Bio-Inorganic Layered Double Hydroxide Nanohybrids in Photochemotherapy: A Mini Review
Abstract
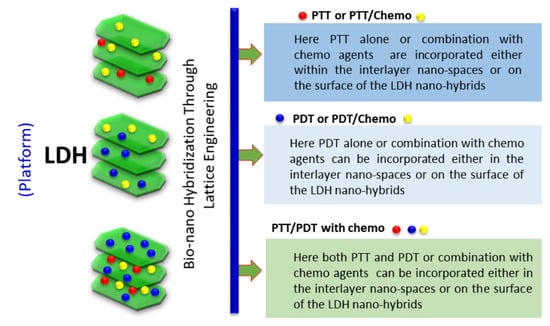
:1. Introduction
2. Inorganic Bio-Nanohybrids
2.1. Improvement of Photochemotherapy Using Inorganic Bio-Nanohybrids
2.2. Production of Inorganic Bio-Nanohybrids
2.3. Photochemotherapy
3. Photothermal Therapy and Photothermal/Chemotherapy
3.1. PTT
3.2. PTT-Chemotherapy
4. PDT and PDT/Chemotherapy
4.1. PDT
4.2. PDT/Chemotherapy
5. Simultaneous Photothermal/Photodynamic Therapy and Chemotherapy
5.1. Photothermal/Photodynamic
5.2. PTT-PDT-Chemotherapy
6. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciardiello, F.; Ciardiello, D.; Martini, G.; Napolitano, S.; Tabernero, J.; Cervantes, A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA A Cancer J. Clin. 2022, 72, 372–401. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, D.; Rampias, T. Cancer Evolution in Precision Medicine Era. Cancers 2022, 14, 1885. [Google Scholar] [CrossRef] [PubMed]
- Curtin, M.; Somayaji, D.; Dickerson, S.S. Precision Medicine Testing and Disparities in Health Care for Individuals With Non-Small Cell Lung Cancer: A Narrative Review. Oncol. Nurs. Forum 2022, 49, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Goulder, A.; Gaillard, S.L. Molecular classification of endometrial cancer: Entering an era of precision medicine. J. Gynecol. Oncol. 2022, 33, e47. [Google Scholar] [CrossRef]
- Singh, R.; Yang, X. A 3D finite element model to study the cavitation induced stresses on blood–vessel wall during the ultrasound-only phase of photo-mediated ultrasound therapy. AIP Adv. 2022, 12, 045020. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Jia, Y.; Tan, Z.; Hou, R.; Lu, J.; Luo, D.; Fu, X.; Wang, L.; Wang, X. Glucose-sensitive delivery of tannic acid by a photo-crosslinked chitosan hydrogel film for antibacterial and anti-inflammatory therapy. J. Biomater. Sci. Polym. Ed. 2022, 33, 1644–1663. [Google Scholar] [CrossRef]
- Qin, Y.; Yu, Y.; Fu, J.; Wang, M.; Yang, X.; Wang, X.; Paulus, Y.M. Photo-mediated ultrasound therapy for the treatment of retinal neovascularization in rabbit eyes. Lasers Surg. Med. 2022, 54, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wang, F.; Zhou, J.-J.; Liu, X.; He, Q.; Wang, C.; Zheng, Y.-W.; Wen, Y.; Xiong, L. Application of photodynamic therapy for liver malignancies. J. Gastrointest. Oncol. 2020, 11, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Lin, Y.; Chen, Y.; Chen, G.; Zhang, J.; Li, L.; Huang, A.; Zhang, L.; Ma, Y.; Xie, Z.-Y.; et al. Black Phosphorus Nanosheets Induced Oxidative Stress In Vitro and Targeted Photo-thermal Antitumor Therapy. ACS Appl. Bio Mater. 2021, 4, 1704–1719. [Google Scholar] [CrossRef] [PubMed]
- Bertel, L.; Mendez-Sanchez, S.C.; Martínez-Ortega, F. Laser photo-thermal therapy of epithelial carcinoma using pterin-6-carboxylic acid conjugated gold nanoparticles. Photochem. Photobiol. Sci. 2021, 20, 1599–1609. [Google Scholar] [CrossRef]
- Behnam, M.A.; Emami, F.; Sobhani, Z. PEGylated Carbon Nanotubes Decorated with Silver Nanoparticles: Fabrication, Cell Cytotoxicity and Application in Photo Thermal Therapy. IJPR 2021, 20, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Xia, Y.; Shao, J.; Guo, B.; Dong, Y.; Pijpers, I.A.B.; Zhong, Z.; Meng, F.; Abdelmohsen, L.K.E.A.; Williams, D.S.; et al. Biodegradable Polymersomes with Structure Inherent Fluorescence and Targeting Capacity for Enhanced Photo-Dynamic Therapy. Angew. Chem. Int. Ed. 2021, 60, 17629–17637. [Google Scholar] [CrossRef]
- Perni, S.; Preedy, E.C.; Prokopovich, P. Amplify antimicrobial photo dynamic therapy efficacy with poly-beta-amino esters (PBAEs). Sci. Rep. 2021, 11, 7275. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Y.; Chang, M.; Ding, B.; Deng, X.; Cui, S.; Hou, Z.; Lin, J. Azo Initiator Loaded Black Mesoporous Titania with Multiple Optical Energy Conversion for Synergetic Photo-Thermal-Dynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 47730–47738. [Google Scholar] [CrossRef] [PubMed]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Photodynamic Therapy: A Compendium of Latest Reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef]
- Orzalesi, M. Advantages and Disadvantages of Phototherapy (PT) in Neonatal Hyperbilirubinemia. In Research in Photobiology; Castellani, A., Ed.; Springer US: Boston, MA, USA, 1977; pp. 419–429. [Google Scholar]
- Kumari, A.; Kumari, K.; Gupta, S. The effect of nanoencapsulation of ICG on two-photon bioimaging. RSC Adv. 2019, 9, 18703–18712. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.-L.; Shen, B.-X.; Lin, M.-T.; Tong, M.-Q.; Zheng, Y.-W.; Jiang, X.; Yang, W.-G.; Yuan, J.-D.; Yao, Q.; Zhao, Y.-Z. Homing of ICG-loaded liposome inlaid with tumor cellular membrane to the homologous xenografts glioma eradicates the primary focus and prevents lung metastases through phototherapy. Biomater. Sci. 2018, 6, 2410–2425. [Google Scholar] [CrossRef]
- Yan, L.; Qiu, L. Indocyanine green targeted micelles with improved stability for near-infrared image-guided photothermal tumor therapy. Nanomedicine 2015, 10, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Guggenheim, S.; Martin, R.T. Definition of clay and clay mineral: Joint report of the AIPEA and CMS Nomenclature Committees. Clay Miner. 1995, 30, 257–259. [Google Scholar] [CrossRef]
- Carretero, M.I.; Gomes, C.S.F.; Tateo, F. Chapter 5.5—Clays, Drugs, and Human Health. Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 711–764. [Google Scholar]
- Limpitlaw, U.G. Ingestion of Earth materials for health by humans and animals. Int. Geol. Rev. 2010, 52, 726–744. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M. Clay and non-clay minerals in the pharmaceutical and cosmetic industries Part II. Active ingredients. Appl. Clay Sci. 2010, 47, 171–181. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M. Clay and non-clay minerals in the pharmaceutical industry: Part I. Excipients and medical applications. Appl. Clay Sci. 2009, 46, 73–80. [Google Scholar] [CrossRef]
- Bibi, I.; Icenhower, J.; Niazi, N.K.; Naz, T.; Shahid, M.; Bashir, S. Chapter 21—Clay Minerals: Structure, Chemistry, and Significance in Contaminated Environments and Geological CO2 Sequestration. In Environmental Materials and Waste; Prasad, M.N.V., Shih, K., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 543–567. [Google Scholar]
- Huggett, J.M. Clay Minerals. In Encyclopedia of Geology; Selley, R.C., Cocks, L.R.M., Plimer, I.R., Eds.; Elsevier: Oxford, UK, 2005; pp. 358–365. [Google Scholar]
- Abu-Thabit, N.Y.; Makhlouf, A.S.H. Chapter 24—Recent Advances in Nanocomposite Coatings for Corrosion Protection Applications. In Handbook of Nanoceramic and Nanocomposite Coatings and Materials; Makhlouf, A.S.H., Scharnweber, D., Eds.; Butterworth-Heinemann: Oxford, UK, 2015; pp. 515–549. [Google Scholar]
- Mousa, M.; Evans, N.D.; Oreffo, R.O.C.; Dawson, J.I. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials 2018, 159, 204–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendibil, X.; Ortiz, R.; de Viteri, V.S.; Ugartemendia, J.M.; Sarasua, J.-R.; Quintana, I. High Throughput Manufacturing of Bio-Resorbable Micro-Porous Scaffolds Made of Poly(L-lactide-co-ε-caprolactone) by Micro-Extrusion for Soft Tissue Engineering Applications. Polymers 2019, 12, 34. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Choi, G.; Elzatahry, A.; Vinu, A.; Bin Choy, Y.; Choy, J.-H. Review of Clay-drug Hybrid Materials for Biomedical Applications: Administration Routes. Clays Clay Miner. 2016, 64, 115–130. [Google Scholar] [CrossRef]
- Yan, Y.; Fu, H.; Wang, J.; Chen, C.; Wang, Q.; Duan, Y.; Hua, J. A photo-stable and reversible pH-responsive nano-agent based on the NIR phenazine dye for photoacoustic imaging-guided photothermal therapy. Chem. Commun. 2019, 55, 10940–10943. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Cheng, Z.; Tan, S.; Zhu, Q. Clay nanoparticles as pharmaceutical carriers in drug delivery systems. Expert Opin. Drug Deliv. 2020, 18, 695–714. [Google Scholar] [CrossRef]
- Ma, H.; Xue, M. Recent advances in the photothermal applications of two-dimensional nanomaterials: Photothermal therapy and beyond. J. Mater. Chem. A 2021, 9, 17569–17591. [Google Scholar] [CrossRef]
- Chimene, D.; Alge, D.L.; Gaharwar, A.K. Two-Dimensional Nanomaterials for Biomedical Applications: Emerging Trends and Future Prospects. Adv. Mater. 2015, 27, 7261–7284. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Piao, H.; Choi, G.; Jin, G.-W.; Choy, J.-H. Niclosamide-Exfoliated Anionic Clay Nanohybrid Repurposed as an Antiviral Drug for Tackling Covid-19; Oral Formulation with Tween 60/Eudragit S100. Clays Clay Miner. 2021, 69, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Piao, H.; Rejinold, N.S.; Choi, G.; Pei, Y.-R.; Jin, G.-W.; Choy, J.-H. Niclosamide encapsulated in mesoporous silica and geopolymer: A potential oral formulation for COVID-19. Microporous Mesoporous Mater. 2021, 326, 111394. [Google Scholar] [CrossRef] [PubMed]
- Freag, M.S. Protein-inorganic Nanohybrids: A Potential Symbiosis in Tissue Engineering. Curr. Drug Targets 2018, 19, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Shinde, R.B.; Padalkar, N.S.; Sadavar, S.V.; Kale, S.B.; Magdum, V.V.; Chitare, Y.M.; Kulkarni, S.P.; Patil, U.M.; Parale, V.G.; Park, H.-H.; et al. 2D–2D lattice engineering route for intimately coupled nanohybrids of layered double hydroxide and potassium hexaniobate: Chemiresistive SO2 sensor. J. Hazard. Mater. 2022, 432, 128734. [Google Scholar] [CrossRef]
- Cao, X.; Huang, A.; Liang, C.; Chen, H.-C.; Han, T.; Lin, R.; Peng, Q.; Zhuang, Z.; Shen, R.; Chen, H.M.; et al. Engineering Lattice Disorder on a Photocatalyst: Photochromic BiOBr Nanosheets Enhance Activation of Aromatic C–H Bonds via Water Oxidation. J. Am. Chem. Soc. 2022, 144, 3386–3397. [Google Scholar] [CrossRef]
- Wang, C.; Zhai, P.; Xia, M.; Wu, Y.; Zhang, B.; Li, Z.; Ran, L.; Gao, J.; Zhang, X.; Fan, Z.; et al. Engineering Lattice Oxygen Activation of Iridium Clusters Stabilized on Amorphous Bimetal Borides Array for Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2021, 60, 27126–27134. [Google Scholar] [CrossRef]
- Zhang, W.; Qiu, F.; Li, Y.; Zhang, R.; Liu, H.; Li, L.; Xie, J.; Hu, W. Lattice Defect Engineering Enables Performance-Enhanced MoS2 Photodetection through a Paraelectric BaTiO3 Dielectric. ACS Nano 2021, 15, 13370–13379. [Google Scholar] [CrossRef]
- Kim, N.; Gu, T.-H.; Shin, D.; Jin, X.; Shin, H.; Kim, M.G.; Kim, H.; Hwang, S.-J. Lattice Engineering to Simultaneously Control the Defect/Stacking Structures of Layered Double Hydroxide Nanosheets to Optimize Their Energy Functionalities. ACS Nano 2021, 15, 8306–8318. [Google Scholar] [CrossRef]
- Stathi, P.; Solakidou, M.; Deligiannakis, Y. Lattice Defects Engineering in W-, Zr-doped BiVO4 by Flame Spray Pyrolysis: Enhancing Photocatalytic O2 Evolution. Nanomaterials 2021, 11, 501. [Google Scholar] [CrossRef]
- Lai, Y.-T.; Wang, T.; O’Dell, S.; Louder, M.K.; Schön, A.; Cheung, C.S.F.; Chuang, G.-Y.; Druz, A.; Lin, B.; McKee, K.; et al. Lattice engineering enables definition of molecular features allowing for potent small-molecule inhibition of HIV-1 entry. Nat. Commun. 2019, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Hur, W.; Choi, G.; Min, H.S.; Choi, T.H.; Bin Choy, Y.; Choy, J.-H. Theranostic Bioabsorbable Bone Fixation Plate with Drug-Layered Double Hydroxide Nanohybrids. Adv. Health Mater. 2016, 5, 2765–2775. [Google Scholar] [CrossRef]
- Choy, J.-H.; Choi, G.; Huiyan, P.; A Alothman, Z.; Vinu, A.; Yun, C.-O. Anionic clay as the drug delivery vehicle: Tumor targeting function of layered double hydroxide-methotrexate nanohybrid in C33A orthotopic cervical cancer model. Int. J. Nanomed. 2016, 11, 337–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, F.; Xu, L.; Zhang, Y.; Meng, Z. Layered Double Hydroxide Assemblies with Controllable Drug Loading Capacity and Release Behavior as well as Stabilized Layer-by-Layer Polymer Multilayers. ACS Appl. Mater. Interfaces 2015, 7, 19104–19111. [Google Scholar] [CrossRef] [PubMed]
- Tuncelli, G.; Ay, A.N.; Zümreoglu-Karan, B. 5-Fluorouracil intercalated iron oxide@layered double hydroxide core-shell nano-composites with isotropic and anisotropic architectures for shape-selective drug delivery applications. Mater. Sci. Eng. C 2015, 55, 562–568. [Google Scholar] [CrossRef]
- Oh, J.-M.; Park, C.-B.; Choy, J.-H. Intracellular Drug Delivery of Layered Double Hydroxide Nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 1632–1635. [Google Scholar] [CrossRef]
- Choy, J.-H.; Jung, J.-S.; Oh, J.-M.; Park, M.; Jeong, J.; Kang, Y.-K.; Han, O.-J. Layered double hydroxide as an efficient drug reservoir for folate derivatives. Biomaterials 2004, 25, 3059–3064. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, W.; Zhang, Q.; Lu, J.; Ye, J.; Gao, X.; Xu, W.; Fahad, A.; Xie, Y.; Wei, Y.; et al. Photoactivation-triggered in situ self-supplied H2O2 for boosting chemodynamic therapy via layered double Hydroxide-mediated catalytic cascade reaction. Chem. Eng. J. 2022, 446, 137310. [Google Scholar] [CrossRef]
- Piao, H.; Choi, G.; Jin, X.; Hwang, S.-J.; Song, Y.J.; Cho, S.-P.; Choy, J.-H. Monolayer Graphitic Carbon Nitride as Metal-Free Catalyst with Enhanced Performance in Photo- and Electro-Catalysis. Nano-Micro Lett. 2022, 14, 55. [Google Scholar] [CrossRef]
- Choi, G.; Rejinold, N.S.; Piao, H.; Choy, J.-H. Inorganic–inorganic nanohybrids for drug delivery, imaging and photo-therapy: Recent developments and future scope. Chem. Sci. 2021, 12, 5044–5063. [Google Scholar] [CrossRef]
- Choi, G.; Piao, H.; Rejinold, N.; Yu, S.; Kim, K.-Y.; Jin, G.-W.; Choy, J.-H. Hydrotalcite–Niclosamide Nanohybrid as Oral Formulation towards SARS-CoV-2 Viral Infections. Pharmaceuticals 2021, 14, 486. [Google Scholar] [CrossRef]
- Yu, S.; Piao, H.; Rejinold, N.; Jin, G.; Choi, G.; Choy, J.-H. Niclosamide–Clay Intercalate Coated with Nonionic Polymer for Enhanced Bioavailability toward COVID-19 Treatment. Polymers 2021, 13, 1044. [Google Scholar] [CrossRef]
- Choi, G.; Choy, J.-H. Recent progress in layered double hydroxides as a cancer theranostic nanoplatform. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1679. [Google Scholar] [CrossRef]
- Park, C.G.; Choi, G.; Kim, M.H.; Kim, S.-N.; Lee, H.; Lee, N.K.; Bin Choy, Y.; Choy, J.-H. Brimonidine–montmorillonite hybrid formulation for topical drug delivery to the eye. J. Mater. Chem. B 2020, 8, 7914–7920. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.-R.; Yang, J.-H.; Choi, G.; Choy, J.-H. A geopolymer route to micro- and meso-porous carbon. RSC Adv. 2020, 10, 6814–6821. [Google Scholar] [CrossRef] [Green Version]
- Piao, H.; Kim, M.H.; Cui, M.; Choi, G.; Choy, J.-H. Alendronate-Anionic Clay Nanohybrid for Enhanced Osteogenic Proliferation and Differentiation. J. Korean Med. Sci. 2019, 34, e37. [Google Scholar] [CrossRef]
- Choi, G.; Eom, S.; Vinu, A.; Choy, J.-H. 2D Nanostructured Metal Hydroxides with Gene Delivery and Theranostic Functions; A Comprehensive Review. Chem. Rec. 2018, 18, 1033–1053. [Google Scholar] [CrossRef]
- Wang, L.; Xu, S.-M.; Yang, X.; He, S.; Guan, S.; Waterhouse, G.I.N.; Zhou, S. Exploiting Co Defects in CoFe-Layered Double Hydroxide (CoFe-LDH) Derivatives for Highly Efficient Photothermal Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 54916–54926. [Google Scholar] [CrossRef]
- Ye, Y.; Bremner, D.H.; Zhang, H.; Chen, X.; Lou, J.; Zhu, L.-M. Functionalized layered double hydroxide nanoparticles as an intelligent nanoplatform for synergistic photothermal therapy and chemotherapy of tumors. Colloids Surfaces B Biointerfaces 2022, 210, 112261. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, W.; Lu, J.; Guo, X.; Chi, Y.; Wang, D.; Takuya, N.; Xu, W.; Ye, J.; Liu, X.; et al. Ferrous ions doped layered double hydroxide: Smart 2D nanotheranostic platform with imaging-guided synergistic chemo/photothermal therapy for breast cancer. Biomater. Sci. 2021, 9, 5928–5938. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Sun, X.-M.; Xu, Z.P.; Liu, R.-T. Development of Multifunctional Clay-Based Nanomedicine for Elimination of Primary Invasive Breast Cancer and Prevention of Its Lung Metastasis and Distant Inoculation. ACS Appl. Mater. Interfaces 2019, 11, 35566–35576. [Google Scholar] [CrossRef]
- Gao, R.; Mei, X.; Yan, D.; Liang, R.; Wei, M. Nano-photosensitizer based on layered double hydroxide and isophthalic acid for singlet oxygenation and photodynamic therapy. Nat. Commun. 2018, 9, 2798. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, R.; Yan, L.; Chen, X.; Zhu, G. Combined chemotherapy and photodynamic therapy using a nanohybrid based on layered double hydroxides to conquer cisplatin resistance. Chem. Commun. 2015, 51, 11587–11590. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Jia, X.; Wang, C.; Zhen, W.; Jiang, X. Mn–Fe layered double hydroxide nanosheets: A new photothermal nanocarrier for O2-evolving phototherapy. Chem. Commun. 2018, 54, 11729–11732. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, L.; Liu, J.; Sun, B.; Li, L.; Xu, Z.P. Biomimetic 2D layered double hydroxide nanocomposites for hyperthermia-facilitated homologous targeting cancer photo-chemotherapy. J. Nanobiotechnol. 2021, 19, 351. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, L.; Li, L.; Zhang, R.; Xu, Z.P. Synergistic Cancer Photochemotherapy via Layered Double Hydroxide-Based Trimodal Nanomedicine at Very Low Therapeutic Doses. ACS Appl. Mater. Interfaces 2021, 13, 7115–7126. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Wang, Z.; Sun, Q.; Dong, S.; Xu, J.; Zhang, F.; Feng, L.; He, F.; Yang, D.; Yang, P.; et al. Intelligent Fe–Mn Layered Double Hydroxides Nanosheets Anchored with Upconversion Nanoparticles for Oxygen-Elevated Synergetic Therapy and Bioimaging. Small 2020, 16, e2001343. [Google Scholar] [CrossRef]
- Sun, L.; Wang, J.; Liu, J.; Li, L.; Xu, Z.P. Creating Structural Defects of Drug-Free Copper-Containing Layered Double Hydroxide Nanoparticles to Synergize Photothermal/Photodynamic/Chemodynamic Cancer Therapy. Small Struct. 2020, 2, 2000112. [Google Scholar] [CrossRef]
- Liao, S.; Yue, W.; Cai, S.; Tang, Q.; Lu, W.; Huang, L.; Qi, T.; Liao, J. Improvement of Gold Nanorods in Photothermal Therapy: Recent Progress and Perspective. Front. Pharmacol. 2021, 12, 664123. [Google Scholar] [CrossRef]
- Thorve, S.M.; Awad, N.T.; Nair, J.P.; Waghmare, S.R.; Gp, G.; Nair, G.S. Comparison in Outcome of Patients with Post TB-Destroyed Lung and COPD Admitted with Respiratory Failure. J. Assoc. Physicians India 2021, 69, 11–12. [Google Scholar]
- Vemuri, S.K.; Banala, R.R.; Mukherjee, S.; Uppula, P.; Subbaiah, G.P.V.; AV, G.R.; Malarvilli, T. Novel biosynthesized gold nanoparticles as anti-cancer agents against breast cancer: Synthesis, biological evaluation, molecular modelling studies. Mater. Sci. Eng. C 2019, 99, 417–429. [Google Scholar] [CrossRef]
- Sampara, P.; Banala, R.R.; Vemuri, S.K.; Av, G.R.; Gpv, S. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: A review. Gene Ther. 2018, 25, 67–82. [Google Scholar] [CrossRef]
- Magalashvili, R.D.; Demetrashvili, Z.M.; Gpodze, L.N.; Mikaberidze, Z.V.; Butkhuzi, D.S. Acute cholelithic intestinal obstruction. Khirurgiia 2007, 2, 51–52. [Google Scholar]
- Gpföert, H. Medical, social and economic state of German thermalism. La Press. Therm. Clim. 1969, 106, 178–180. [Google Scholar]
- Ma, K.; Li, Y.; Wang, Z.; Chen, Y.; Zhang, X.; Chen, C.; Yu, H.; Huang, J.; Yang, Z.; Wang, X.; et al. Core–Shell Gold Nanorod@Layered Double Hydroxide Nanomaterial with Highly Efficient Photothermal Conversion and Its Application in Antibacterial and Tumor Therapy. ACS Appl. Mater. Interfaces 2019, 11, 29630–29640. [Google Scholar] [CrossRef] [PubMed]
- Ülkütaş, H.; Özçelik, B.; Güler, I. Designing and application of a new medical instrument sterilization system using reactive oxygen species. Rev. Sci. Instruments 2021, 92, 114105. [Google Scholar] [CrossRef]
- Kovacic, P.; Somanathan, R. Ototoxicity and noise trauma: Electron transfer, reactive oxygen species, cell signaling, electrical effects, and protection by antioxidants: Practical medical aspects. Med. Hypotheses 2008, 70, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P. Unifying mechanism for bacterial cell signalers (4,5-dihydroxy-2,3-pentanedione, lactones and oligopeptides): Electron transfer and reactive oxygen species. Practical medical features. Med. Hypotheses 2007, 69, 1105–1110. [Google Scholar] [CrossRef]
- Liu, C.-G.; Tang, H.-X.; Zheng, X.; Yang, D.-Y.; Zhang, Y.; Zhang, J.-T.; Kankala, R.K.; Wang, S.-B.; Liu, G.; Chen, A.-Z. Near-Infrared-Activated Lysosome Pathway Death Induced by ROS Generated from Layered Double Hydroxide-Copper Sulfide Nanocomposites. ACS Appl. Mater. Interfaces 2020, 12, 40673–40683. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Liu, C.; Yan, Y.; Yang, Z.; Chen, X.; Liu, G. Effect of Extracellular Matrix Coating on Cancer Cell Membrane-Encapsulated Polyethyleneimine/DNA Complexes for Efficient and Targeted DNA Delivery In Vitro. Mol. Pharm. 2021, 18, 2803–2822. [Google Scholar] [CrossRef]
- Cunha, V.R.R.; De Souza, R.B.; da Fonseca Martins, A.M.C.R.P.; Koh, I.H.J.; Constantino, V.R.L. Accessing the biocompatibility of layered double hydroxide by intramuscular implantation: Histological and microcirculation evaluation. Sci. Rep. 2016, 6, 30547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.-J.; Ge, Y.-W.; Fan, Z.-H.; Li, Y.; Jia, W.-T.; Guo, Y.-P. Graphene oxide-modified layered double hydroxide/chitosan nacre-mimetic scaffolds treat breast cancer metastasis-induced bone defects. Carbon 2022, 200, 63–74. [Google Scholar] [CrossRef]





| Clay Hybrids | Phototherapeutic Agent or Chemo Drug Used | Applications | Physico/Chemical Characteristics | Preparation Method | Remarks | Ref. |
|---|---|---|---|---|---|---|
| Co-Fe-LDH | Co | PTT, NIR, MRI, and PAI | The TEM image of CoFe-LDH revealed nanosheets with a characteristic hexagonal morphology and a lateral size of 200 ± 20 nm. | Sintering at 200–800 °C under an Ar atmosphere | Theranostic | [61] |
| B3int, DOX, and ICG loaded in LDH | ICG and DOX | PTT and Chemo | 80 nm sized LDH NPs | LDH NPs were prepared by a hydrothermal method followed by surface modification of the LDH NPs with H2N-PEG-NH2. The targeting peptide B3int was introduced through an amide condensation reaction and finally, DOX and ICG were loaded using a physical adsorption method | Targeted cancer therapy and PTT | [62] |
| Fe-LDH | Fe-LDH/DOX | MRI, PTT, and chemo | Photothermal conversion efficiency of 45.67% with good stability and pH sensitivity | Doping of ferrous ions into Fe-LDH and DOX loading through physical methods | In vivo results on 4T1 tumor model proved its theranostic properties | [63] |
| DOX/ICG-CpG-LDH | ICG and DOX | PTT-Chemo-Immuno therapy | The average particle size (DLS) increased from 84 nm (LDH) to 120 nm (BSA coated-LDH) at the BSA/LDH mass ratio of 5:2 | Solution mixing | Theranosis toward breast cancers | [64] |
| Isophthalic acid (IPA)/LDH nanohybrids | Isophthalic acid | PDT | The tapping mode AFM image demonstrates ~50 nm diameter and ~4 nm thickness of IPA/LDH, confirming the formation of ultrathin nanosheets | Co-precipitation method | Combined PDT/chemotherapy | [65] |
| Ce6/Pt(IV)-LDH | PDT/Chemo | Combined PDT and Chemo | Average hydrodynamic size for LDH is 52 ± 2 nm; the size increases to 150 ± 7, 184 ± 3, and 2633 ± 685 nm for Ce6-Pt(IV)/LDH with the Ce6:Pt(IV) of 0.81, 1.92, and 4.99, respectively | LDH was prepared by hydrothermal method and the Ce6 and Pt(IV) were loaded by anion-exchange reaction | Combined PDT and Chemo approach cisplatin-resistant human cancer cells | [66] |
| Fe-Mn-LDH | Methylene blue | PTT/PDT | It has 2D nanosheet structure with particle size of about 200 nm and thickness of 2.8 nm comprising 2–3 layers with good dispersibility in water and cell culture medium, with an average hydrated particle size of about 220 nm/zeta potential of −25.4 mV in water and exhibits good stability in both water and cell culture medium | Co-precipitation method | Improved efficacy on U14 tumor model | [67] |
| LIPC[a] nanosheets | CCM, ICG, and PTX loaded with LDHs | Combined PDT and Chemo | TEM images reveal that LI possessed a typical hexagonal plate-like morphology, with the particle lateral dimension in the range from 50 to 100 nm. After loading PTX-BSA/BSA and coating CCM, LIPC displayed a core–shell structure with a shell of 6–10 nm in length | Physical mixing | Theranostic approach to treat colorectal cancers | [68] |
| ICG/Cu-LDH@BSA−DOX | ICG, Cu, and DOX | Dual phototherapy and imaging | Hydrodynamic diameter and the zeta potential of Cu-LDH NPs in deionized water were measured to be 58.4 ± 2.5 nm and 37.0 ± 0.6 mV, respectively. The hybrids were in the range of ~200 nm | Physical mixing | Theranostic applications at lower dosage | [69] |
| Fe-Mn-LDH | Ce6 and mesoporous silica | Trimodal therapy | The Ce6 and silica coating on UCNPs@Ce6@mSiO2 (UCS) possesses an average diameter of 47 nm. The pure FeMn-LDH exhibits a 2D ultrathin structure and hexagon structure comprising several layers with the size of ≈100 nm. After anchoring with UCSP NPs, it can be observed that UCSP can be anchored on the FeMn-LDH nanosheets with an increased size of ≈200 nm with good monodispersity. The DLS particle sizes of UCSP, FeMn-LDH, and UCSP-LDH are 58.8, 130, and 230 nm, respectively with excellent stability in both water and cell culture medium after two days’ standing | Physical mixing | The trimodal theranostic approach was validated on a U14-bearing tumor model | [70] |
| d-Cu-LDH/ICG | d-Cu-LDH and ICG | Trimodal therapy | Cu-LDH, d-Cu-LDH, and LDH/ICG NPs showed typical plate-like morphology with almost the same particle size distribution with the average hydrodynamic particle size from 25.9 ± 1.1 to 38.8 ± 1.8 nm and zeta potential of around 34–35 mV | Intercalation of ICG into the interlayers of d-Cu-LDH | PTT and PDT-Chemo | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rejinold, N.S.; Choi, G.; Choy, J.-H. Bio-Inorganic Layered Double Hydroxide Nanohybrids in Photochemotherapy: A Mini Review. Int. J. Mol. Sci. 2022, 23, 11862. https://doi.org/10.3390/ijms231911862
Rejinold NS, Choi G, Choy J-H. Bio-Inorganic Layered Double Hydroxide Nanohybrids in Photochemotherapy: A Mini Review. International Journal of Molecular Sciences. 2022; 23(19):11862. https://doi.org/10.3390/ijms231911862
Chicago/Turabian StyleRejinold, N. Sanoj, Goeun Choi, and Jin-Ho Choy. 2022. "Bio-Inorganic Layered Double Hydroxide Nanohybrids in Photochemotherapy: A Mini Review" International Journal of Molecular Sciences 23, no. 19: 11862. https://doi.org/10.3390/ijms231911862