Comparative Physiological and Transcriptomic Analyses of Improved Heat Stress Tolerance in Celery (Apium Graveolens L.) Caused by Exogenous Melatonin
Abstract
:1. Introduction
2. Results
2.1. Growth and Physiological Analysis under Heat Stress
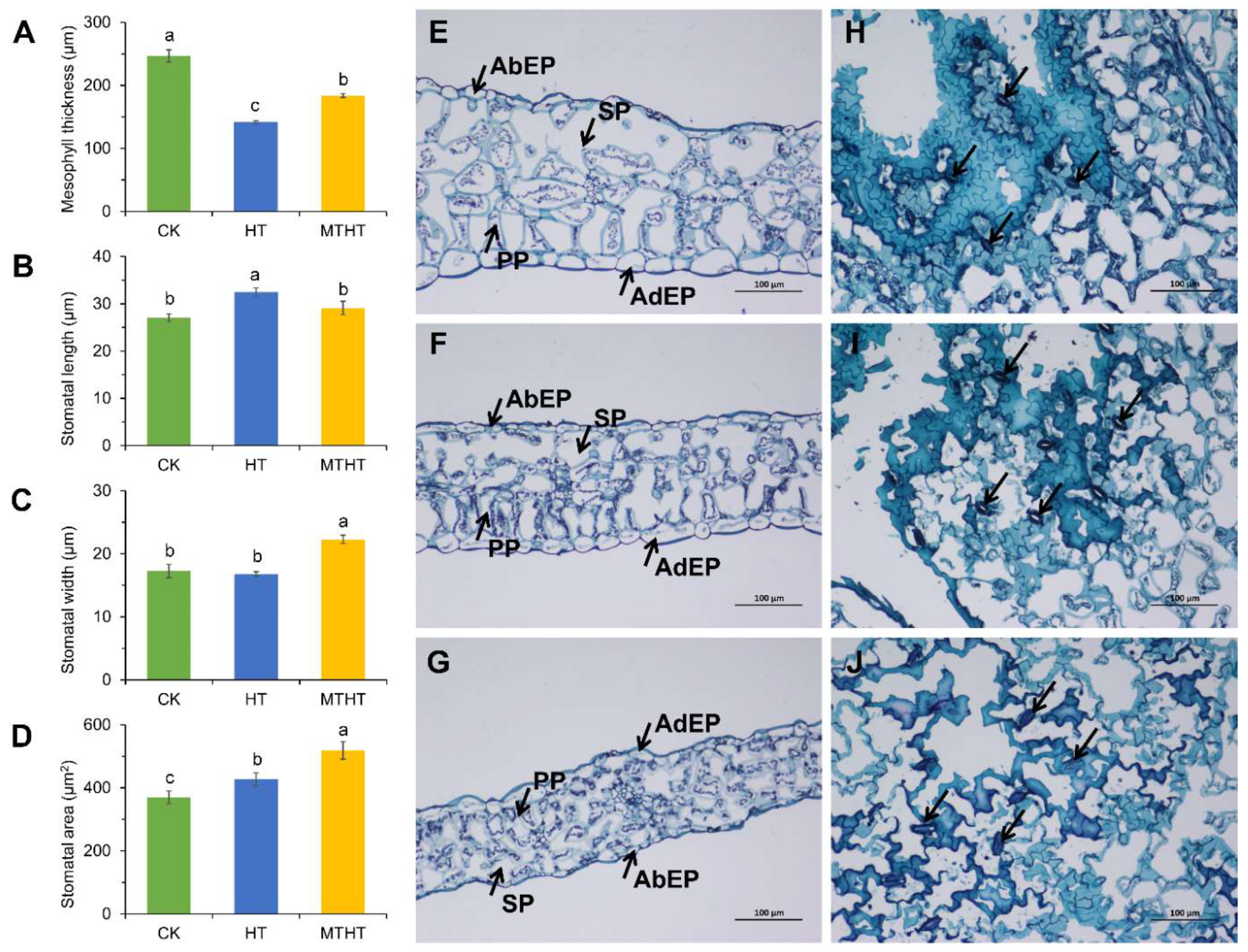
2.2. Changes in Stomatal Movement and Cross-Section of Celery Leaves under Heat Stress
2.3. Transcriptome Sequencing and Assembly
2.4. Differentially Expressed Genes (DEGs) Analysis
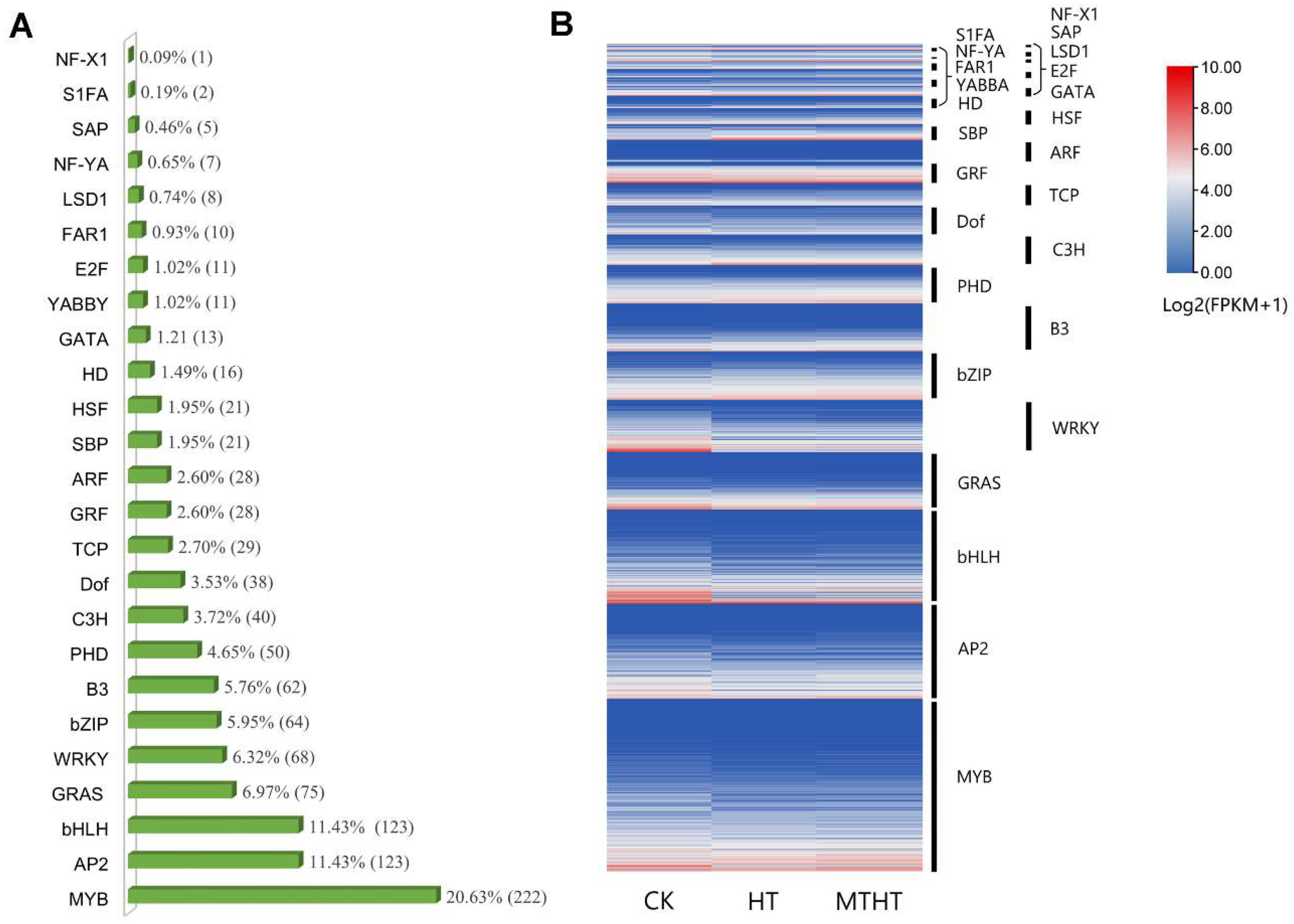
2.5. Analysis of Differentially Expressed Transcription Factors (TFs)
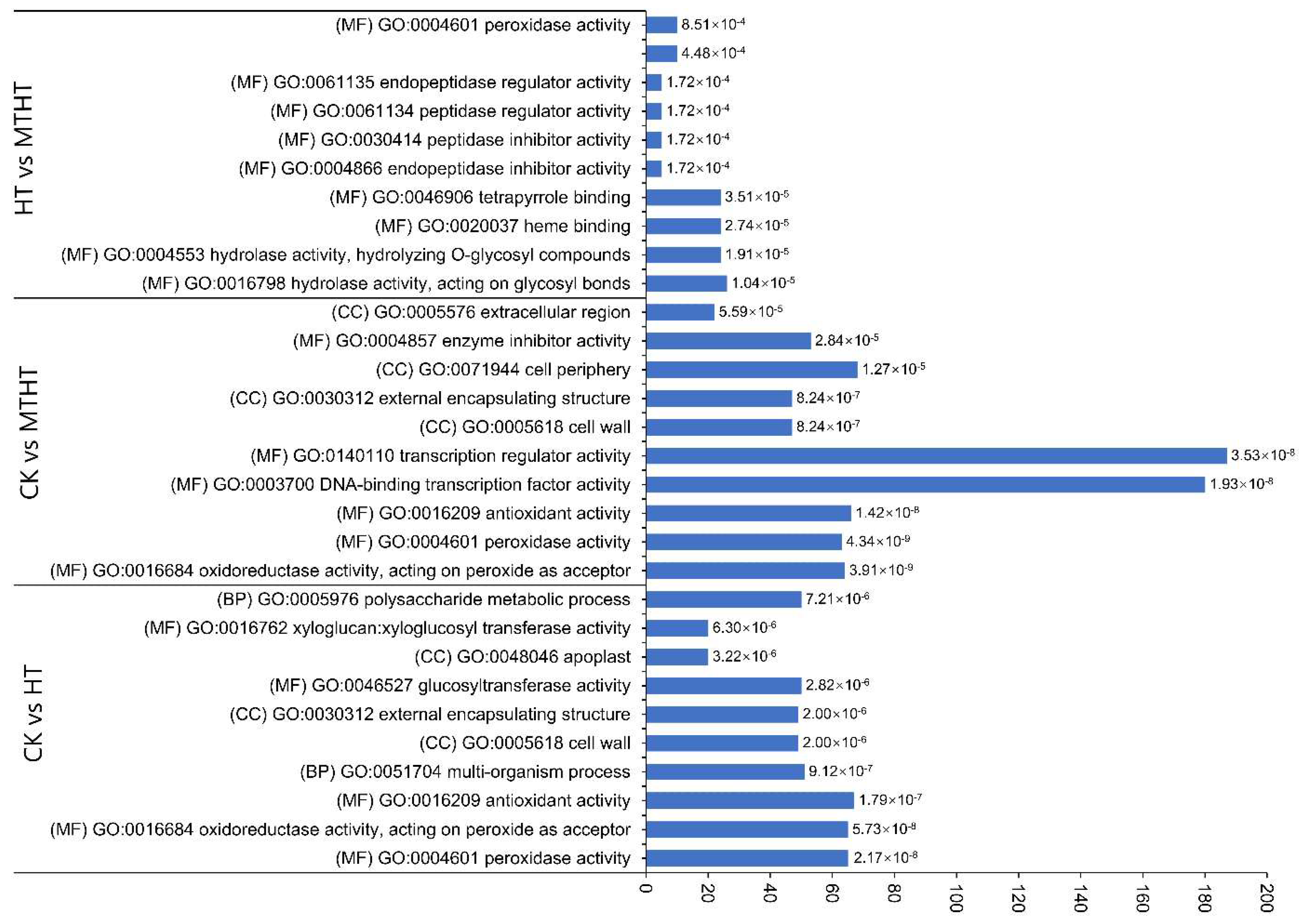
2.6. DEGs Annotation Analysis
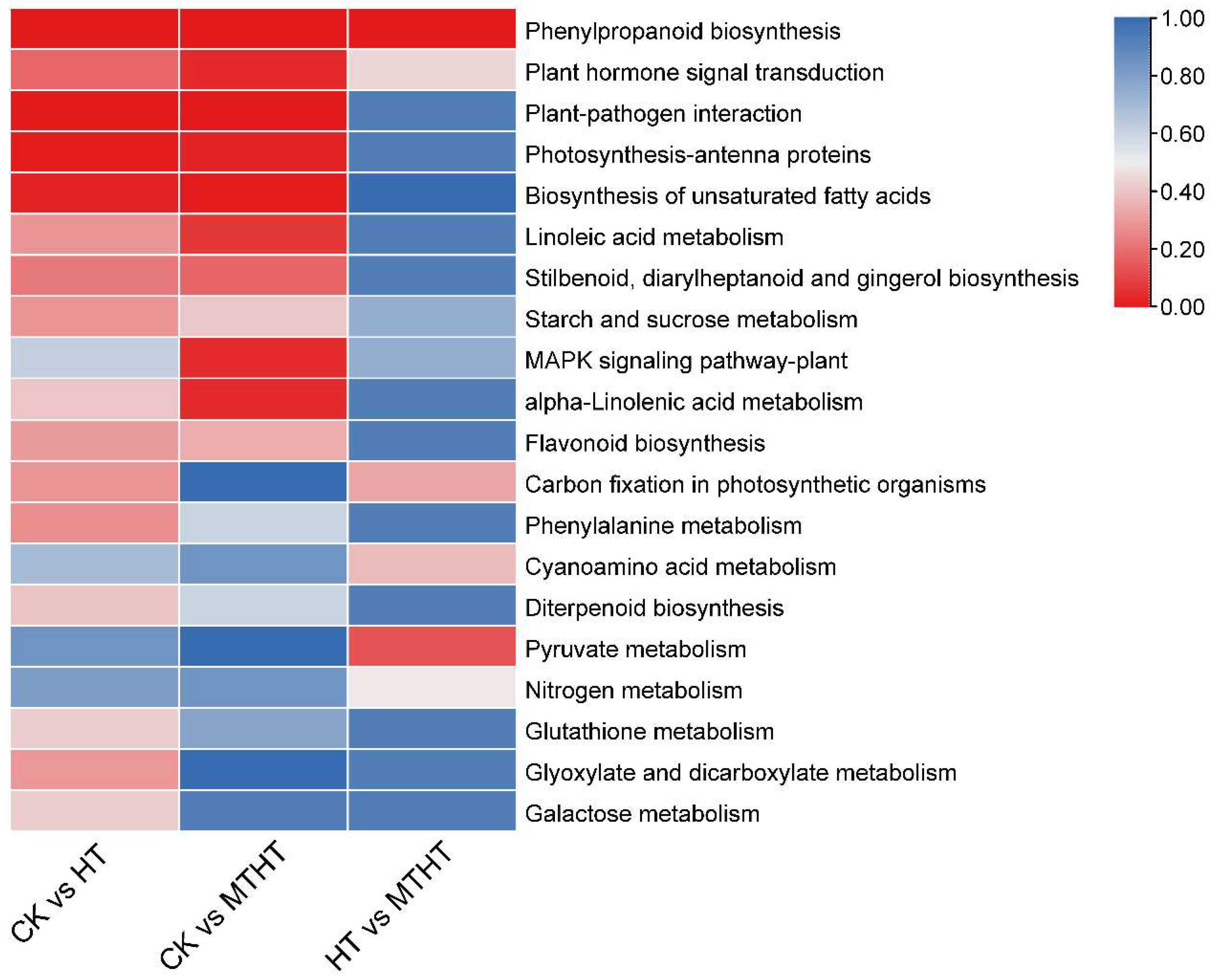
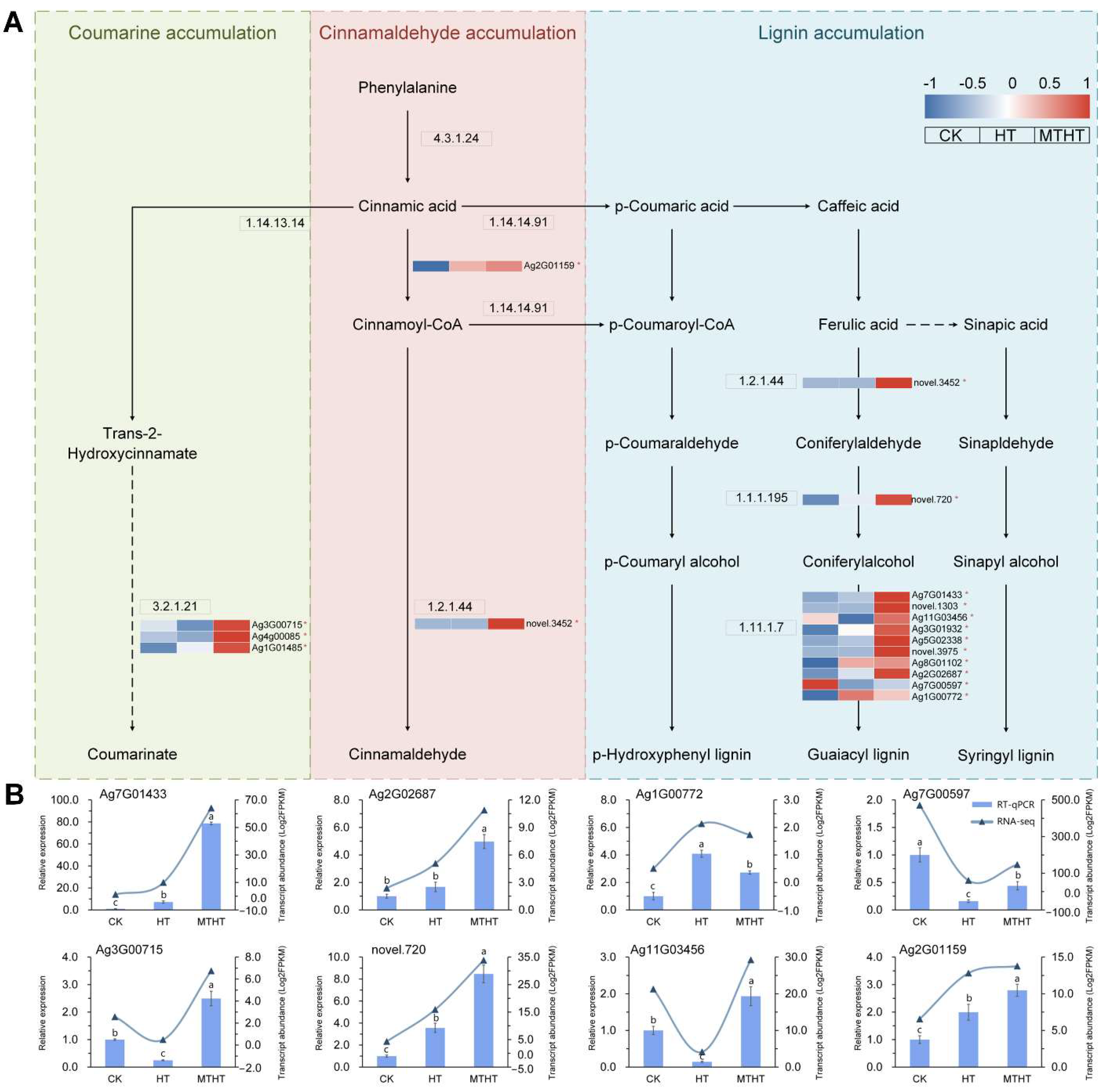
2.7. Special Metabolic Pathway of Celery in Response to Heat Stress
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatment
4.2. Physiological Indicators Assay and Leaf Structure Observation
4.3. RNA Extraction, Library Construction, Sequencing, and Data Assembly
4.4. Screening and Classification of Differentially Expressed Genes (DEGs)
4.5. Validation of Gene Expression Levels
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schiermeier, Q. Droughts, heatwaves and floods: How to tell when climate change is to blame. Nature 2018, 560, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Zheng, J.S.; Li, Y.Q.; Guo, W.D. Effects of high temperature on photosynthesis, chlorophyll fluorescence, chloroplast ultrastructure, and antioxidant activities in fingered citron. Russ. J. Plant Physiol. 2012, 59, 732–740. [Google Scholar] [CrossRef]
- He, Y.; Huang, B.R. Differential responses to heat stress in activities and isozymes of four antioxidant enzymes for two cultivars of kentucky bluegrass contrasting in heat tolerance. J. Am. Soc. Hortic. Sci. 2010, 135, 116–124. [Google Scholar] [CrossRef]
- Kidwai, M.; Ahmad, I.Z.; Chakrabarty, D. Class III peroxidase: An indispensable enzyme for biotic/abiotic stress tolerance and a potent candidate for crop improvement. Plant Cell Rep. 2020, 39, 1381–1393. [Google Scholar] [CrossRef]
- Haque, M.S.; Kjaer, K.H.; Rosenqvist, E.; Sharma, D.K.; Ottosen, C.O. Heat stress and recovery of photosystem II efficiency in wheat (Triticum aestivum L.) cultivars acclimated to different growth temperatures. Environ. Exp. Bot. 2014, 99, 1–8. [Google Scholar] [CrossRef]
- Wang, X.M. Regulatory functions of phospholipase D and phosphatidic acid in plant growth, development, and stress responses. Plant Physiol. 2005, 139, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Geng, J.; Du, Y.L.; Zhao, Q.; Zhang, W.J.; Fang, Q.X.; Yin, Z.G.; Li, J.H.; Yuan, X.K.; Fan, Y.R.; et al. Heat shock transcription factor (Hsf) gene family in common bean (Phaseolus vulgaris): Genome-wide identification, phylogeny, evolutionary expansion and expression analyses at the sprout stage under abiotic stress. BMC Plant Biol. 2022, 22, 33. [Google Scholar] [CrossRef]
- Tian, F.X.; Hu, X.L.; Yao, T.; Yang, X.H.; Chen, J.G.; Lu, M.Z.; Zhang, J. Recent advances in the roles of HSFs and HSPs in heat stress response in woody plants. Front. Plant Sci. 2021, 12, 704905. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.Y.; Wang, H.H.; Li, B.; Yi, Y.J.; Kong, F.J.; Liu, J.Y.; Zhang, H.X. Constitutive expression of a tomato small heat shock protein gene LeHSP21 improves tolerance to high-temperature stress by enhancing antioxidation capacity in Tobacco. Plant Mol. Biol. Rep. 2016, 34, 399–409. [Google Scholar] [CrossRef]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef]
- Bokszczanin, K.L.; (SPOT-ITN) Consortium; Fragkostefanakis, S. Perspectives on deciphering mechanisms underlying plant heat stress response and thermotolerance. Front. Plant Sci. 2013, 4, 315. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.J.; Huang, Q.; Chen, J.B.; Li, J.X.; Liu, Z.B.; Yang, Y.; Li, X.F.; Wang, J.M. SYTA has positive effects on the heat resistance of Arabidopsis. Plant Growth Regul. 2017, 81, 467–476. [Google Scholar] [CrossRef]
- Anckar, J.; Sistonen, L. Regulation of HSF1 function in the heat stress response: Implications in aging and disease. Annu. Rev. Biochem. 2011, 80, 1089–1115. [Google Scholar] [CrossRef] [PubMed]
- Zahid, K.R.; Ali, F.; Shah, F.; Younas, M.; Shah, T.; Shahwar, D.; Hassan, W.; Ahmad, Z.; Qi, C.; Lu, Y.L.; et al. Response and tolerance mechanism of cotton Gossypium hirsutum L. to elevated temperature stress: A review. Front. Plant Sci. 2016, 7, 937. [Google Scholar] [CrossRef]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.Z.; Huang, L.J.; Kim, W.Y. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef]
- Li, M.; Tan, S.; Tan, G.; Luo, Y.; Sun, B.; Zhang, Y.; Chen, Q.; Wang, Y.; Zhang, F.; Zhang, Y.; et al. Transcriptome Analysis Reveals Important Transcription Factor Families and Reproductive Biological Processes of Flower Development in Celery (Apium graveolens L.). Agronomy 2020, 10, 653. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiao, J.; Zheng, K.; Ma, J.; He, M.; Li, J.; Li, M. Transcriptome Profiling Reveals the Effects of Nitric Oxide on the Growth and Physiological Characteristics of Watermelon under Aluminum Stress. Genes 2021, 12, 1735. [Google Scholar] [CrossRef]
- Callwood, J.; Melmaiee, K.; Kulkarni, K.P.; Vennapusa, A.R.; Aicha, D.; Moore, M.; Vorsa, N.; Natarajan, P.; Reddy, U.K.; Elavarthi, S. Differential morpho-physiological and transcriptomic responses to heat stress in two blueberry species. Int. J. Mol. Sci. 2021, 22, 2481. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.X.; Acuna-Castroviejo, D.; Qin, L.L.; Yang, S.F.; Xu, K.X. Melatonin, a full service anti-cancer agent: Inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 2017, 18, 843. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Liu, L.T.; Wang, H.; Li, D.X.; Bai, Z.Y.; Zhang, Y.J.; Sun, H.C.; Zhang, K.; Li, C.D. Exogenous melatonin accelerates seed germination in cotton (Gossypium hirsutum L.). PLoS ONE 2019, 14, e0216575. [Google Scholar] [CrossRef] [PubMed]
- Erdal, S. Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant Cell Rep. 2019, 38, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.Q.; Zhang, N.; Wang, J.F.; Zhang, H.J.; Li, D.B.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.X.; et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef]
- Lin, X.X.; Wang, L.; Hou, Y.Y.; Zheng, Y.H.; Jin, P. A combination of melatonin and ethanol treatment improves postharvest quality in bitter melon fruit. Foods 2020, 9, 1376. [Google Scholar] [CrossRef]
- Poeggeler, B.; Saarela, S.; Reiter, R.J.; Tan, D.X.; Chen, L.D.; Manchester, L.C.; Barlow-Walden, L.R. Melatonin—A highly potent endogenous radical scavenger and electron donor: New aspects of the oxidation chemistry of this indole accessed in vitro. Ann. N. Y. Acad. Sci. 1994, 738, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Bawa, G.; Feng, L.Y.; Shi, J.Y.; Chen, G.P.; Cheng, Y.J.; Luo, J.; Wu, W.S.; Ngoke, B.; Cheng, P.; Tang, Z.Q.; et al. Evidence that melatonin promotes soybean seedlings growth from low-temperature stress by mediating plant mineral elements and genes involved in the antioxidant pathway. Funct. Plant Biol. 2020, 4, 815–824. [Google Scholar] [CrossRef]
- Li, C.; Tan, D.X.; Liang, D.; Chang, C.; Jia, D.F.; Ma, F.W. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef]
- Shi, H.T.; Tan, D.X.; Reiter, R.J.; Ye, T.T.; Yang, F.; Chan, Z.L. Melatonin induces class A1 heat-shock factors (HSFA1s) and their possible involvement of thermotolerance in Arabidopsis. J. Pineal Res. 2015, 58, 335–342. [Google Scholar] [CrossRef]
- Fleta-Soriano, E.; Díaz, L.; Bonet, E.; Munné-Bosch, S. Melatonin may exert a protective role against drought stress in maize. J. Agron. Crop Sci. 2017, 302, 286–294. [Google Scholar] [CrossRef]
- Gao, W.Y.; Feng, Z.; Bai, Q.Q.; He, J.J.; Wang, Y.J. Melatonin-mediated regulation of growth and antioxidant capacity in salt-tolerant naked oat under salt stress. Int. J. Mol. Sci. 2019, 20, 1176. [Google Scholar] [CrossRef] [Green Version]
- Amjadi, Z.; Namdjoyan, S.; Soorki, A.A. Exogenous melatonin and salicylic acid alleviates cadmium toxicity in safflower (Carthamus tinctorius L.) seedlings. Ecotoxicology 2021, 30, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Manafi, H.; Baninasab, B.; Gholami, M.; Talebi, M.; Khanizadeh, S. Exogenous melatonin alleviates heatinduced oxidative damage in strawberry (Fragaria × ananassa Duch. cv. Ventana) plant. J. Plant. Growth Regul. 2022, 41, 52–64. [Google Scholar] [CrossRef]
- Li, M.Y.; Feng, K.; Hou, X.L.; Jiang, Q.; Xu, Z.S.; Wang, G.L.; Liu, J.X.; Wang, F.; Xiong, A.S. The genome sequence of celery (Apium graveolens L.), an important leaf vegetable crop rich in apigenin in the Apiaceae family. Hortic. Res. 2020, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, J.; Zhang, R.; Lin, Y.; Xiong, A.; Tan, G.; Luo, Y.; Zhang, Y.; Chen, Q.; Wang, Y.; et al. Combined Analysis of the Metabolome and Transcriptome to Explore Heat Stress Responses and Adaptation Mechanisms in Celery (Apium graveolens L.). Int. J. Mol. Sci. 2022, 23, 3367. [Google Scholar] [CrossRef]
- Corey Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, Q.Q.; Zhang, H.J.; Cao, Y.Y.; Weeda, S.; Ren, S.X.; Guo, Y.D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.H.; Rizwan, M.; Fahad, S.; Xu, Z.H.; Hu, L.Y. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Shen, H.F.; Zhao, B.; Xu, J.J. Relationship between leaf anatomy and heat tolerance of 15 rhododendron cultivars. Chin. J. Appl. Ecol. 2016, 27, 3895–3904. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Y.; Zhang, X.Z.; Du, H.M.; Xu, B.; Huang, B.R. Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.). Environ Exp. Bot. 2017, 138, 36–45. [Google Scholar] [CrossRef]
- Moradpour, M.; Abdullah, S.N.A.; Namasivayam, P. The impact of heat stress on morpho-physiological response and expression of specific genes in the heat stress-responsive transcriptional tegulatory network in Brassica oleracea. Plants 2021, 10, 1064. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, X.D.; Li, T.L.; Cao, X.J. Photosynthesis and ultrastructure of photosynthetic apparatus in tomato leaves under elevated temperature. Photosynthetica 2014, 52, 430–436. [Google Scholar] [CrossRef]
- Cui, G.B.; Zhao, X.X.; Liu, S.D.; Sun, F.L.; Zhang, C.; Xi, Y.J. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Chen, Y.E.; Zhao, Y.Q.; Ding, C.B.; Liao, J.Q.; Hu, C.; Zhou, L.J.; Zhang, Z.W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef]
- Yin, L.H.; Wang, P.; Li, M.J.; Ke, X.W.; Li, C.Y.; Liang, D.; Wu, S.; Ma, X.L.; Li, C.; Zou, Y.J.; et al. Exogenous melatonin improves Malus resistance to marssonina apple blotch. J. Pineal Res. 2013, 54, 426–434. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Wang, T.; Liu, G.S.; Hu, M.J.; Yun, Z.; Duan, X.W.; Cai, K.; Jiang, G.X. Inhibition of downy blight and enhancement of resistance in litchi fruit by postharvest application of melatonin. Food Chem. 2021, 347, 129009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Jin, J.H.; He, Y.M.; Lu, B.Y.; Li, D.W.; Chai, W.G.; Khan, A.; Gong, Z.H. Genome-wide identification and analysis of the SBP-Box family genes under phytophthora capsici stress in pepper (Capsicum annuum L.). Front. Plant Sci. 2016, 7, 504. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.J.; Chen, D.; Zheng, Y.C.; Jin, S.; Yang, J.F.; Ye, N.X. Identification and expression analyses of SBP-Box genes reveal their involvement in abiotic stress and hormone response in tea plant (Camellia sinensis). Int. J. Mol. Sci. 2018, 19, 3404. [Google Scholar] [CrossRef]
- Feng, X.; Yu, Q.; Zeng, J.B.; He, X.Y.; Liu, W.X. Genome-wide identification and characterization of GATA family genes in wheat. BMC Plant Biol. 2022, 22, 372. [Google Scholar] [CrossRef]
- Babbar, R.; Karpinska, B.; Grover, A.; Foyer, C.H. Heat-induced oxidation of the nuclei and cytosol. Front. Plant Sci. 2021, 11, 617779. [Google Scholar] [CrossRef]
- Martinez, V.; Nieves-Cordones, M.; Lopez-Delacalle, M.; Rodenas, R.; Mestre, T.C.; Garcia-Sanchez, F.; Rubio, F.; Nortes, P.A.; Mittler, R.; Rivero, R.M. Tolerance to stress combination in tomato plants: New insights in the protective role of melatonin. Molecules 2018, 23, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Yu, X.L.; Lou, W.; Wei, S.Q.; Wang, R.; Wan, Q.; Shen, W.B. Transgenic Arabidopsis overexpressing MsSNAT enhances salt tolerance via the increase in autophagy, and the reestablishment of redox and ion homeostasis. Environ. Exp. Bot. 2019, 164, 20–28. [Google Scholar] [CrossRef]
- Shanga, F.Z.; Liu, R.L.; Wu, W.J.; Han, Y.C.; Fang, X.J.; Chen, H.J.; Haiyan Gao, H.Y. Effects of melatonin on the components, quality and antioxidant activities of blueberry fruits. LWT 2021, 147, 111582. [Google Scholar] [CrossRef]
- Zhang, X.; St Leger, R.J.; Fang, W.G. Pyruvate accumulation is the first line of cell defense against heat stress in a fungus. mBio 2017, 8, e01284-17. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhou, J.; Du, J.; Li, X.; Sun, Y.; Wang, Z.; Lin, Y.; Zhang, Y.; Wang, Y.; He, W.; et al. Comparative Physiological and Transcriptomic Analyses of Improved Heat Stress Tolerance in Celery (Apium Graveolens L.) Caused by Exogenous Melatonin. Int. J. Mol. Sci. 2022, 23, 11382. https://doi.org/10.3390/ijms231911382
Li M, Zhou J, Du J, Li X, Sun Y, Wang Z, Lin Y, Zhang Y, Wang Y, He W, et al. Comparative Physiological and Transcriptomic Analyses of Improved Heat Stress Tolerance in Celery (Apium Graveolens L.) Caused by Exogenous Melatonin. International Journal of Molecular Sciences. 2022; 23(19):11382. https://doi.org/10.3390/ijms231911382
Chicago/Turabian StyleLi, Mengyao, Jin Zhou, Jiageng Du, Xiaoyan Li, Yue Sun, Zhuo Wang, Yuanxiu Lin, Yunting Zhang, Yan Wang, Wen He, and et al. 2022. "Comparative Physiological and Transcriptomic Analyses of Improved Heat Stress Tolerance in Celery (Apium Graveolens L.) Caused by Exogenous Melatonin" International Journal of Molecular Sciences 23, no. 19: 11382. https://doi.org/10.3390/ijms231911382
APA StyleLi, M., Zhou, J., Du, J., Li, X., Sun, Y., Wang, Z., Lin, Y., Zhang, Y., Wang, Y., He, W., Wang, X., Chen, Q., Zhang, Y., Luo, Y., & Tang, H. (2022). Comparative Physiological and Transcriptomic Analyses of Improved Heat Stress Tolerance in Celery (Apium Graveolens L.) Caused by Exogenous Melatonin. International Journal of Molecular Sciences, 23(19), 11382. https://doi.org/10.3390/ijms231911382







