Bifidobacterium longum subsp. infantis CECT 7210 Reduces Inflammatory Cytokine Secretion in Caco-2 Cells Cultured in the Presence of Escherichia coli CECT 515
Abstract
:1. Introduction
2. Results
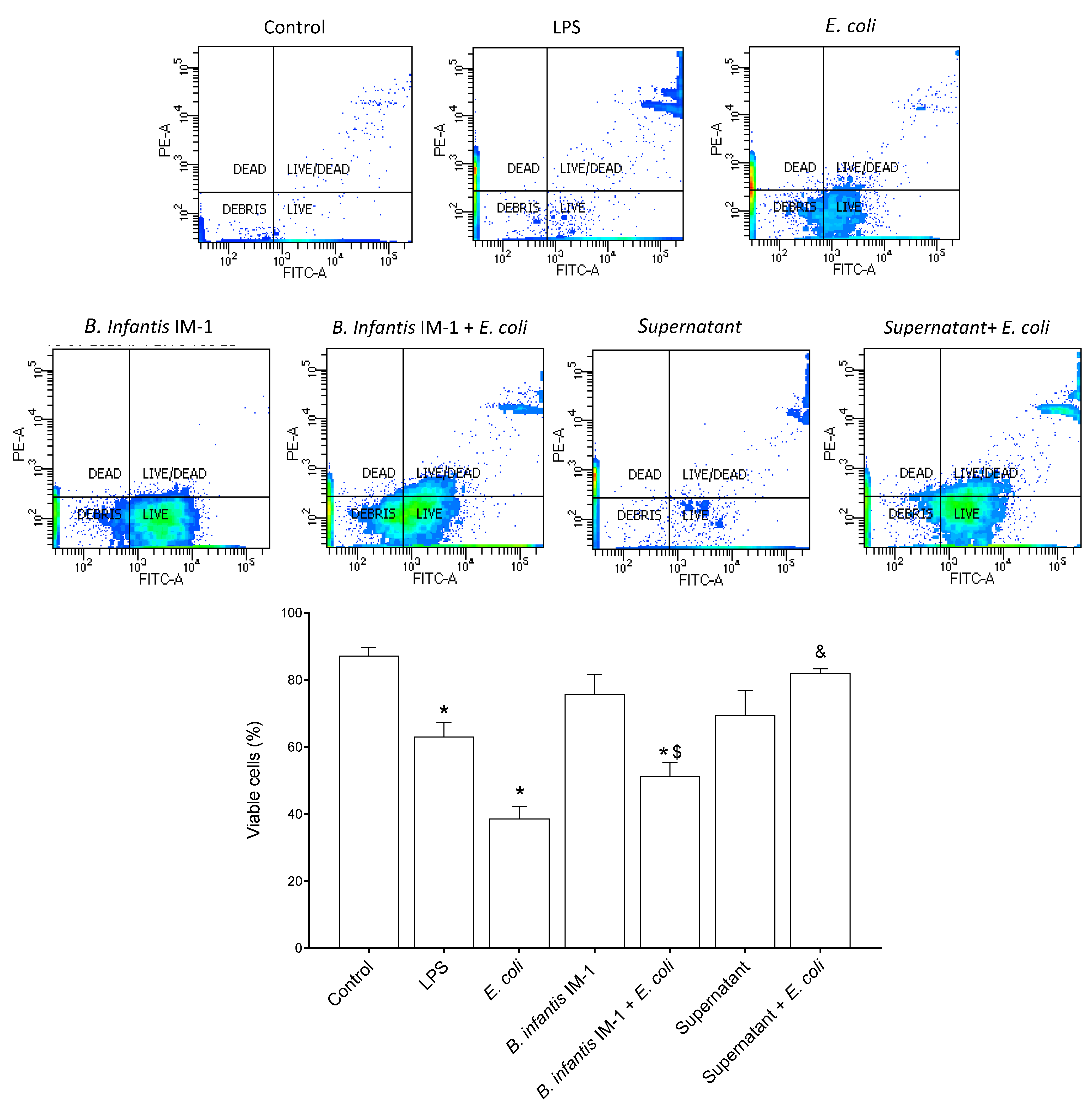
2.1. B. infantis IM-1® Supernatant Protects Caco-2 Cells against Escherichia coli CECT 515
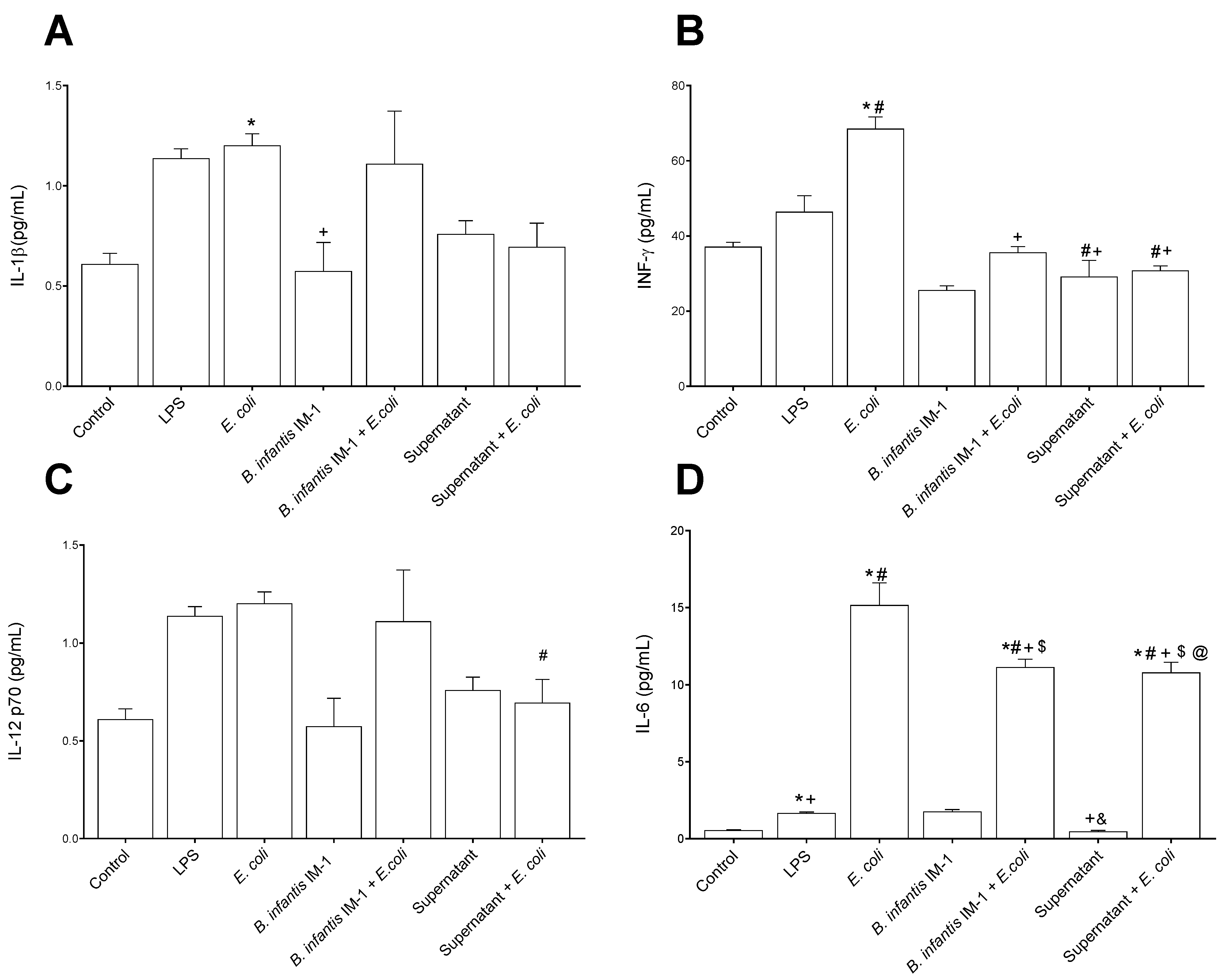
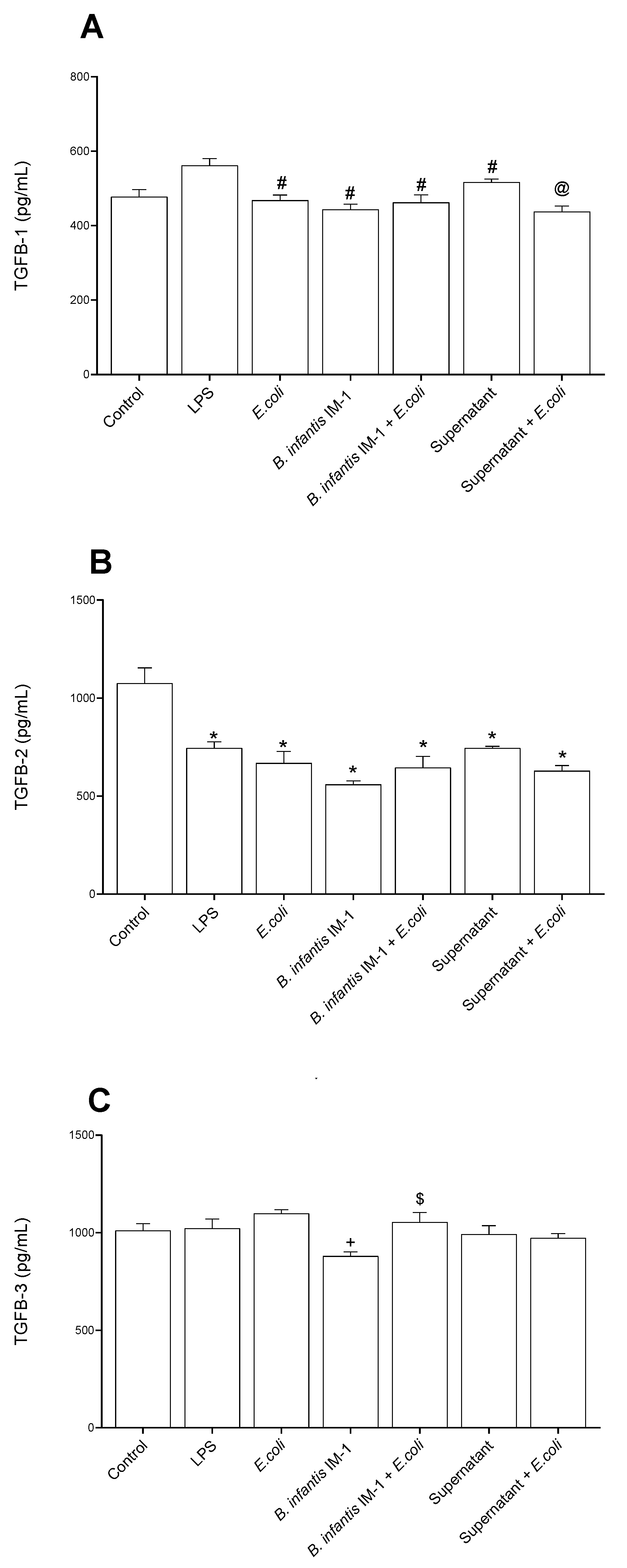
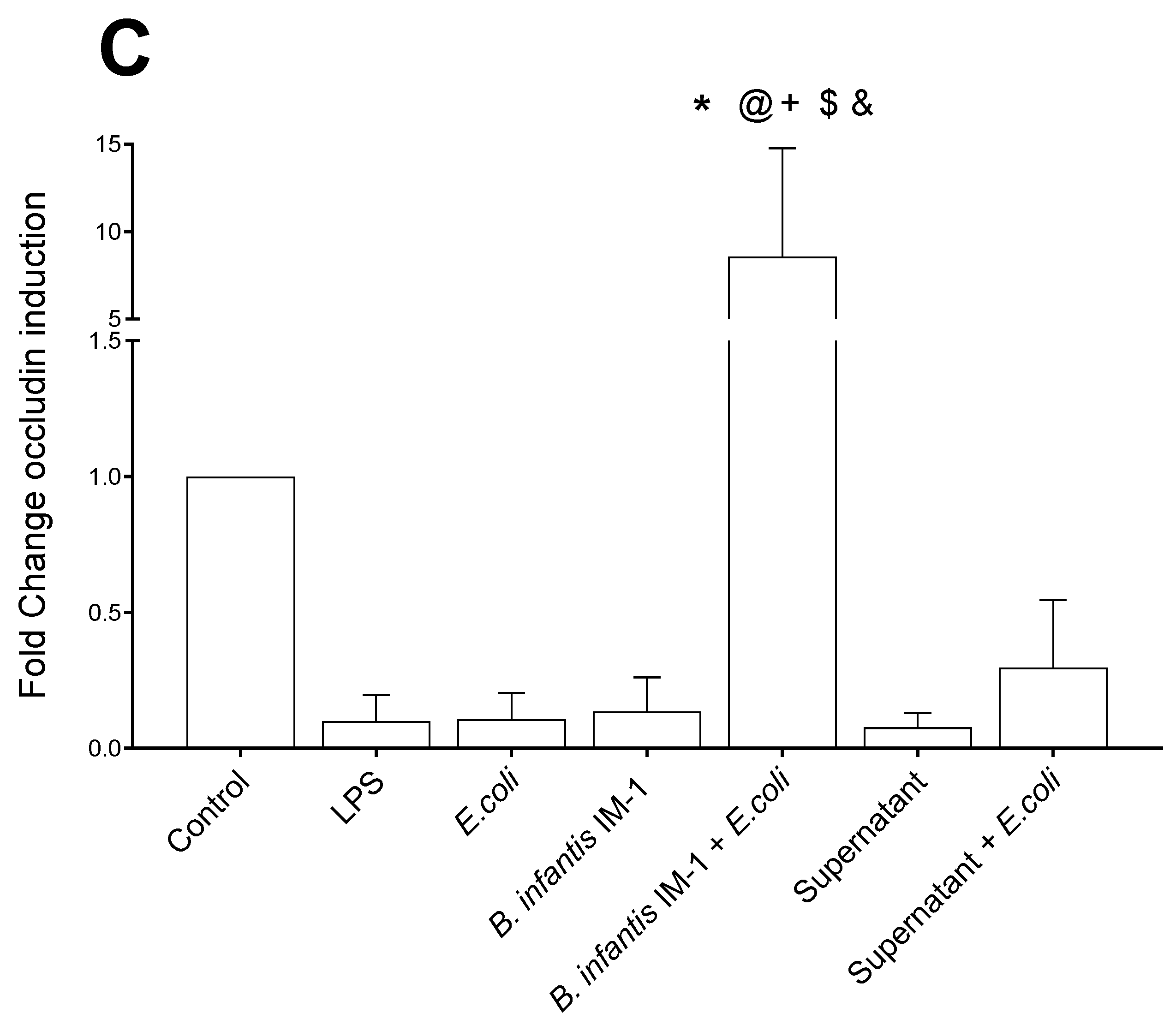
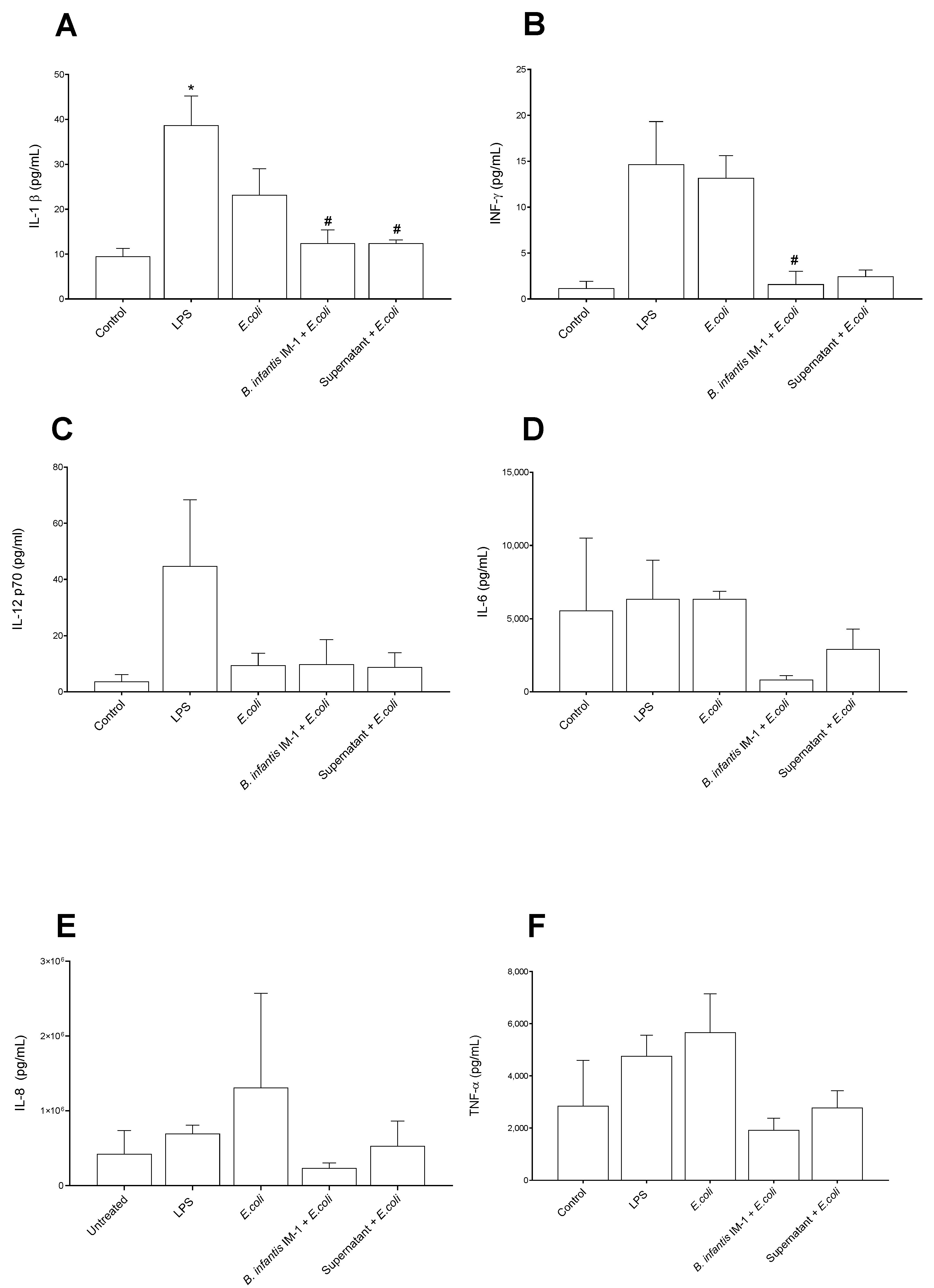
2.2. B. infantis IM-1® and Its Supernatant Decrease Secretion of Pro-Inflammatory Cytokines by Caco-2 Cells
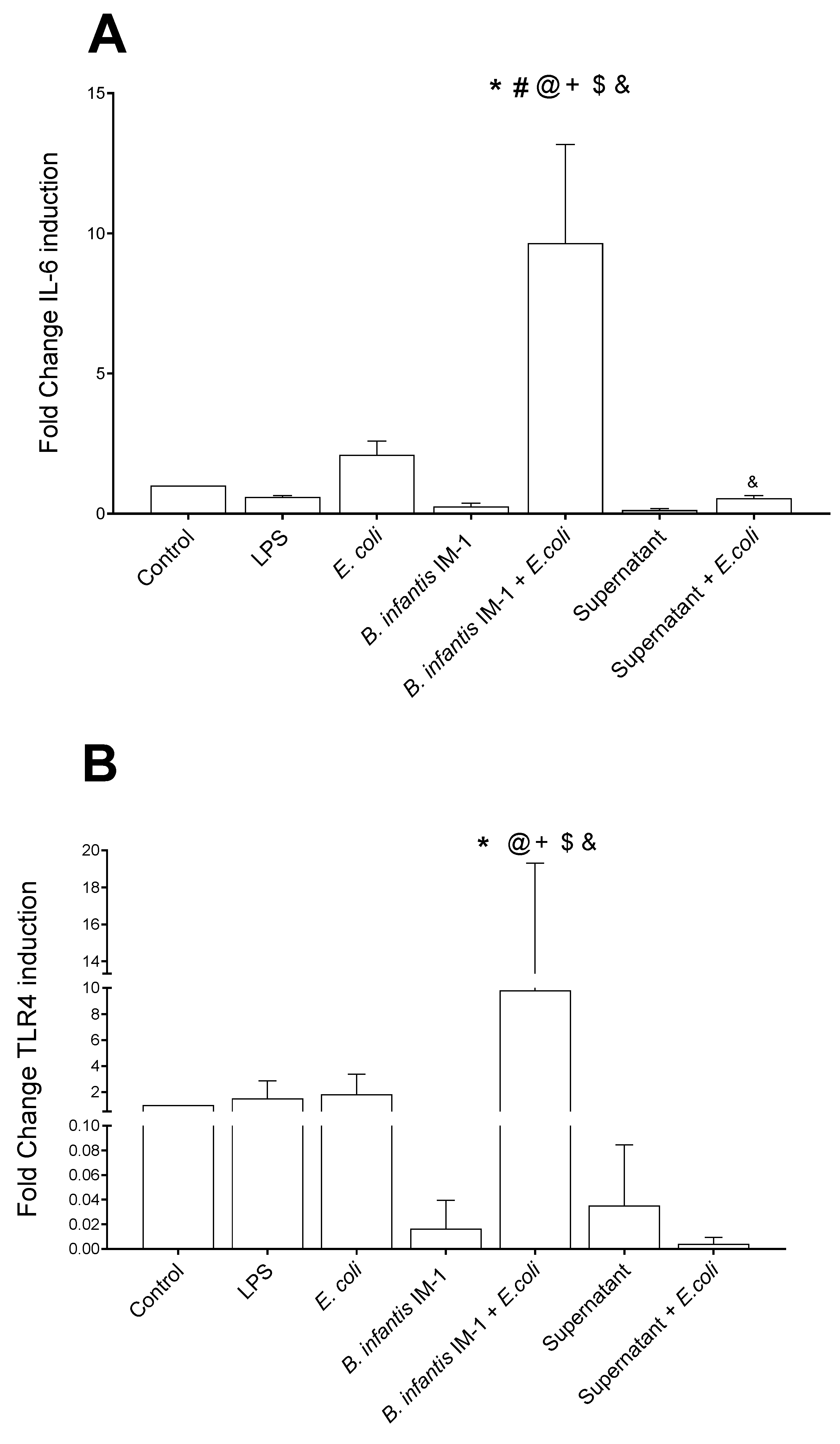
2.3. B. infantis IM-1® and Its Supernatant Reduce the Pro-Inflammatory Response by Co-Cultures of Caco-2 and DCs
3. Discussion
4. Materials and Methods
4.1. Probiotic Strain
4.2. Preparation of Bacteria and Cell-Free Culture Supernatant
4.3. Cells
4.4. Co-Cultures of Caco-2 and DCs
4.5. Cytokine Quantification in Culture Supernatants
4.6. RNA Isolation from Cell Lysates and qRT-PCR
4.7. Flow Cytometry
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: A systematic review of randomized human clinical trials. BioMed Res. Int. 2015, 2015, 505878. [Google Scholar] [CrossRef]
- Lai, H.-H.; Chiu, C.-H.; Kong, M.-S.; Chang, C.-J.; Chen, C.-C. Probiotic Lactobacillus casei: Effective for managing childhood diarrhea by altering gut microbiota and attenuating fecal inflammatory markers. Nutrients 2019, 11, 1150. [Google Scholar] [CrossRef]
- Skrzydło-Radomańska, B.; Prozorow-Król, B.; Cichoż-Lach, H.; Majsiak, E.; Bierła, J.B.; Kanarek, E.; Sowińska, A.; Cukrowska, B. The effectiveness and safety of multi-strain probiotic preparation in patients with diarrhea-predominant irritable bowel syndrome: A randomized controlled study. Nutrients 2021, 13, 756. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Górska, S. Impact of Probiotic Bacteria on Respiratory Allergy Disorders. Front. Microbiol. 2021, 12, 688137. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Lamas, A.; del Carmen Mondragón, A.; Cardelle-Cobas, A.; Regal, P.; Rodriguez-Avila, J.A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Probiotic effects against virus infections: New weapons for an old war. Foods 2021, 10, 130. [Google Scholar] [CrossRef]
- Zhai, R.; Xue, X.; Zhang, L.; Yang, X.; Zhao, L.; Zhang, C. Strain-specific anti-inflammatory properties of two Akkermansia muciniphila strains on chronic colitis in mice. Front. Cell. Infect. Microbiol. 2019, 9, 239. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Garssen, J.; Ben Amor, K.; Knol, J.; Franch, À.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. Strain-specific probiotic properties of bifidobacteria and lactobacilli for the prevention of diarrhea caused by rotavirus in a preclinical model. Nutrients 2020, 12, 498. [Google Scholar] [CrossRef]
- Park, H.; Lee, M.; Jeong, D.; Park, S.; Ji, Y.; Todorov, S.D.; Holzapfel, W.H. Safety Evaluation and In vivo Strain-Specific Functionality of Bacillus Strains Isolated from Korean Traditional Fermented Foods. Probiotics Antimicrob. Proteins 2021, 13, 60–71. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wu, C. Modulation of Gut Microbiota and Immune System by Probiotics, Pre-biotics, and Post-biotics. Front. Nutr. 2022, 8, 634897. [Google Scholar] [CrossRef]
- Suda, K.; Matsuda, K. How Microbes Affect Depression: Underlying Mechanisms via the Gut-Brain Axis and the Modulating Role of Probiotics. Int. J. Mol. Sci. 2022, 23, 1172. [Google Scholar] [CrossRef]
- Zeuthen, L.H.; Fink, L.N.; Frøkiær, H. Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut-derived lactobacilli and bifidobacteria in dendritic cells. Immunology 2008, 124, 489–502. [Google Scholar] [CrossRef]
- Moreno-Muñoz, J.A.; Chenoll, E.; Casinos, B.; Bataller, E.; Ramón, D.; Genovés, S.; Montava, R.; Ribes, J.M.; Buesa, J.; Fàbrega, J.; et al. Novel probiotic Bifidobacterium longum subsp. infantis CECT 7210 strain active against rotavirus infections. Appl. Environ. Microbiol. 2011, 77, 8775–8783. [Google Scholar] [CrossRef]
- Chenoll, E.; Rivero, M.; Codoñer, F.M.; Martinez-Blanch, J.F.; Ramón, D.; Genovés, S.; Muñoz, J.A.M. Complete genome sequence of Bifidobacterium longum subsp. infantis strain CECT 7210, a probiotic strain active against rotavirus infections. Genome Announc. 2015, 3, e00105-15. [Google Scholar] [CrossRef]
- Ruiz, L.; Flórez, A.B.; Sánchez, B.; Moreno-Muñoz, J.A.; Rodriguez-Palmero, M.; Jiménez, J.; de los Reyes Gavilán, C.G.; Gueimonde, M.; Ruas-Madiedo, P.; Margolles, A. Bifidobacteriumlongum subsp. Infantis CECT7210 (b. infantis im-1®) displays in vitro activity against some intestinal pathogens. Nutrients 2020, 12, 3259. [Google Scholar] [CrossRef]
- Chenoll, E.; Casinos, B.; Bataller, E.; Buesa, J.; Ramón, D.; Genovés, S.; Fábrega, J.; Urgell, M.R.; Muñoz, J.A.M. Identification of a peptide produced by Bifidobacterium longum CECT 7210 with antirotaviral activity. Front. Microbiol. 2016, 7, 655. [Google Scholar] [CrossRef]
- Barba-Vidal, E.; Castillejos, L.; López-Colom, P.; Urgell, M.R.; Muñoz, J.A.M.; Martín-Orúe, S.M. Evaluation of the probiotic strain Bifidobacterium longum subsp. infantis CECT 7210 capacities to improve health status and fight digestive pathogens in a piglet model. Front. Microbiol. 2017, 8, 533. [Google Scholar] [CrossRef]
- Barba-Vidal, E.; Castillejos, L.; Roll, V.F.B.; Cifuentes-Orjuela, G.; Moreno Muñoz, J.A.; Martín-Orúe, S.M. The Probiotic combination of Bifidobacterium longum subsp. infantis CECT 7210 and Bifidobacterium animalis subsp. lactis BPl6 reduces pathogen loads and improves gut health of weaned piglets orally challenged with Salmonella Typhimurium. Front. Microbiol. 2017, 8, 1570. [Google Scholar] [CrossRef]
- Rodríguez-Sorrento, A.; Castillejos, L.; López-Colom, P.; Cifuentes-Orjuela, G.; Rodríguez-Palmero, M.; Moreno-Muñoz, J.A.; Martín-Orúe, S.M. Effects of Bifidobacterium longum Subsp. infantis CECT 7210 and Lactobacillus rhamnosus HN001, Combined or Not With Oligofructose-Enriched Inulin, on Weaned Pigs Orally Challenged With Salmonella Typhimurium. Front. Microbiol. 2020, 11, 2012. [Google Scholar] [CrossRef]
- Rodríguez-Sorrento, A.; Castillejos, L.; López-Colom, P.; Cifuentes-Orjuela, G.; Rodríguez-Palmero, M.; Moreno-Muñoz, J.A.; Luise, D.; Trevisi, P.; Martín-Orúe, S.M. Effects of the Administration of Bifidobacterium longum subsp. infantis CECT 7210 and Lactobacillus rhamnosus HN001 and Their Synbiotic Combination With Galacto-Oligosaccharides Against Enterotoxigenic Escherichia coli F4 in an Early Weaned Piglet Model. Front. Microbiol. 2021, 12, 642549. [Google Scholar] [CrossRef]
- Escribano, J.; Ferré, N.; Gispert-Llaurado, M.; Luque, V.; Rubio-Torrents, C.; Zaragoza-Jordana, M.; Polanco, I.; Codoñer, F.M.; Chenoll, E.; Morera, M.; et al. Bifidobacterium longum subsp infantis CECT7210-supplemented formula reduces diarrhea in healthy infants: A randomized controlled trial. Pediatr. Res. 2018, 83, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.-P.; Ricciardi-Castagnoli, P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001, 2, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Matencio, E.; Romero, F.; Gil, A. Lactobacillus paracasei CNCM I-4034 and its culture supernatant modulate Salmonella-induced inflammation in a novel transwell co-culture of human intestinal-like dendritic and Caco-2 cells. BMC Microbiol. 2015, 15, 79. [Google Scholar] [CrossRef]
- Muñoz-Quezada, S.; Bermúdez-Brito, M.; Gómez-Llorente, C.; Matencio, E.; Bernal, M.; Romero, F.; Gil, A. Lactobacillus paracasei CNCM I-4034 disminuye la respuesta inflamatoria inducida por Salmonella typhi en células Caco-2. Nutr. Hosp. 2012, 27, 66–67. [Google Scholar]
- Viljanen, M.; Pohjavuori, E.; Haahtela, T.; Korpela, R.; Kuitunen, M.; Sarnesto, A.; Vaarala, O.; Savilahti, E. Induction of inflammation as a possible mechanism of probiotic effect in atopic eczema-dermatitis syndrome. J. Allergy Clin. Immunol. 2005, 115, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- D’Arienzo, R.; Maurano, F.; Lavermicocca, P.; Ricca, E.; Rossi, M. Modulation of the immune response by probiotic strains in a mouse model of gluten sensitivity. Cytokine 2009, 48, 254–259. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Muñoz-Quezada, S.; Gomez-Llorente, C.; Matencio, E.; Bernal, M.J.; Romero, F.; Gil, A. Human intestinal dendritic cells decrease cytokine release against Salmonella infection in the presence of Lactobacillus paracasei upon TLR activation. PLoS ONE 2012, 7, e43197. [Google Scholar] [CrossRef]
- Jayashree, S.; Karthikeyan, R.; Nithyalakshmi, S.; Ranjani, J.; Gunasekaran, P.; Rajendhran, J. Anti-adhesion property of the potential probiotic strain Lactobacillus fermentum 8711 against methicillin-resistant Staphylococcus aureus (MRSA). Front. Microbiol. 2018, 9, 411. [Google Scholar] [CrossRef]
- Poormontaseri, M.; Hosseinzadeh, S.; Shekarforoush, S.S.; Kalantari, T. The effects of probiotic Bacillus subtilis on the cytotoxicity of Clostridium perfringens type a in Caco-2 cell culture. BMC Microbiol. 2017, 17, 150. [Google Scholar] [CrossRef]
- Ballester, M.; Quintanilla, R.; Ortega, F.J.; Serrano, J.C.E.; Cassanyé, A.; Rodríguez-Palmero, M.; Moreno-Muñoz, J.A.; Portero-Otin, M.; Tibau, J. Dietary intake of bioactive ingredients impacts liver and adipose tissue transcriptomes in a porcine model of prepubertal early obesity. Sci. Rep. 2020, 10, 37–72. [Google Scholar] [CrossRef]
- Valent, D.; Arroyo, L.; Fàbrega, E.; Font-I-Furnols, M.; Rodríguez-Palmero, M.; Moreno-Muñoz, J.A.; Tibau, J.; Bassols, A. Effects of a high-fat-diet supplemented with probiotics and ω3-fatty acids on appetite regulatory neuropeptides and neurotransmitters in a pig model. Benef. Microbes 2020, 11, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Leveque, M.; Buisson-Brenac, C.; Bueno, L.; Fioramonti, J.; Houdeau, E. Oestradiol decreases colonic permeability through oestrogen receptor β-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J. Physiol. 2009, 587, 3317–3328. [Google Scholar] [CrossRef] [PubMed]
- Dokladny, K.; Ye, D.; Kennedy, J.C.; Moseley, P.L.; Ma, T.Y. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: Regulatory role of heat shock factor-1. Am. J. Pathol. 2008, 172, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Christensen, H.R.; Zeuthen, L.H.; Vogensen, F.K.; Jakobsen, M.; Frøkiær, H. Lactobacilli and bifidobacteria induce differential interferon-β profiles in dendritic cells. Cytokine 2011, 56, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Allocca, M.; Jovani, M.; Fiorino, G.; Schreiber, S.; Danese, S. Anti-IL-6 Treatment for Inflammatory Bowel Diseases: Next Cytokine, Next Target. Curr. Drug Targets 2013, 14, 1508–1521. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Muñoz-Quezada, S.; Gomez-Llorente, C.; Romero, F.; Gil, A. Lactobacillus rhamnosus and its cell-free culture supernatant differentially modulate inflammatory biomarkers in Escherichia coli-challenged human dendritic cells. Br. J. Nutr. 2014, 111, 1727–1737. [Google Scholar] [CrossRef] [Green Version]
- Castellani, A.; Chambers, A.J. Manual of Tropical Medicine, 3rd ed.; Wood, W., Ed.; Baillière, Tindall and Cox: London, UK, 1919. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Mercado, A.I.; Plaza-Díaz, J.; de Almagro, M.C.; Gil, Á.; Moreno-Muñoz, J.A.; Fontana, L. Bifidobacterium longum subsp. infantis CECT 7210 Reduces Inflammatory Cytokine Secretion in Caco-2 Cells Cultured in the Presence of Escherichia coli CECT 515. Int. J. Mol. Sci. 2022, 23, 10813. https://doi.org/10.3390/ijms231810813
Álvarez-Mercado AI, Plaza-Díaz J, de Almagro MC, Gil Á, Moreno-Muñoz JA, Fontana L. Bifidobacterium longum subsp. infantis CECT 7210 Reduces Inflammatory Cytokine Secretion in Caco-2 Cells Cultured in the Presence of Escherichia coli CECT 515. International Journal of Molecular Sciences. 2022; 23(18):10813. https://doi.org/10.3390/ijms231810813
Chicago/Turabian StyleÁlvarez-Mercado, Ana I., Julio Plaza-Díaz, M. Cristina de Almagro, Ángel Gil, José Antonio Moreno-Muñoz, and Luis Fontana. 2022. "Bifidobacterium longum subsp. infantis CECT 7210 Reduces Inflammatory Cytokine Secretion in Caco-2 Cells Cultured in the Presence of Escherichia coli CECT 515" International Journal of Molecular Sciences 23, no. 18: 10813. https://doi.org/10.3390/ijms231810813
APA StyleÁlvarez-Mercado, A. I., Plaza-Díaz, J., de Almagro, M. C., Gil, Á., Moreno-Muñoz, J. A., & Fontana, L. (2022). Bifidobacterium longum subsp. infantis CECT 7210 Reduces Inflammatory Cytokine Secretion in Caco-2 Cells Cultured in the Presence of Escherichia coli CECT 515. International Journal of Molecular Sciences, 23(18), 10813. https://doi.org/10.3390/ijms231810813







