Comparative Study of Chemical Stability of a PK20 Opioid–Neurotensin Hybrid Peptide and Its Analogue [Ile9]PK20—The Effect of Isomerism of a Single Amino Acid
Abstract
:1. Introduction
2. Results
PK20 and [Ile9]PK20 Degradation Depending on Conditions
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. LC Apparatus and LC-MS Conditions
4.3. The Stock Solution of Investigated Compounds
4.4. Quantification of Peptides Using LC-MS
4.5. Method Validation
4.6. Degradation and Analysis Procedures of the Stressed Compounds
4.6.1. Acid and Base Hydrolysis
4.6.2. Thermal Degradation
4.6.3. UV Degradation
4.6.4. Oxidative Degradation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powell, M.F. Chapter 30. Peptide Stability in Drug Development: In vitro Peptide Degradation in Plasma and Serum. Ann. Rep. Med. Chem. 1993, 28, 285–294. [Google Scholar] [CrossRef]
- Battersby, J.E.; Hancock, W.S.; Canova-Davis, E.; Oeswein, J.; O’Onnor, B. Diketopiperazine formation and N-terminal degradation in recombinant human growth hormone. Int. J. Pept. Protein Res. 2009, 44, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Kertscher, U.; Bienert, M.; Krause, E.; Sepetov, N.F.; Mehlis, B. Spontaneous chemical degradation of substance P in the solid phase and in solution. Int. J. Pept. Protein Res. 2009, 41, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Yin, J.; Brooks, B.R.; Wang, D.I.; Ricci, M.S.; Brems, D.N.; Trout, B.L. A comprehensive picture of non-site specific oxidation of methionine residues by peroxides in protein pharmaceuticals. J. Pharm. Sci. 2004, 93, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Pogocki, D.; Ghezzo-Schöneich, E.; Schöneich, C. Conformational Flexibility Controls Proton Transfer between the Methionine Hydroxy Sulfuranyl Radical and the N-Terminal Amino Group in Thr−(X)n−Met Peptides. J. Phys. Chem. B 2001, 105, 1250–1259. [Google Scholar] [CrossRef]
- García-Garayoa, E.; Bläuenstein, P.; Bruehlmeier, M.; Blanc, A.; Iterbeke, K.; Conrath, P.; Tourwé, D.; Schubiger, P.A. Preclinical evaluation of a new, stabilized neurotensin(8–13) pseudopeptide radiolabeled with (99m)Tc. J. Nucl. Med. 2002, 43, 374–383. [Google Scholar] [PubMed]
- Kleczkowska, P.; Bojnik, E.; Lesniak, A.; Kosson, P.; Eynde, I.V.D.; Ballet, S.; Benyhe, S.; Tourwé, D.; Lipkowski, A.W. Identification of Dmt-D-Lys-Phe-Phe-OH as a highly antinociceptive tetrapeptide metabolite of the opioid-neurotensin hybrid peptide PK20. Pharmacol. Rep. 2013, 65, 836–846. [Google Scholar] [CrossRef]
- Kleczkowska, P.; Kosson, P.; Ballet, S.; Van den Eynde, I.; Tsuda, Y.; Tourwé, D.; Lipkowski, A.W. PK20, a New Opioid-Neurotensin Hybrid Peptide That Exhibits Central and Peripheral Antinociceptive Effects. Mol. Pain 2010, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Kleczkowska, P.; Hermans, E.; Kosson, P.; Kowalczyk, A.; Lesniak, A.; Pawlik, K.; Bojnik, E.; Benyhe, S.; Nowicka, B.; Bujalska-Zadrozny, M.; et al. Antinociceptive effect induced by a combination of opioid and neurotensin moieties vs. their hybrid peptide [Ile(9)]PK20 in an acute pain treatment in rodents. Brain Res. 2016, 1648, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Kleczkowska, P.; Kawalec, M.; Bujalska-Zadrozny, M.; Filip, M.; Zablocka, B.; Lipkowski, A.W. Effects of the Hybridization of Opioid and Neurotensin Pharmacophores on Cell Survival in Rat Organotypic Hippocampal Slice Cultures. Neurotox. Res. 2015, 28, 352–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsante, K.M.; Huynh-Ba, K.; Baertschi, S.; Reed, R.A.; Landis, M.S.; Kleinman, M.H.; Foti, C.; Rao, V.M.; Meers, P.; Abend, A.; et al. Recent Trends in Product Development and Regulatory Issues on Impurities in Active Pharmaceutical Ingredient (API) and Drug Products. Part 1: Predicting Degradation Related Impurities and Impurity Considerations for Pharmaceutical Dosage Forms. AAPS PharmSciTech 2013, 15, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Stefanucci, A.; Pinnen, F.; Feliciani, F.; Cacciatore, I.; Lucente, G.; Mollica, A. Conformationally Constrained Histidines in the Design of Peptidomimetics: Strategies for the χ-Space Control. Int. J. Mol. Sci. 2011, 12, 2853–2890. [Google Scholar] [CrossRef] [PubMed]
- Wu, L. Regulatory considerations for peptide therapeutics. In Peptide Therapeutics: Strategy and Tactics for Chemistry, Manufacturing, and Controls; Srivastava, V., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 1–30. [Google Scholar]
- Baertschi, S.W.; Jansen, P.J.; Alsante, K.M. Stress testing: A predictive tool (Chapter 2). In Pharmaceutical Stress Testing: Predicting Drug Degradation; Baertschi, S.W., Alsante, K.M., Reed, R.A., Eds.; Informa Life Sciences: London, UK, 2011; pp. 10–48. [Google Scholar]
- Alsante, K.M.; Baertschi, S.W. Reviewing advances in knowledge of drug degradation chemistry. In Proceedings of the Forced Degradation for Pharmaceuticals: Conference Proceedings, London, UK, 18–19 January 2012. [Google Scholar]
- Xu, G.; Chance, M.R. Hydroxyl Radical-Mediated Modification of Proteins as Probes for Structural Proteomics. Chem. Rev. 2007, 107, 3514–3543. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Meddah, B.; Kamel, S.; Giroud, C.; Brazier, M. Effects of ultraviolet light on free and peptide-bound pyridinoline and deoxypyridinoline cross-links. Protective effect of acid pH against photolytic degradation. J. Photochem. Photobiol. B Biol. 2000, 54, 168–174. [Google Scholar] [CrossRef]
- Sakura, S.; Fujimoto, D.; Sakamoto, K.; Mizuno, A.; Motegi, K. Photolysis of pyridinoline, a cross-linking amino acid of collagen, by ultraviolet light. Can. J. Biochem. 1982, 60, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Pinnen, F.; Stefanucci, A.; Constante, R. The evolution of peptide synthesis: From early days to small molecular machines. Curr. Bioact. Comp. 2013, 9, 184–202. [Google Scholar] [CrossRef]
- Available online: https://somatek.com/wp-content/uploads/2014/06/sk140605h.pdf (accessed on 20 August 2022).



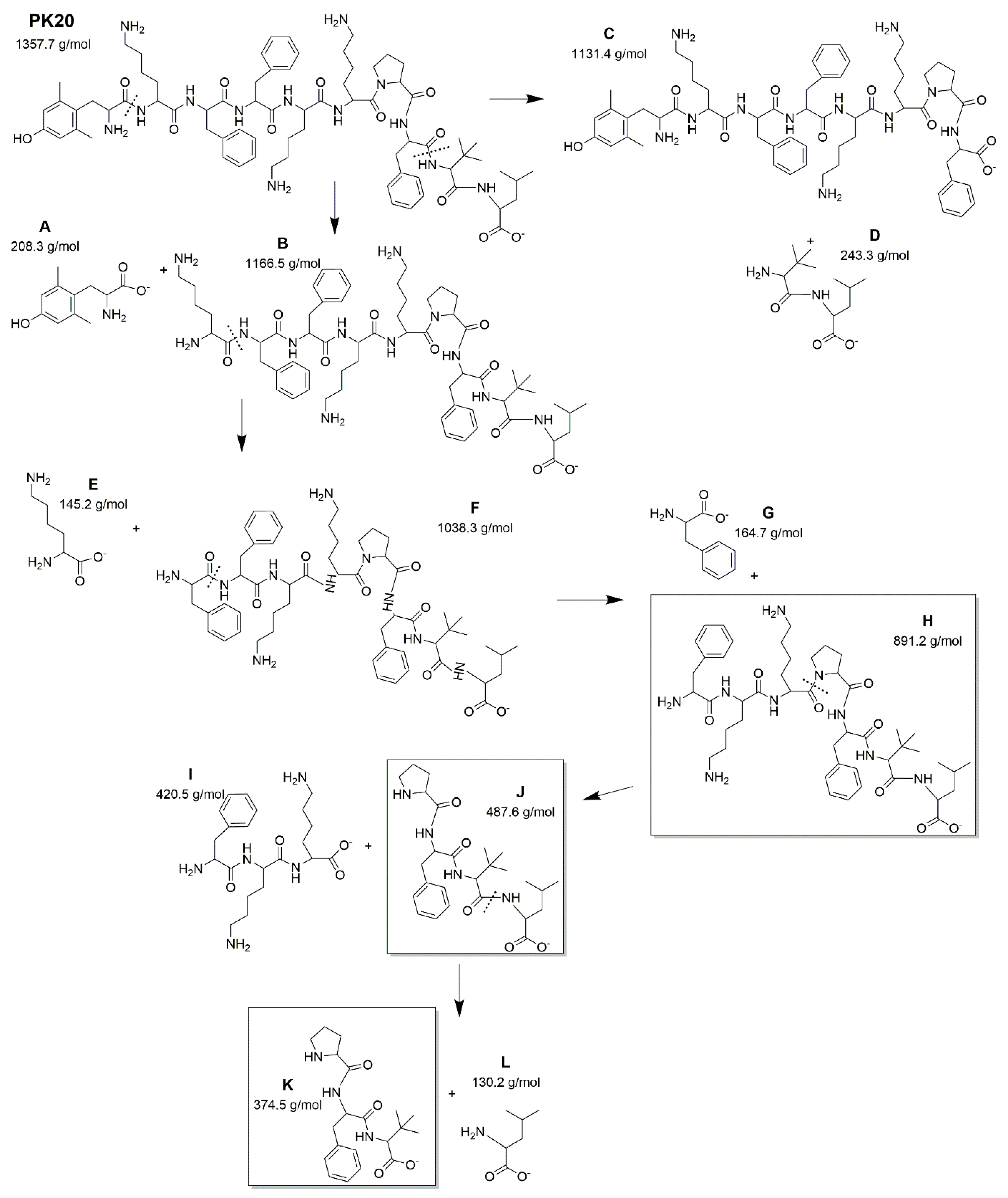
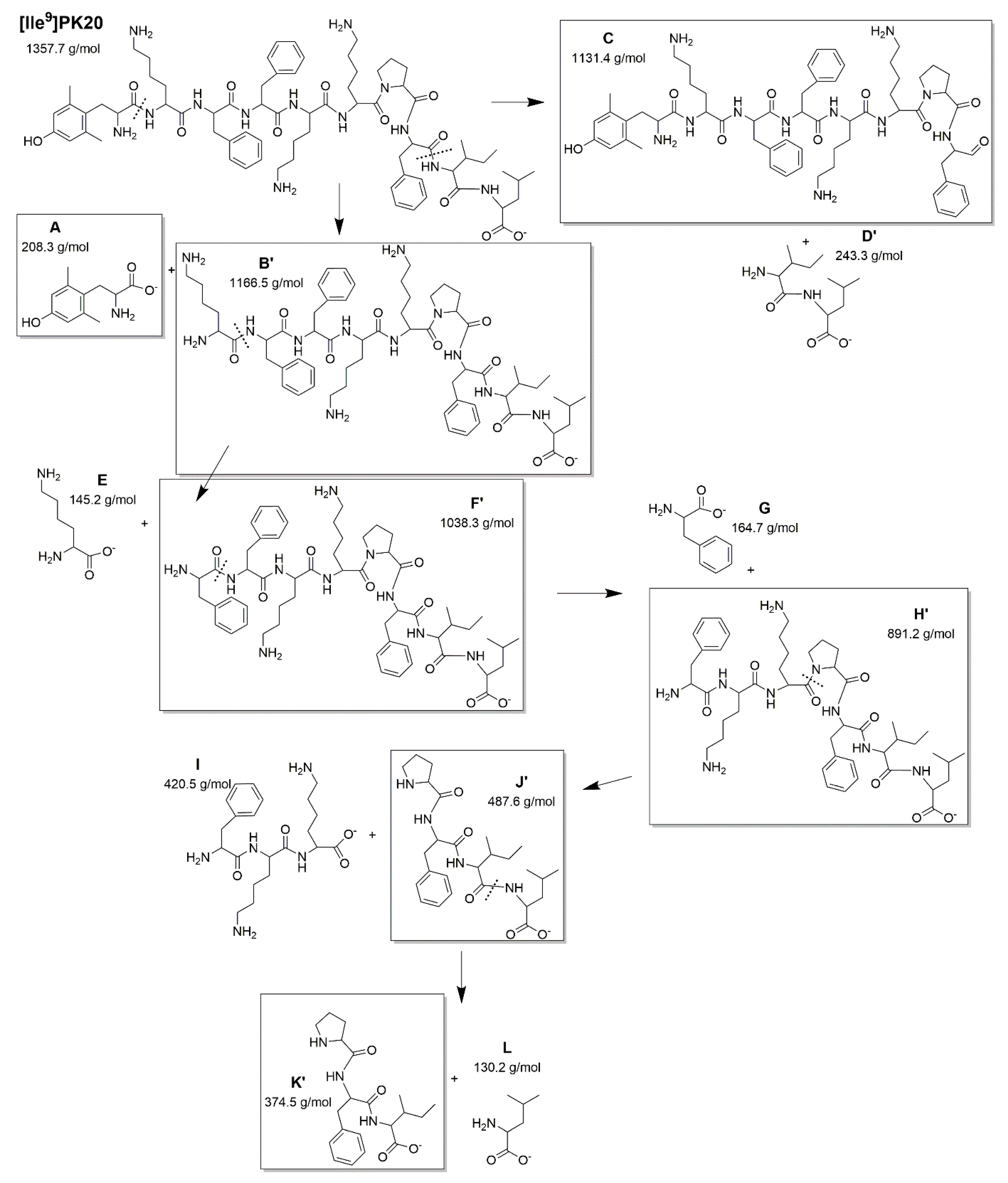
| Condition | Drug Concentration Injected (μg/mL) | Concentration Found (Mean ± SD, μg/mL) | Recovery (%) | RSD (%) * | |||
|---|---|---|---|---|---|---|---|
| PK20 | [Ile9]PK20 | PK20 | [Ile9]PK20 | PK20 | [Ile9]PK20 | ||
| Acidic degradation (1 M HCl) | |||||||
| 37 °C (24 h) | 50 | 39.09 ± 6.33 | 44.39 ± 4.48 | 78.19 | 88.79 | 16.18 | 10.10 |
| 80 °C (12 h) | 50 | 27.22 ± 2.75 | 26.12 ± 1.52 | 54.43 | 52.25 | 10.09 | 5.83 |
| Basic degradation (1 M NaOH) | |||||||
| 37 °C (24 h) | 50 | 15.16 ± 0.34 | 18.56 ± 0.59 | 30.32 | 37.13 | 2.27 | 3.19 |
| 80 °C (12 h) | 50 | 5.71 ± 1.67 | 5.61 ± 1.83 | 11.44 | 11.23 | 29.17 | 32.69 |
| Oxidative degradation (30% H2O2) | |||||||
| 37 °C (24 h) | 50 | 30.52 ± 0.49 | 31.39 ± 0.27 | 61.04 | 62.79 | 1.60 | 0.87 |
| 80 °C (12 h) | 50 | 9.80 ± 0.2 | 8.24 ± 6.69 | 19.60 | 16.48 | 2.07 | 81.14 |
| Thermal degradation | |||||||
| Room temperature (24 h) | 50 | 51.36 ± 1.64 | 49.71 ± 2.24 | 102.72 | 99.41 | 3.19 | 4.50 |
| 37 °C (24 h) | 50 | 49.98 ± 0.35 | 44.16 ± 0.99 | 99.97 | 88.32 | 0.70 | 2.24 |
| 80 °C (12 h) | 50 | 48.67 ± 2.74 | 43.27 ± 1.18 | 97.34 | 86.54 | 5.63 | 2.73 |
| −80 °C (24 h) | 50 | 51.90 ± 0.20 | 51.87 ± 0.40 | 103.80 | 103.74 | 0.38 | 0.77 |
| Two freeze–thaw cycles (24 h (−80 °C), –2 h (22 °C), 24 h (−20 °C)) | 50 | 43.25 ± 9.42 | 49.04 ± 1.03 | 86.50 | 98.08 | 21.78 | 2.09 |
| Photolytic degradation (365 nm UV light 7 h) | 50 | 18.72 ± 0.94 | 9.63 ± 0.11 | 37.44 | 19.25 | 5.04 | 1.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żyżyńska-Granica, B.; Mollica, A.; Stefanucci, A.; Granica, S.; Kleczkowska, P. Comparative Study of Chemical Stability of a PK20 Opioid–Neurotensin Hybrid Peptide and Its Analogue [Ile9]PK20—The Effect of Isomerism of a Single Amino Acid. Int. J. Mol. Sci. 2022, 23, 10839. https://doi.org/10.3390/ijms231810839
Żyżyńska-Granica B, Mollica A, Stefanucci A, Granica S, Kleczkowska P. Comparative Study of Chemical Stability of a PK20 Opioid–Neurotensin Hybrid Peptide and Its Analogue [Ile9]PK20—The Effect of Isomerism of a Single Amino Acid. International Journal of Molecular Sciences. 2022; 23(18):10839. https://doi.org/10.3390/ijms231810839
Chicago/Turabian StyleŻyżyńska-Granica, Barbara, Adriano Mollica, Azzurra Stefanucci, Sebastian Granica, and Patrycja Kleczkowska. 2022. "Comparative Study of Chemical Stability of a PK20 Opioid–Neurotensin Hybrid Peptide and Its Analogue [Ile9]PK20—The Effect of Isomerism of a Single Amino Acid" International Journal of Molecular Sciences 23, no. 18: 10839. https://doi.org/10.3390/ijms231810839





