–52574. [Google Scholar] [CrossRef] [Green Version]
- Newton, A.D.; Predina, J.D.; Frenzel-Sulyok, L.G.; Low, P.S.; Singhal, S.; Roses, R.E. Intraoperative Molecular Imaging Utilizing a Folate Receptor-Targeted Near-Infrared Probe Can Identify Macroscopic Gastric Adenocarcinomas. Mol. Imaging Biol. 2021, 23, 11–17. [Google Scholar] [CrossRef]
- Mahalingam, S.M.; Kularatne, S.A.; Myers, C.H.; Gagare, P.; Norshi, M.; Liu, X. Evaluation of Novel Tumor-Targeted Near-Infrared Probe for Fluorescence-Guided Surgery of Cancer. J. Med. Chem. 2018, 61, 9637–9646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Gondos, A.; Bray, F.; Hakulinen, T.; Brenner, H.; Aareleid, T.; Willem, J. Trends in cancer survival in 11 European populations from 1990 to 2009: A model-based analysis. Ann. Oncol. 2009, 20, 564–573. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T. Cancer Statistics, 2008. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef]
- Aletti, G.D.; Gallenberg, M.M.; Cliby, W.A.; Jatoi, A.; Hartmann, L.C. Current management strategies for ovarian cancer. Mayo Clin. Proc. 2007, 82, 751–770. [Google Scholar] [CrossRef]
- Winter, W.E.; Maxwell, G.L.; Tian, C.; Sundborg, M.J.; Rose, G.S.; Rose, P.G. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: A Gynecologic Oncology Group study. J. Clin. Oncol. 2008, 26, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Ibeanu, O.A.; Bristow, R.E. Predicting the outcome of cytoreductive surgery for advanced ovarian cancer: A review. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2010, 20 (Suppl. 1), S1–S11. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.J.; Bristow, R.E.; Ryu, H.S. Impact of complete cytoreduction leaving no gross residual disease associated with radical cytoreductive surgical procedures on survival in advanced ovarian cancer. Ann. Surg. Oncol. 2012, 19, 4059–4067. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef] [Green Version]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef]
- Hoskins, W.J.; McGuire, W.P.; Brady, M.F.; Homesley, H.D.; Creasman, W.T.; Berman, M. The effect of diameter of largest residual disease on survival after primary. Am. J. Obstet. Gynecol. 1994, 170, 974–979. [Google Scholar] [CrossRef]
- Bristow, R.E.; Berek, J.S. Surgery for ovarian cancer: How to improve survival. Lancet 2006, 367, 1558–1560. [Google Scholar] [CrossRef]
- Koppe, M.J.; Boerman, O.C.; Oyen, W.J.; Bleichrodt, R.P. Peritoneal carcinomatosis of colorectal origin: Incidence and current treatment strategies. Ann. Surg. 2006, 243, 212–222. [Google Scholar] [CrossRef]
- Vergote, I.; Van Gorp, T.; Amant, F.; Leunen, K.; Neven, P.; Berteloot, P. Timing of debulking surgery in advanced ovarian cancer. Int. J. Gynecol. Cancer 2008, 18, 11–19. [Google Scholar] [CrossRef]
- Aletti, G.D.; Dowdy, S.C.; Gostout, B.S.; Jones, M.B.; Stanhope, C.R.; Wilson, T.O. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet. Gynecol. 2006, 107, 77–85. [Google Scholar] [CrossRef]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Gunning, W.; Ratnam, M. Expression of folate receptor type alpha in relation to cell type, malignancy, and differentiation in ovary, uterus, and cervix. Cancer Epidemiol. Biomark. Prev. 1999, 8, 775–782. [Google Scholar]
- Shen, J.; Hilgenbrink, A.R.; Xia, W.; Feng, Y.; Dimitrov, D.S.; Lockwood, M.B. Folate receptor-β constitutes a marker for human proinflammatory monocytes. J. Leukoc. Biol. 2014, 96, 563–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Shannessy, D.J.; Somers, E.B.; Wang, L.C.; Wang, H.; Hsu, R. Expression of folate receptors alpha and beta in normal and cancerous gynecologic tissues: Correlation of expression of the beta isoform with macrophage markers. J. Ovarian Res. 2015, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Puig-Kröger, A.; Sierra-Filardi, E.; Domínguez-Soto, A.; Samaniego, R.; Corcuera, M.T.; Gómez-Aguado, F. Folate receptor β is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory Macrophages. Cancer Res. 2009, 6, 9395–9403. [Google Scholar] [CrossRef] [Green Version]
- Kurahara, H.; Takao, S.; Kuwahata, T.; Nagai, T.; Ding, Q.; Maeda, K. Clinical Significance of Folate Receptor β–expressing Tumor-associated Macrophages in Pancreatic Cancer. Ann. Surg. Oncol. 2012, 19, 2264–2271. [Google Scholar] [CrossRef]
- Smith, H.A.; Kang, Y. The metastasis-promoting roles of tumor-associated immune cells. J. Mol. Med. 2013, 91, 411–429. [Google Scholar] [CrossRef]
- Joyce, J.A.; Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef]
- Lewis, C.E.; Pollard, J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006, 66, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Randall, L.M.; Wenham, R.M.; Low, P.S.; Dowdy, S.C.; Tanyi, J.L. A phase II, multicenter, open-label trial of OTL38 injection for the intra-operative imaging of folate receptor-alpha positive ovarian cancer. Gynecol. Oncol. 2019, 155, 63–68. [Google Scholar] [CrossRef]
- Shen, J.; Putt, K.S.; Visscher, D.W.; Murphy, L.; Cohen, C.; Singhal, S. Assessment of folate receptor-β expression in human neoplastic tissues. Oncotarget 2015, 6, 14700–14709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Hu, Y.; Putt, K.S.; Singhal, S.; Han, H.; Visscher, D.W. Assessment of folate receptor alpha and beta expression in selection of lung and pancreatic cancer patients for receptor targeted therapies. Oncotarget 2018, 9, 4485–4495. [Google Scholar] [CrossRef]
- Yale Medicine. Available online: https://www.yalemedicine.org/conditions/non-small-cell-lung-cancer (accessed on 25 August 2022).
- Kelsey, C.R.; Marks, L.B.; Hollis, D.; Hubbs, J.L.; Ready, N.E.; D’Amico, T.A. Local recurrence after surgery for early-stage lung cancer: An 11-year experience with 975 patients. Cancer 2009, 115, 5218–5227. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; Bryant, A.S. Is palpation of the nonresected pulmonary lobe(s) required for patients with non-small cell lung cancer? A prospective study. J. Thorac. Cardiovasc. Surg. 2008, 135, 261–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.W.; Rizzo, S.; Ma, L.H.; Qiu, X.Y.; Warth, A.; Seki, N. Pulmonary ground-glass opacity: Computed tomography features, histopathology and molecular pathology. Transl. Lung Cancer Res. 2017, 6, 68–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tipirneni, K.E.; Warram, J.M.; Moore, L.S.; Prince, A.C.; De Boer, E.; Jani, A.H. Oncologic Procedures Amenable to Fluorescence-guided Surgery. Ann. Surg. 2017, 266, 36–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tipirneni, K.E.; Rosenthal, E.L.; Moore, L.S.; Haskins, A.D.; Udayakumar, N.; Jani, A.H. Fluorescence Imaging for Cancer Screening and Surveillance. Mol. Imaging Biol. 2017, 19, 645–655. [Google Scholar] [CrossRef]
- Azari, F.; Kennedy, G.; Bernstein, E.; Delikatny, J.; Lee, J.Y.; Kucharczuk, J. Evaluation of OTL38-Generated Tumor-to-Background Ratio in Intraoperative Molecular Imaging-Guided Lung Cancer Resections. Mol. Imaging Biol. 2021. [Google Scholar] [CrossRef]
- Predina, J.D.; Newton, A.; Corbett, C.; Xia, L.; Sulyok, L.F.; Shin, M. Localization of Pulmonary Ground-Glass Opacities with Folate Receptor–Targeted Intraoperative Molecular Imaging. J. Thorac. Oncol. 2018, 13, 1028–1036. [Google Scholar] [CrossRef] [Green Version]
- Newton, A.D.; Predina, J.D.; Nie, S.; Low, P.S.; Singhal, S. Intraoperative fluorescence imaging in thoracic surgery. J. Surg. Oncol. 2018, 118, 344–355. [Google Scholar] [CrossRef]
- Fisher, R.E.; Siegel, B.A.; Edell, S.L.; Oyesiku, N.M.; Morgenstern, D.E.; Messmann, R.A. Exploratory study of 99mTc-EC20 imaging for identifying patients with folate receptor-positive solid tumors. J. Nucl. Med. 2008, 49, 899–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shum, C.F.; Bahler, C.D.; Low, P.S.; Ratliff, T.L.; Kheyfets, S.V.; Natarajan, J.P. Novel Use of Folate-Targeted Intraoperative Fluorescence, OTL38, in Robot-Assisted Laparoscopic Partial Nephrectomy: Report of the First Three Cases. J. Endourol. Case Rep. 2016, 2, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Hekman, M.C.; Rijpkema, M.; Langenhuijsen, J.F.; Boerman, O.C.; Oosterwijk, E.; Mulders, P.F. Intraoperative Imaging Techniques to Support Complete Tumor Resection in Partial Nephrectomy. Eur. Urol. Focus 2018, 4, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, T.J.; Jiang, J.; Keating, J.; DeJesus, E.; Judy, R.; Nie, S. Intraoperative Molecular Diagnostic Imaging Can Identify Renal Cell Carcinoma. J. Urol. 2016, 195, 748–755. [Google Scholar] [CrossRef]
- Wallis, C.J.; Garbens, A.; Chopra, S.; Gill, I.S.; Satkunasivam, R. Robotic Partial Nephrectomy: Expanding Utilization, Advancing Innovation. J. Endourol. 2017, 31, 348–354. [Google Scholar] [CrossRef]
- Ghani, K.R.; Sukumar, S.; Sammon, J.D.; Rogers, C.G.; Trinh, Q.D.; Menon, M. Practice patterns and outcomes of open and minimally invasive partial nephrectomy since the introduction of robotic partial nephrectomy: Results from the nationwide inpatient sample. J. Urol. 2014, 191, 907–912. [Google Scholar] [CrossRef]
- Rogers, C.G.; Laungani, R.; Bhandari, A.; Krane, L.S.; Eun, D.; Patel, M.N. Maximizing console surgeon independence during robot-assisted renal surgery by using the Fourth Arm and TilePro. J. Endourol. 2009, 23, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Krane, L.S.; Manny, T.B.; Hemal, A.K. Is near infrared fluorescence imaging using indocyanine green dye useful in robotic partial nephrectomy: A prospective comparative study of 94 patients. Urology 2012, 80, 110–116. [Google Scholar] [CrossRef]
- Cho, S.S.; Lee, J.Y. Intraoperative Fluorescent Visualization of Pituitary Adenomas. Neurosurg. Clin. N. Am. 2019, 30, 401–412. [Google Scholar] [CrossRef]
- Lakomkin, N.; Van Gompel, J.J.; Post, K.D.; Cho, S.S.; Lee, J.Y.; Hadjipanayis, C.G. Fluorescence guided surgery for pituitary adenomas. J. Neurooncol. 2021, 151, 403–413. [Google Scholar] [CrossRef]
- Galt, J.R.; Halkar, R.K.; Evans, C.O.; Osman, N.A.; LaBorde, D.; Fox, T.H. In vivo assay of folate receptors in nonfunctional pituitary adenomas with99mTc-Folate SPECT/CT. J. Nucl. Med. 2010, 51, 1716–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, C.O.; Yao, C.; LaBorde, D.; Oyesiku, N.M. Chapter 8 Folate Receptor Expression in Pituitary Adenomas. Cellular and Molecular Analysis. Vitam. Horm. 2008, 79, 235–266. [Google Scholar] [PubMed]
- Evans, C.O.; Reddy, P.; Brat, D.J.; O’Neill, E.B.; Craige, B.; Stevens, V.L. Differential expression of folate receptor in pituitary adenomas. Cancer Res. 2003, 63, 4218–4224. [Google Scholar]
- Evans, C.O.; Young, A.N.; Brown, M.R.; Brat, D.J.; Parks, J.S.; Neish, A.S. Novel patterns of gene expression in pituitary adenomas identified by complementary deoxyribonucleic acid microarrays and quantitative reverse transcription-polymerase chain reaction. J. Clin. Endocrinol. Metab. 2001, 86, 3097–3107. [Google Scholar] [PubMed] [Green Version]
- Lee, J.Y.; Cho, S.S.; Zeh, R.; Pierce, J.T.; Martinez-Lage, M.; Adappa, N.D. Folate receptor overexpression can be visualized in real time during pituitary adenoma endoscopic transsphenoidal surgery with near-infrared imaging. J. Neurosurg. 2018, 129, 390–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.S.; Jeon, J.; Buch, L.; Nag, S.; Nasrallah, M.; Low, P.S. Intraoperative near-infrared imaging with receptor-specific versus passive delivery of fluorescent agents in pituitary adenomas. J. Neurosurg. 2019, 131, 1974–1984. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.S.; Zeh, R.; Pierce, J.T.; Jeon, J.; Nasrallah, M.; Adappa, N.D. Folate Receptor Near-Infrared Optical Imaging Provides Sensitive and Specific Intraoperative Visualization of Nonfunctional Pituitary Adenomas. Oper. Neurosurg. 2019, 16, 59–70. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Bentrem, D.; Gerdes, H.; Tang, L.; Brennan, M.; Coit, D. Clinical correlation of endoscopic ultrasonography with pathologic stage and outcome in patients undergoing curative resection for gastric cancer. Ann. Surg. Oncol. 2007, 14, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Spolverato, G.; Ejaz, A.; Kim, Y.; Squires, M.H.; Poultsides, G.A.; Fields, R.C. Use of endoscopic ultrasound in the preoperative staging of gastric cancer: A multi-institutional study of the US gastric cancer collaborative. J. Am. Coll. Surg. 2015, 220, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.D.; Zhao, C.; Ren, G.S. Endoscopic ultrasonography in preoperative staging of gastric cancer: Determination of tumor invasion depth, nodal involvement and surgical resectability. World J. Gastroenterol. 2003, 9, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Bucur, O. Emerging technologies for diagnostic pathology. Discoveries 2015, 3, e46. [Google Scholar] [CrossRef] [Green Version]
- Dindere, M.E.; Bucur, O. Cancer detection during surgery: FDA-approved use of pafolacianine. Discov. Rep. 2022, 5, e30. [Google Scholar] [CrossRef]
- Bucur, O.; Fu, F.; Calderon, M.; Mylvaganam, G.H.; Ly, N.L.; Day, J. Nanoscale imaging of clinical specimens using conventional and rapid-expansion pathology. Nat. Protoc. 2020, 15, 1649–1672. [Google Scholar] [CrossRef] [Green Version]
- Mediu, R.; Rama, A.; Mediu, N. Screening for prostate cancer: A study on the free and total prostate specific antigen. Discoveries 2021, 9, e143. [Google Scholar] [CrossRef]
- Klimas, A.; Bucur, O.; Njeri, B.; Zhao, Y. Nanoscopic Imaging of Human Tissue Sections via Physical and Isotropic Expansion. J. Vis. Exp. 2019, 151, e60195. [Google Scholar] [CrossRef]
- Nishith, N.; Rao, R.N.; Rai, P. Cytologic Categorization with Risk Stratification of Endoscopic Ultrasound-Guided Fine Needle Aspiration from Pancreatic Lesions Based on Guidelines of the Papanicolaou Society of Cytopathology: 12-Year Tertiary Care Experience. Discoveries 2021, 9, e134. [Google Scholar] [CrossRef]
- Zhao, Y.; Bucur, O.; Irshad, H.; Chen, F.; Weins, A.; Stancu, A.L. Nanoscale imaging of clinical specimens using pathology-optimized expansion microscopy. Nat. Biotechnol. 2017, 35, 757–764. [Google Scholar] [CrossRef]
- Lee, C.M.; Tian, X.; Tsao, C.; Chen, P.; Huang, T.N.; Hsueh, Y.P. Macro photography with Lightsheet Illumination Enables Whole Expanded Brain Imaging with Single-cell Resolution. Discoveries 2021, 9, e133. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, R.; Kamath, R.; Pandav, K.; Mehendale, M. Transvaginal ultrasonography versus hysteroscopy in endometrial pathology diagnosis among women with abnormal uterine bleeding. Discov. Rep. 2021, 4, e24. [Google Scholar] [CrossRef]
- Predina, J.D.; Newton, A.D.; Keating, J.; Barbosa, E.M.; Okusanya, O.; Xia, L. Intraoperative Molecular Imaging Combined with Positron Emission Tomography Improves Surgical Management of Peripheral Malignant Pulmonary Nodules. Ann. Surg. 2017, 266, 479–488. [Google Scholar] [CrossRef]
- De Jesus, E.; Keating, J.J.; Kularatne, S.A.; Jiang, J.; Judy, R.; Predina, J. Comparison of Folate Receptor Targeted Optical Contrast Agents for Intraoperative Molecular Imaging. Int. J. Mol. Imaging 2015, 2015, 469047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boogerd, L.S.; Hoogstins, C.E.; Gaarenstroom, K.N.; de Kroon, C.D.; Beltman, J.J.; Bosse, T. Folate receptor-α targeted near-infrared fluorescence imaging in high-risk endometrial cancer patients: A tissue microarray and clinical feasibility study. Oncotarget 2018, 9, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Predina, J.D.; Newton, A.; Deshpande, C.; Low, P.; Singhal, S. Utilization of targeted near-infrared molecular imaging to improve pulmonary metastasectomy of osteosarcomas. J. Biomed. Opt. 2018, 23, 016005. [Google Scholar] [CrossRef] [Green Version]
- Stewart, H.L.; Birch, D.J. Fluorescence Guided Surgery. Methods Appl. Fluoresc. 2021, 9, 042002. [Google Scholar] [CrossRef]
- Barth, C.W.; Gibbs, S.L. Fluorescence Image-Guided Surgery—A Perspective on Contrast Agent Development. Proc. SPIE Int. Soc. Opt. Eng. 2020, 11222, 112220J. [Google Scholar]
- Yamada, M.; Miller, D.M.; Lowe, M. A first-in-human study of BLZ-100 (tozuleristide) demonstrates tolerability and safety in skin cancer patients. Contemp. Clin. Trials Commun. 2021, 23, 100830. [Google Scholar] [CrossRef]
- Olson, M.T.; Ly, Q.P.; Mohs, A.M. Fluorescence Guidance in Surgical Oncology: Challenges, Opportunities, and Translation. Mol. Imaging Biol. 2019, 21, 200–218. [Google Scholar] [CrossRef]
- Gutowski, M.; Framery, B.; Boonstra, M.C.; Garambois, V.; Quenet, F.; Dumas, K.; Scherninski, F.; Cailler, F.; Vahrmeijer, A.L.; Pèlegrin, A.; et al. SGM-101: An innovative near-infrared dye-antibody conjugate that targets CEA for fluorescence-guided surgery. Surg. Oncol. 2017, 26, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E.L.; Warram, J.M.; de Boer, E.; Chung, T.K.; Korb, M.L.; Brandwein-Gensler, M. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 3658–3666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martoccia, C.; Zellweger, M.; Lovisa, B.; Jichlinski, P.; van den Bergh, H.; Wagnières, G. Optical spectroscopy of the bladder washout fluid to optimize fluorescence cystoscopy with Hexvix®. J. Biomed. Opt. 2014, 19, 97002. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Stummer, W. 5-ALA and FDA approval for glioma surgery. J. Neurooncol. 2019, 141, 479–486. [Google Scholar] [CrossRef]
- Sylantiev, C.; Schoenfeld, N.; Mamet, R.; Groozman, G.B.; Drory, V.E. Acute neuropathy mimicking porphyria induced by aminolevulinic acid during photodynamic therapy. Muscle Nerve 2005, 31, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Falco, J.; Cavallo, C.; Vetrano, I.G.; de Laurentis, C.; Siozos, L.; Schiariti, M. Fluorescein Application in Cranial and Spinal Tumors Enhancing at Preoperative MRI and Operated with a Dedicated Filter on the Surgical Microscope: Preliminary Results in 279 Patients Enrolled in the Fluocertum Prospective Study. Front. Surg. 2019, 6, 49. [Google Scholar] [CrossRef] [Green Version]
- Cavallo, C.; De Laurentis, C.; Vetrano, I.G.; Falco, J.; Broggi, M.; Schiariti, M. The utilization of fluorescein in brain tumor surgery: A systematic review. J. Neurosurg. Sci. 2018, 62, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, 37–61. [Google Scholar] [CrossRef]
- Gioux, S.; Choi, H.S.; Frangioni, J.V. Image-guided surgery using invisible near-infrared light: Fundamentals of clinical translation. Mol. Imaging 2010, 9, 237–255. [Google Scholar] [CrossRef] [Green Version]
- Boni, L.; David, G.; Mangano, A.; Dionigi, G.; Rausei, S.; Spampatti, S. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg. Endosc. 2015, 29, 2046–2055. [Google Scholar] [CrossRef] [Green Version]
- Weissleder, R.; Ntziachristos, V. Shedding light onto live molecular targets. Nat. Med. 2003, 9, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Keereweer, S.; Van Driel, P.B.; Snoeks, T.J.; Kerrebijn, J.D.; De Jong, R.J.; Vahrmeijer, A.L. Optical image-guided cancer surgery: Challenges and limitations. Clin. Cancer Res. 2013, 19, 3745–3754. [Google Scholar] [CrossRef] [PubMed]

| ClinicalTrials.gov | Study Title | Condition | Status | Locations |
|---|---|---|---|---|
| Ovarian cancer detection | ||||
| NCT02317705 | Phase 2 Study of OTL38 for Intra-operative Imaging of Folate Receptor-alpha Positive Ovarian Cancer | Ovarian cancer | Completed | University of CA at Irvine, CA, USA; Moffitt Cancer Center Tampa, FL USA; Mayo Clinic, Rochester, MI, USA; University of Pennsylvania, PA, USA |
| NCT03180307 | OTL38 for Intra-Operative Imaging of Folate Receptor Positive Ovarian Cancer | Ovarian cancer | Completed | The Mayo Clinic, Phoenix, AZ, USA; University of Arizona, Tucson, AZ, USA; City of Hope Medical Center; Duarte, CA, USA (and 8 others) |
| NCT04941378 | OTL38 Injection (OTL38) for Intra-Operative Imaging of Folate Receptor Positive Ovarian Cancer | Ovarian cancer | Withdrawn | Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA, USA |
| Other cancers/diseases | ||||
| NCT02602119 | Intraoperative Imaging of Pulmonary Nodules by OTL38 | Neoplasms | Completed | Hospital of the University of Pennsylvania, Philadelphia, PA, USA |
| NCT03938701 | Fluorescence Imaging of Disease Activity in IBD and Rheumatoid Arthritis Using OTL38 | Inflammatory bowel disease, Rheumatoid arthritis | Not yet recruiting | University Medical Center Groningen, Netherlands |
| NCT02872701 | OTL38 Injection for Intraoperative Imaging of Folate Receptor Positive Lung Nodules | Lung neoplasms | Completed | Beth Israel Deaconess Medical Center Boston, MA, USA; Cleveland Clinic Cleveland, OH, USA; University of Pennsylvania, Philadelphia, PA, USA (and 3 others) |
| NCT02852252 | Solid Tumor Cancer Surgery with or without Intraoperative Imaging: A Registry | Bladder cancer, Gastric cancer | Completed | Hospital of the University of Pennsylvania, Philadelphia, PA, USA |
| NCT04241315 | ELUCIDATE: Enabling Lung Cancer Identification Using Folate Receptor Targeting | Lung neoplasms, Lung cancer | Completed | Stamford, CT, US; University of Iowa, Iowa, USA; Beth Israel Deaconess Medical Center Boston, MA, USA (and 9 others) |
| NCT02629549 | Intraoperative Imaging of Pituitary Adenomas by OTL | Neoplasms, Pituitary adenomas | Terminated | Hospital of the University of Pennsylvania, Philadelphia, PA, USA |
| Agents Used for Intraoperative Molecular Imaging | Comments |
|---|---|
| 5-Aminolevulinic acid | - It emits fluorescence in the visible spectrum, compared with pafolacianine, which emits fluorescence in the near-infrared spectrum [67]; - It provides an inferior depth of penetration and significantly higher background signal compared with pafolacianine [67]; - It is used predominantly for targeting bladder cancer [100] and malignant gliomas (approved by the FDA in 2017 for intraoperative molecular imaging in patients with suspected high-grade gliomas) [101]; - It requires patients to be protected from the sun and ultraviolet radiation for 24 hours after surgery, compared with pafolacianine, which does not limit patient activity or restrict discharge from the hospital [67]; - It may cause liver damage, chest pain, neuropathy, and sudden death [102], while pafolacianine shows minor side effects and no associated toxicity [6]. |
| Folate-fluorescein isothiocyanate | - It exhibits fluorescence in the visible range, compared with pafolacianine, which emits fluorescence in the near-infrared spectrum [13]; - It overlaps with the absorption spectrum of hemoglobin, reducing the signal in a surgical field covered by blood [47]; - It binds FRα, having high specificity [60]; - It differs from pafolacianine in regard to the associated fluorochrome, the folate-fluorescein isothiocyanate carrying fluorescein, while pafolacianine carries an indocyanine green analogue called SO456; - It provides a higher background autofluorescence, inferior contrast, increased scattering, and limited tissue penetration than pafolacianine [91]; - It has significant patient safety advantages, with no associated toxicity [13]. |
| Sodium fluorescein | - It can be visualized under visible light [103]; - It does not have a specific target, but its uptake in the cancer lesions can be estimated by the endothelial breakdown and high vascular permeability [103,104]; - It is safe, implying no toxicity [68]; - It provides a good contrast, but with an inferior depth of penetration [74,75]. |
| Indocyanine green | - It is a near-infrared contrast agent [13]; - It is associated with a similar depth of detection and autofluorescence to pafolacianine, due to its decreased light spread and blood absorption [105,106]; - It does not have tumor specificity, and it may also accumulate in areas of inflammation, creating background autofluorescence [58]; - It maintains fluorescence for a couple of minutes, compared to pafolacianine, which exhibits fluorescence for hours [91,107]; - It exhibits few to no adverse reactions [13]. |
| Criteria | Benefits | Limitations |
|---|---|---|
| Relatively new technology | Near-infrared imaging with pafolacianine is a relatively new technology, recently approved by the FDA for tumor detection in ovarian cancer patients, which has promising outcomes in terms of safety and efficacy [6]. | As a newly developed technique, it needs to be popularized among both surgeons and patients in order to reap the benefits of improved tumor detection. Hospitals also need to provide the necessary logistics to enable surgical procedures of this kind and to provide surgeons with special training on their use. |
| Administration | Pafolacianine is intravenously administered within a couple of hours prior to the surgery [6]. In comparison with other targeted fluorophores that are administered up to 1 week before the surgery, pafolacianine seems to be efficient even when delivered just a few hours before resection [13]. | It is recommended to avoid the use of folate, folic acid, and folate-based supplements during the 48 h prior to pafolacianine administration [6]. |
| Safety | Pafolacianine has a low toxicity profile, the most frequent side effects being dyspepsia, vomiting, nausea, abdominal pain, chest discomfort, flushing, pruritus, and hypersensitivity [6]. | It can lead to fetal harm when delivered to pregnant women [6]. |
| Efficacy | Pafolacianine binds FRα with a ∼1 nM affinity, and it is cleared from tissues which do not express the receptor, with a half-time of <30 minutes. It is demonstrated that pafolacianine enables tumor detection at concentrations of less than 100-fold those needed to cause signs of toxicity [23]. It also provides excellent contrast against the healthy background and a long residence time in the malignant lesions. It exhibits extremely low autofluorescence and a great depth of penetration, with cancer lesions being visible up to 1 cm below the tissue surface [24,108,109,110]. Additionally, intraoperative imaging using pafolacianine provided a 2-fold improvement in surgeons’ ability to identify malignant lesions [4]. | Image interpretation errors may also occur, including both false negatives and false positives [6]. False positive errors can be produced due to pinolcaine’s binding of FRβ, overexpressed on the surface of the macrophages accumulated in the non-malignant regional lymph nodes [4]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dindere, M.E.; Tanca, A.; Rusu, M.; Liehn, E.A.; Bucur, O. Intraoperative Tumor Detection Using Pafolacianine. Int. J. Mol. Sci. 2022, 23, 12842. https://doi.org/10.3390/ijms232112842
Dindere ME, Tanca A, Rusu M, Liehn EA, Bucur O. Intraoperative Tumor Detection Using Pafolacianine. International Journal of Molecular Sciences. 2022; 23(21):12842. https://doi.org/10.3390/ijms232112842
Chicago/Turabian StyleDindere, Mihaela Elisabeta, Antoanela Tanca, Mihaela Rusu, Elisa Anamaria Liehn, and Octavian Bucur. 2022. "Intraoperative Tumor Detection Using Pafolacianine" International Journal of Molecular Sciences 23, no. 21: 12842. https://doi.org/10.3390/ijms232112842






