Cadmium–Zinc Interaction in Mus musculus Fibroblasts
Abstract
:1. Introduction
2. Results
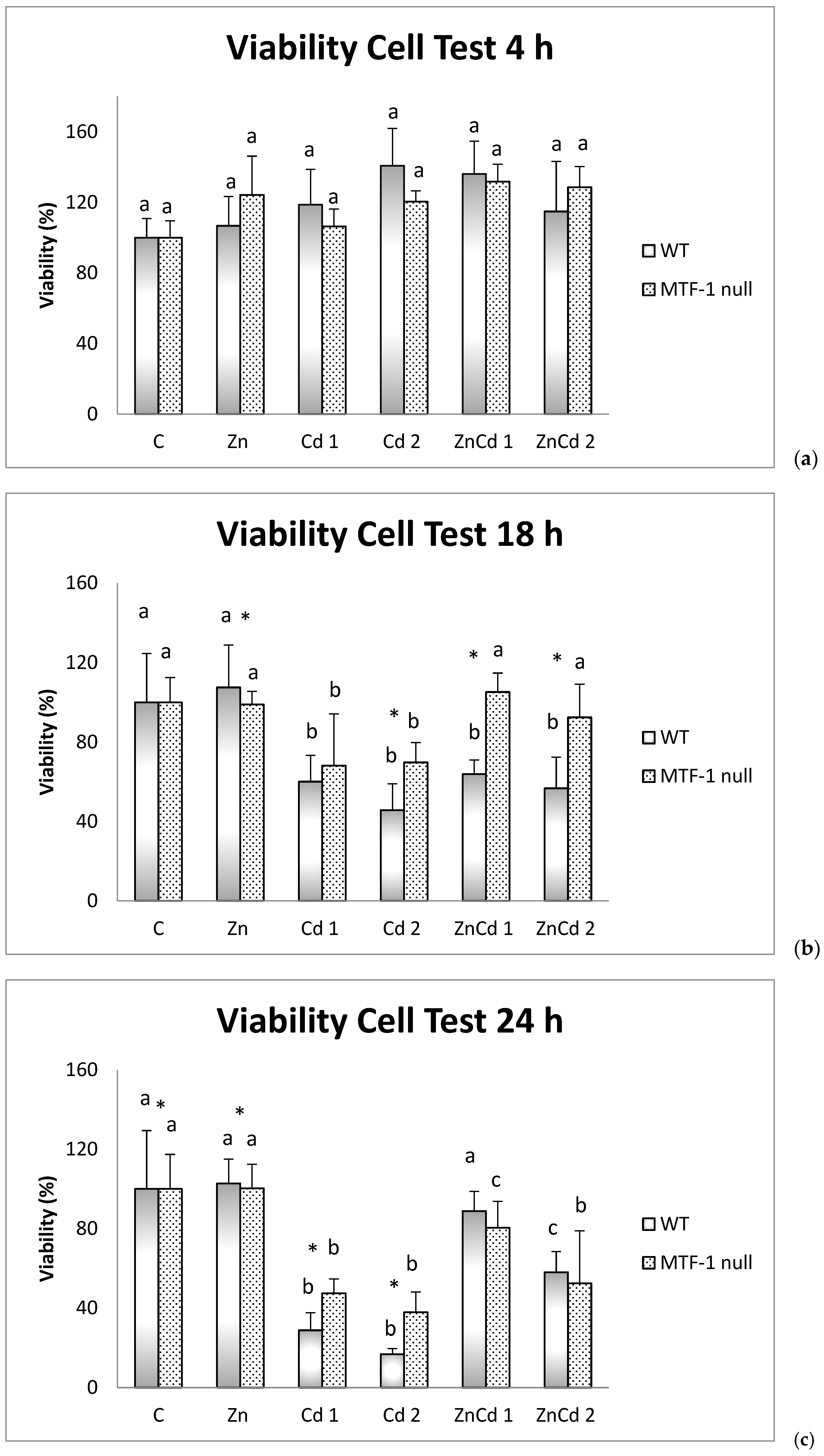
2.1. Cell Viability
2.2. Accumulation of Cd
2.3. Accumulation of Zn
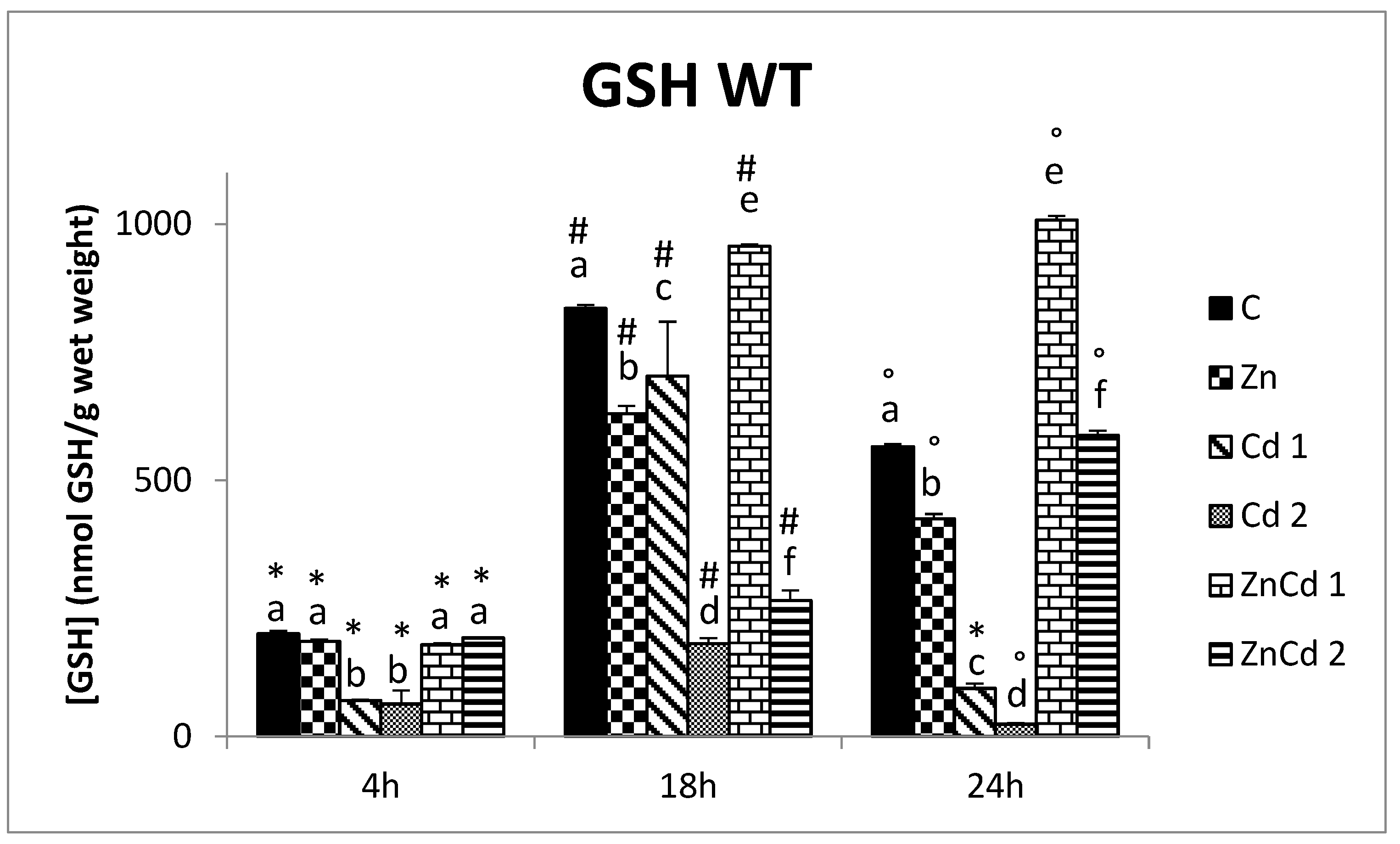
2.4. GSH Content
2.5. Metallothionein Content
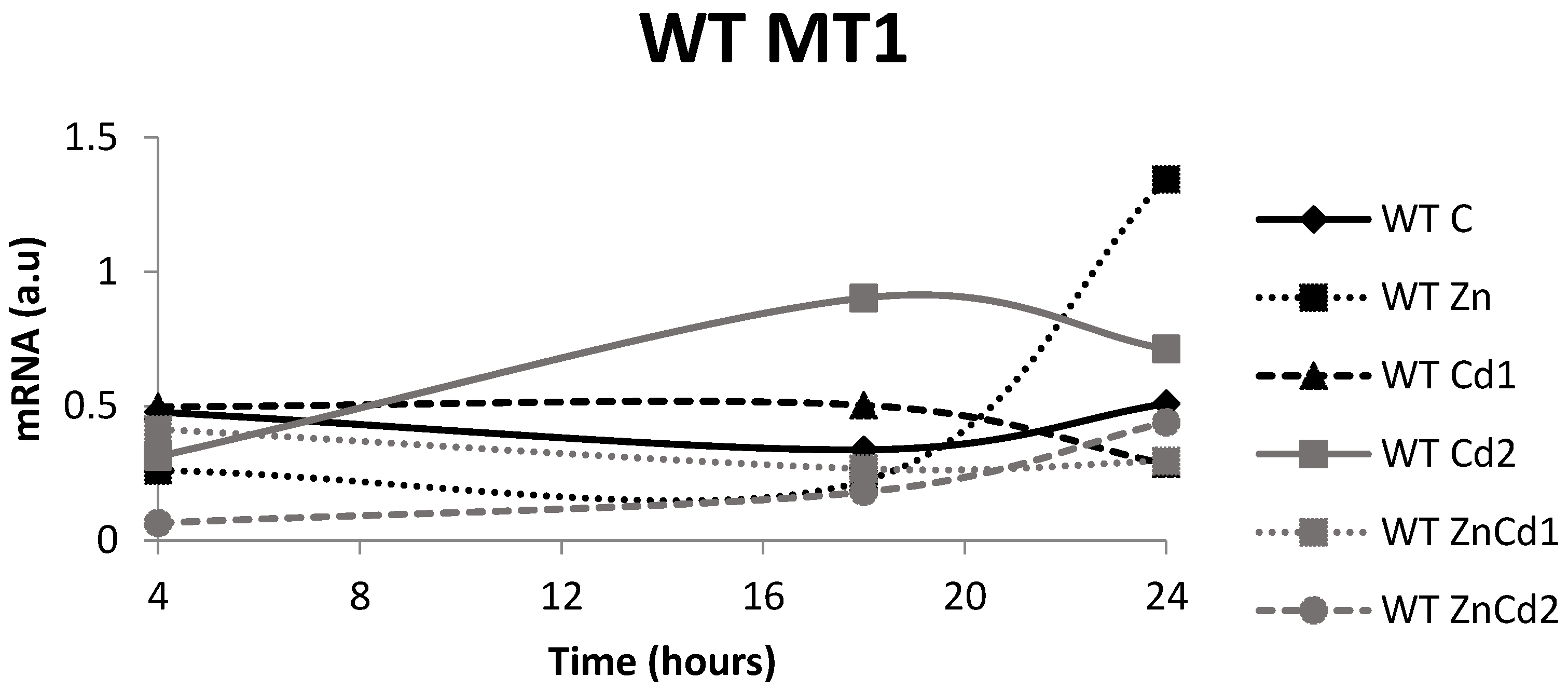
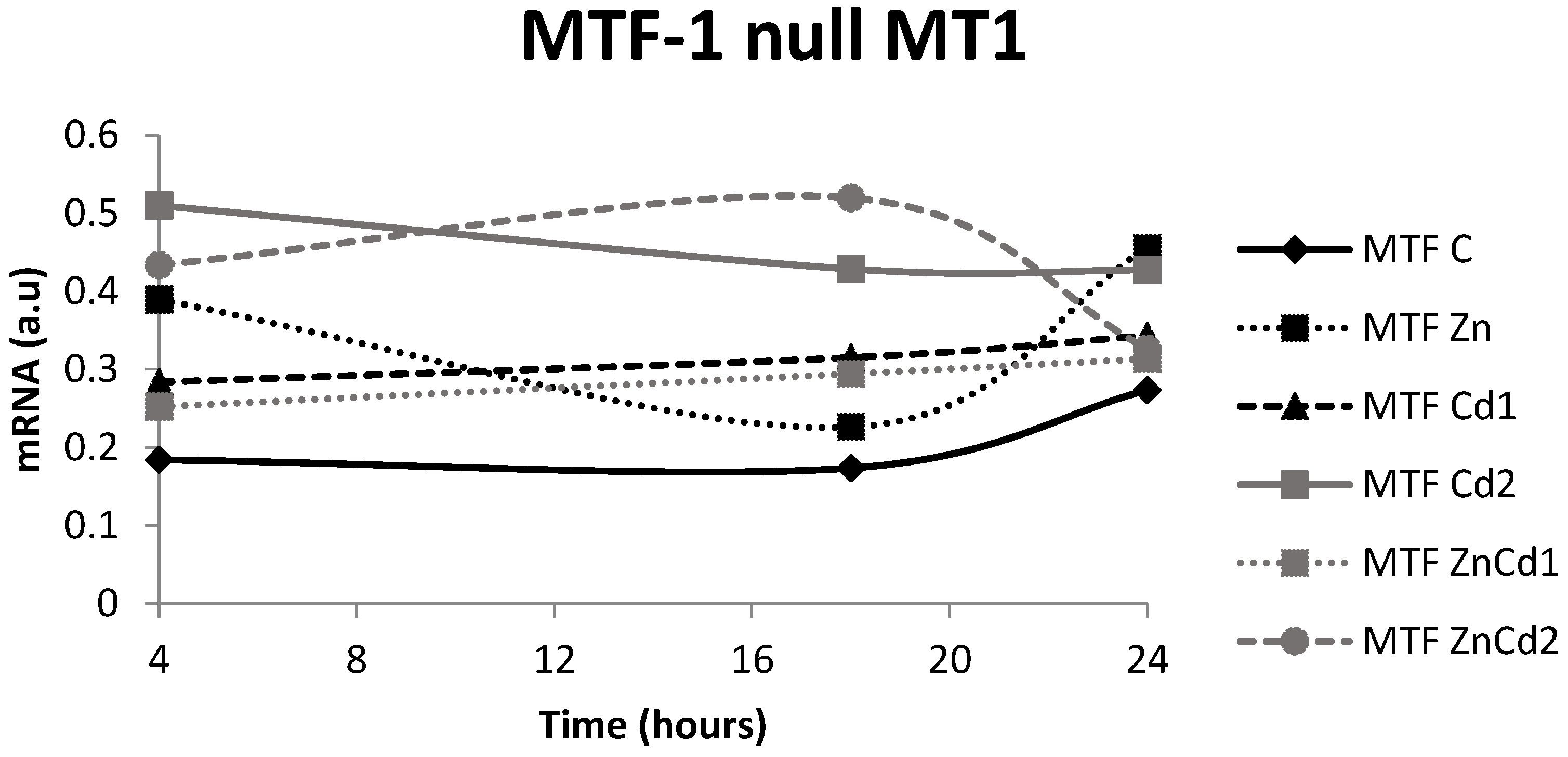
2.6. MT-1 mRNA Gene Expression
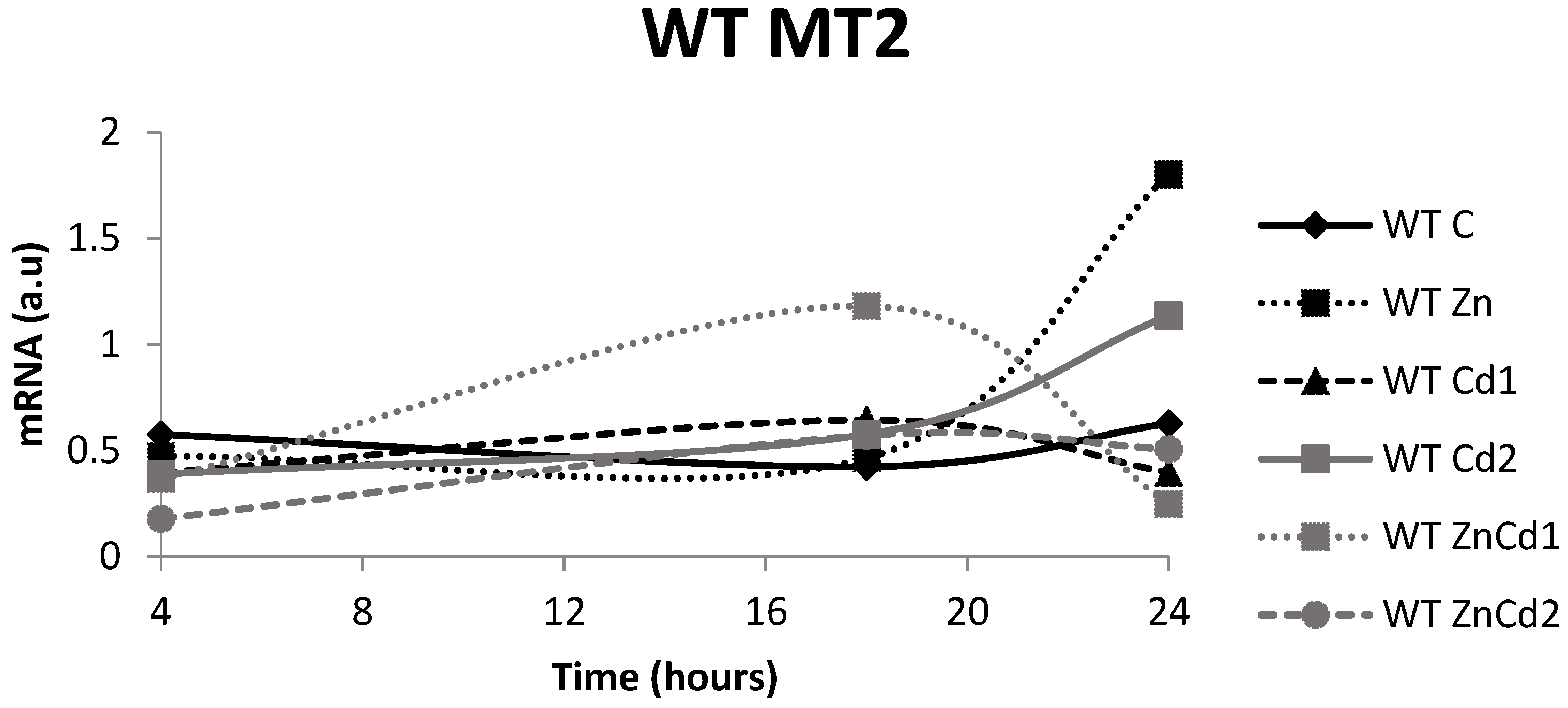
2.7. MT-2 mRNA Gene Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Treatment with Heavy Metals
4.3. Determination of Metals and MT Contents
4.4. Glutathione Quantification
4.5. qRT-PCR Analysis
4.6. Statistical Analysis
5. Conclusions
- In co-treatments, both Cd and Zn are accumulated in less quantity and this determines the greater tolerance to Cd-induced cytotoxicity.
- In the WT strain in the treatment with Zn alone, there does not appear to be an increase in GSH content, which probably occurs only when cells are subjected to oxidative stress. In contrast, treatments with Cd alone cause a reduction in GSH due to the probable oxidative stress that this metal entails. Instead, in co-treatments, Zn causes an increase in the GSH content compared to treatments with Cd alone, promoting its expression and preventing oxidation through indirect mechanisms, demonstrating its protective role.
- The MTF-1 null strain, although lacking the MTF-1 factor, still shows a high GSH content, generally higher than that of the WT strain, thanks to an alternative mechanism of expression of y-glutamylcysteine synthetase, independent of MTF-1.
- The two strains have different threshold doses of activation of MT production: lower for the MTF-1 null strain and higher for the WT strain.
- The MTF-1 null strain expresses only isoform 1 of the MT through the USF/ARE sequences, making up for the lack of isoform 2 and ensuring a higher protein production than that of the WT strain for treatments with Cd only.
- The time courses of the mRNAs of the MT-1 and MT-2 genes and those of the protein found are variable, caused by the different half-lives of the messenger and protein and/or by the intervention of down-regulation mechanisms of gene expression.
- Although the MTF-1 null strain in the literature is considered more sensitive than the WT strain, it generally exhibits greater viability following Cd-only treatments than the WT strain, possibly due to the more significant amount of MT it presents.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Riva, S.; Abelmoschi, M.; Magi, E.; Soggia, F. The utilization of the Antarctic environmental specimen bank (BCAA) in monitoring Cd and Hg in an Antarctic coastal area in Terra Nova Bay (Ross Sea––Northern Victoria Land). Chemosphere 2004, 56, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fernández, A.C.; Páez-Osuna, F.; Hillaire-Marcel, C.; Soto-Jiménez, M.; Ghaleb, B. Principal Component Analysis Applied to the Assessment of Metal Pollution from Urban Wastes in the Culiacán River Estuary. Bull. Environ. Contam. Toxicol. 2001, 67, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Morabito, R.; Remigante, A.; Arcuri, B.; Giammanco, M.; La Spada, G.; Marino, A. Effect of cadmium on anion exchange capability through Band 3 protein in human erythrocytes. J. Biol. Res. 2018, 91, 1–7. [Google Scholar] [CrossRef]
- Bray, T.M.; Bettger, W.J. The physiological role of zinc as an antioxidant. Free Radic. Biol. Med. 1990, 8, 281–291. [Google Scholar] [CrossRef]
- Singhal, R.K.; Anderson, M.E.; Meister, A. Glutathione, a first line of defense against cadmium toxicity. FASEB J. 1987, 1, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Lazo, J.S. A hypothesis regarding the protective role of metallothioneins against the toxicity of DNA interactive anticancer drugs. Toxicol. Lett. 1990, 50, 123–135. [Google Scholar] [CrossRef]
- Amiard, J.-C.; Amiard-Triquet, C.; Barka, S.; Pellerin, J.; Rainbow, P.S. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquat. Toxicol. 2006, 76, 160–202. [Google Scholar] [CrossRef]
- Banni, M.; Dondero, F.; Jebali, J.; Guerbej, H.; Boussetta, H.; Viarengo, A. Assessment of heavy metal contamination using real-time PCR analysis of mussel metallothionein mt10 and mt20 expression: A validation along the Tunisian coast. Biomarkers 2007, 12, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Santovito, G.; Boldrin, F.; Irato, P. Metal and metallothionein distribution in different tissues of the Mediterranean clam Venerupis philippinarum during copper treatment and detoxification. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 174–175, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kägi, J.H. Overview of metallothionein. Methods Enzymol. 1991, 205, 613–626. [Google Scholar]
- Santovito, G.; Piccinni, E.; Irato, P. An Improved method for rapid determination of the reduced and oxidized states of metallothioneins in biological samples. In Marine Pollution: New research; Hofer, T.N., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2008; Chapter 3; pp. 101–123. [Google Scholar]
- Freedman, J.H.; Ciriolo, M.R.; Peisach, J. The Role of Glutathione in Copper Metabolism and Toxicity. J. Biol. Chem. 1989, 264, 5598–5605. [Google Scholar] [CrossRef]
- Formigari, A.; Alberton, P.; Cantale, V.; De Nadal, V.; Feltrin, M.; Ferronato, S.; Santon, A.; Schiavon, L.; Irato, P. Relationship Between Metal Transcription Factor-1 and Zinc in Resistance to Metals Producing Free Radicals. Curr. Chem. Biol. 2008, 2, 256–266. [Google Scholar]
- Heuchel, R.; Radtke, F.; Georgiev, O.; Stark, G.; Aguet, M.; Schaffner, W. The transcription factor MTF-1 is essential for basal and heavy metal-induced metallothionein gene expression. EMBO J. 1994, 13, 2870–2875. [Google Scholar] [CrossRef] [PubMed]
- Langmade, S.J.; Ravindra, R.; Daniels, P.J.; Andrews, G.K. The Transcription Factor MTF-1 Mediates Metal Regulation of the Mouse ZnT1 Gene. J. Biol. Chem. 2000, 275, 34803–34809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunes, C.; Heuchel, R.; Georgiev, O.; Müller, K.; Lichtlen, P.; Blüthmann, H.; Marino, S.; Aguzzi, A.; Schaffner, W. Embryonic lethality and liver degeneration in mice lacking the metal-responsive transcriptional activator MTF-1. EMBO J. 1998, 17, 2846–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, M.J.; Walker, P.; Brown, R.W.; Hogstrand, C. ZINC-mediated gene expression offers protection against H2O2-induced cytotoxicity. Toxicol. Appl. Pharmacol. 2005, 205, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Mishima, A.; Kaji, T.; Yamamoto, C.; Sakamoto, M.; Kozuka, H. Zinc-induced tolerance to cadmium cytotoxicity without metallothionein induction in cultured bovine aortic endothelial cells. Toxicol. Lett. 1995, 75, 85–92. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Gao, J.; Shahzad, M.; Han, Z.; Wang, Z.; Li, J.; Sjölinder, H. Zinc Supplementation Protects against Cadmium Accumulation and Cytotoxicity in Madin-Darby Bovine Kidney Cells. PLoS ONE 2014, 9, e103427. [Google Scholar] [CrossRef] [PubMed]
- Mishima, A.; Yamamoto, C.; Fujiwara, Y.; Kaji, T. Tolerance to cadmium cytotoxicity is induced by zinc through non-metallothionein mechanisms as well as metallothionein induction in cultured cells. Toxicology 1997, 118, 85–92. [Google Scholar] [CrossRef]
- He, L.; Girijashanker, K.; Dalton, T.P.; Reed, J.; Li, H.; Soleimani, M.; Nebert, D.W. ZIP8, Member of the Solute-Carrier-39 (SLC39) Metal-Transporter Family: Characterization of Transporter Properties. Mol. Pharmacol. 2006, 70, 171–180. [Google Scholar] [CrossRef]
- Gachot, B.; Poujeol, P. Effects of cadmium and copper on zinc transport kinetics by isolated renal proximal cells. Biol. Trace Elem. Res. 1992, 35, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Szuster-Ciesielska, A.; Stachura, A.; Słotwińska, M.; Kamińska, T.; Śnieżko, R.; Paduch, R.; Abramczyk, D.; Filar, J.; Kandefer-Szerszeń, M. The inhibitory effect of zinc on cadmium-induced cell apoptosis and reactive oxygen species (ROS) production in cell cultures. Toxicology 2000, 145, 159–171. [Google Scholar] [CrossRef]
- Brzóska, M.; Moniuszko-Jakoniuk, J. Interactions between cadmium and zinc in the organism. Food Chem. Toxicol. 2001, 39, 967–980. [Google Scholar] [CrossRef]
- Song, I.-S.; Chen, H.H.W.; Aiba, I.; Hossain, A.; Liang, Z.D.; Klomp, L.W.J.; Kuo, M.T. Transcription Factor Sp1 Plays an Important Role in the Regulation of Copper Homeostasis in Mammalian Cells. Mol. Pharmacol. 2008, 74, 705–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhazza, I. Effect of zinc on cadmium induced oxidative stress: An in vivo study. World Allergy Organ. J. 2015, 8, A258. [Google Scholar] [CrossRef] [Green Version]
- Jihen, E.H.; Sonia, S.; Fatima, H.; Tahar, S.M.; Abdelhamid, K. Interrelationships between cadmium, zinc and antioxidants in the liver of the rat exposed orally to relatively high doses of cadmium and zinc. Ecotoxicol. Environ. Saf. 2011, 74, 2099–2104. [Google Scholar] [CrossRef] [PubMed]
- Tandon, S.; Singh, S.; Prasad, S.; Khandekar, K.; Dwivedi, V.; Chatterjee, M.; Mathur, N. Reversal of cadmium induced oxidative stress by chelating agent, antioxidant or their combination in rat. Toxicol. Lett. 2003, 145, 211–217. [Google Scholar] [CrossRef]
- Rana, S.V.S.; Verma, S. Protective effects of GSH, vitamin E, and selenium on lipid peroxidation in cadmium-fed rats. Biol. Trace Elem. Res. 1996, 51, 161–168. [Google Scholar] [CrossRef]
- Wang, Y.; Wimmer, U.; Lichtlen, P.; Inderbitzin, D.; Stieger, B.; Meier, P.J.; Hunziker, L.; Stallmach, T.; Forrer, R.; Rülicke, T.; et al. Metal-responsive transcription factor-1 (MTF-1) is essential for embryonic liver development and heavy metal detoxification in the adult liver. FASEB J. 2004, 18, 1071–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1830, 3143–3153. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Sadovic, S.; Shaikh, Z.A. Nephrotoxicity of Cadmium-Metallothionein: Protection by Zinc and Role of Glutathione. Toxicol. Appl. Pharmacol. 1998, 151, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Walther, U.I.; Mückter, H.; Fichtl, B.; Forth, W. Influence of glutathione on zinc-mediated cellular toxicity. Biol. Trace Elem. Res. 1998, 67, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, U.; Wang, Y.; Georgiev, O.; Schaffner, W. Two major branches of anti-cadmium defense in the mouse: MTF-1/metallothioneins and glutathione. Nucleic Acids Res. 2005, 33, 5715–5727. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, N.; Daggett, M.A.F.; Bittel, D.; Andrews, G.K.; Chu, W.A.; Johnson, J.A. Participation of upstream stimulator factor (USF) in cadmium-induction of the mouse metallothionein-I gene. Nucleic Acids Res. 1998, 26, 5182–5189. [Google Scholar] [CrossRef] [Green Version]
- Andrews, G.K. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 2000, 59, 95–104. [Google Scholar] [CrossRef]
- Santon, A.; Formigari, A.; Albergoni, V.; Irato, P. Effect of Zn treatment on wild type and MT-null cell lines in relation to apoptotic and/or necrotic processes and on MT isoform gene expression. Biochim. Biophys. Acta 2006, 1763, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Santovito, G.; Irato, P.; Piccinni, E. Regulation of Metallothionein (MT) in Tetrahymena: Induction of MT-mRNA and Protein by Cadmium Exposure. Eur. J. Protistol. 2000, 36, 437–442. [Google Scholar] [CrossRef]
- Boldrin, F.; Santovito, G. Metal Interaction and Regulation of Tetrahymena pigmentosa Metallothionein Genes. Protist 2002, 153, 283–291. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Scheuhammer, A.; Cherian, M.G. Quantification of metallothionein by silver saturation. Methods Enzymol. 1991, 205, 78–83. [Google Scholar] [CrossRef]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985, 113, 548–555. [Google Scholar] [CrossRef] [PubMed]



| 4 h | 18 h | 24 h | ||
|---|---|---|---|---|
| µg Cd/mg protein | ||||
| WT | Cd 1 | 0.002 ± 0.001 a* | 0.033 ± 0.003 a# | 0.029 ± 0.005 a# |
| Cd 2 | 0.004 ± 0.0001 a* | 0.006 ± 0.0018 b# | 0.168 ± 0.0077 b° | |
| ZnCd 1 | 0.027 ± 0.0013 b* | 0.018 ± 0.0002 c# | 0.021 ± 0.0002 a° | |
| ZnCd 2 | 0.029 ± 0.0023 b* | 0.031 ± 0.0003 d* | 0.038 ± 0.0008 c# | |
| MTF-1 null | Cd 1 | 0.021 ± 0.0016 a* | 0.090 ± 0.0059 a# | 0.022 ± 0.001 a* |
| Cd 2 | 0.030 ± 0.0003 b* | 0.266 ± 0.0479 b# | 0.088 ± 0.0141 b* | |
| ZnCd 1 | 0.013 ± 0.0001 c* | 0.023 ± 0.0005 c# | 0.055 ± 0.0012 c° | |
| ZnCd 2 | 0.014 ± 0.0001 c* | 0.018 ± 0.0008 c# | 0.051 ± 0.0017 c° | |
| µg Zn/mg protein | ||||
| WT | C | 0.027 ± 0.0049 a* | 0.024 ± 0.0119 a* | 0.022 ± 0.0022 a* |
| Zn | 0.105 ± 0.0053 b* | 0.050 ± 0.001 b# | 0.066 ± 0.0038 b° | |
| Cd 1 | 0.023 ± 0.0008 a* | 0.017 ± 0.0002 a# | 0.023 ± 0.0038 a° | |
| Cd 2 | 0.025 ± 0.0003 a* | 0.016 ± 0.0004 a# | 0.065 ± 0.003 b° | |
| ZnCd 1 | 0.030 ± 0.0014 a* | 0.040 ± 0.0004 b# | 0.054 ± 0.0006 c° | |
| ZnCd 2 | 0.039 ± 0.0031 c* | 0.040 ± 0.0004 b* | 0.057 ± 0.0011 c° | |
| MTF-1 null | C | 0.024 ± 0.0104 a* | 0.024 ± 0.0064 a* | 0.028 ± 0.0015 a* |
| Zn | 0.044 ± 0.0005 b* | 0.099 ± 0.0046 b# | 0.080 ± 0.0038 b° | |
| Cd 1 | 0.026 ± 0.002 a* | 0.027 ± 0.0018 a* | 0.024 ± 0.001 a* | |
| Cd 2 | 0.023 ± 0.0002 a* | 0.067 ± 0.012 c# | 0.040 ± 0.0063 c° | |
| ZnCd 1 | 0.031 ± 0.0003 a* | 0.039 ± 0.0008 d# | 0.089 ± 0.002 d° | |
| ZnCd 2 | 0.032 ± 0.0002 a* | 0.041 ± 0.0017 d# | 0.063 ± 0.0021 e° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priante, E.; Pietropoli, E.; Piva, E.; Santovito, G.; Schumann, S.; Irato, P. Cadmium–Zinc Interaction in Mus musculus Fibroblasts. Int. J. Mol. Sci. 2022, 23, 12001. https://doi.org/10.3390/ijms231912001
Priante E, Pietropoli E, Piva E, Santovito G, Schumann S, Irato P. Cadmium–Zinc Interaction in Mus musculus Fibroblasts. International Journal of Molecular Sciences. 2022; 23(19):12001. https://doi.org/10.3390/ijms231912001
Chicago/Turabian StylePriante, Ettore, Edoardo Pietropoli, Elisabetta Piva, Gianfranco Santovito, Sophia Schumann, and Paola Irato. 2022. "Cadmium–Zinc Interaction in Mus musculus Fibroblasts" International Journal of Molecular Sciences 23, no. 19: 12001. https://doi.org/10.3390/ijms231912001








