Biophysical Characterization of LTX-315 Anticancer Peptide Interactions with Model Membrane Platforms: Effect of Membrane Surface Charge
Abstract
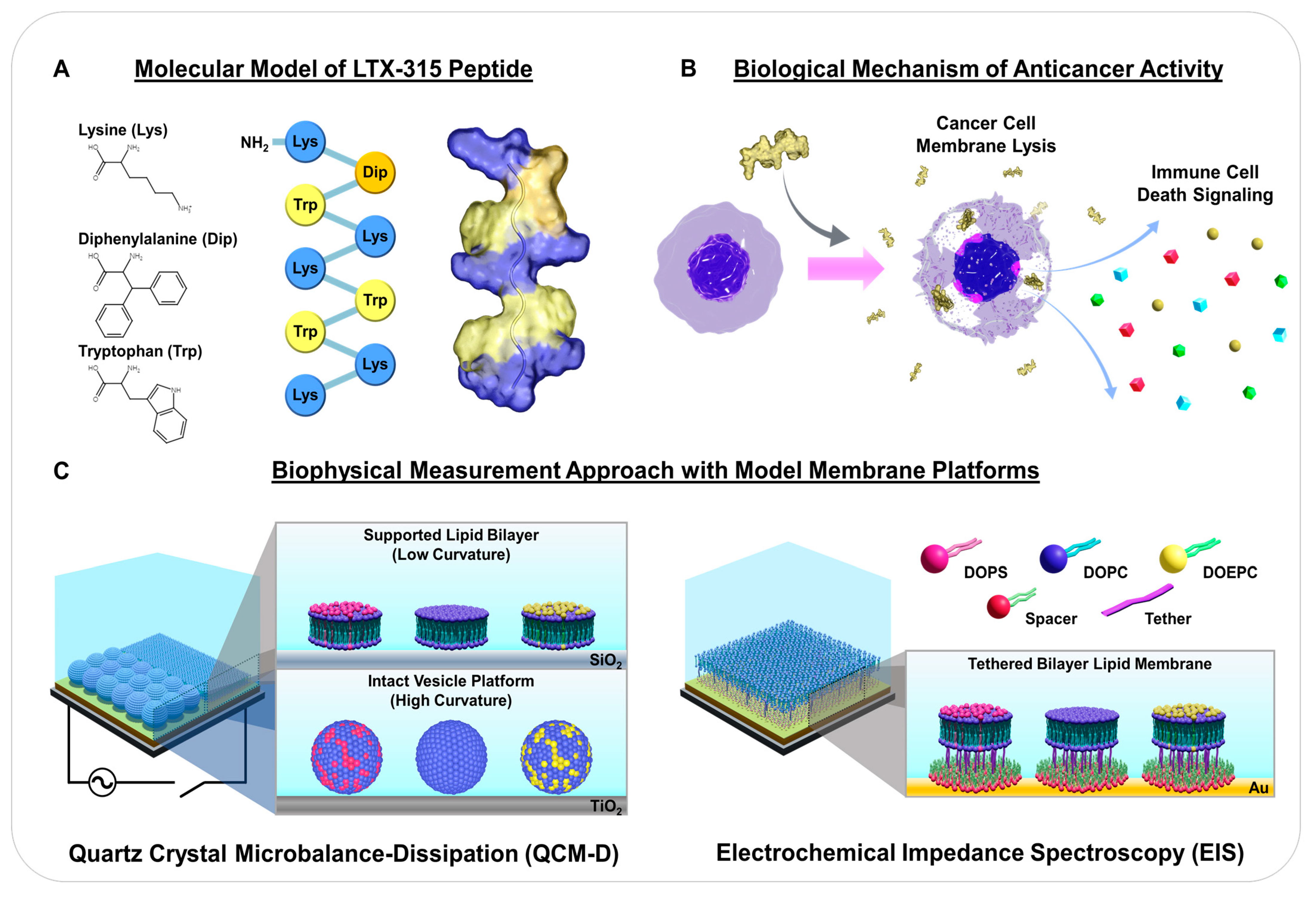
:1. Introduction
2. Results and Discussion
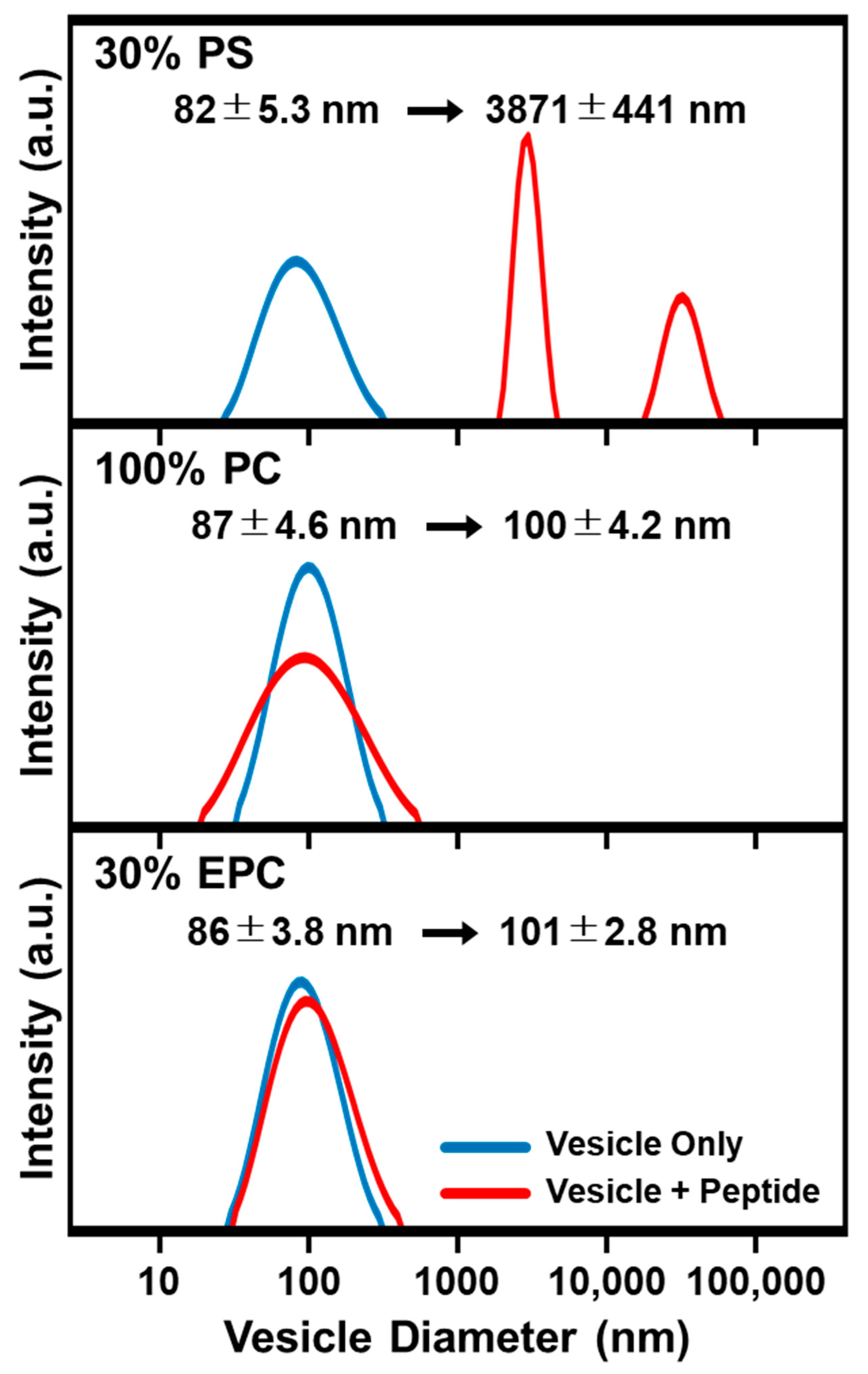
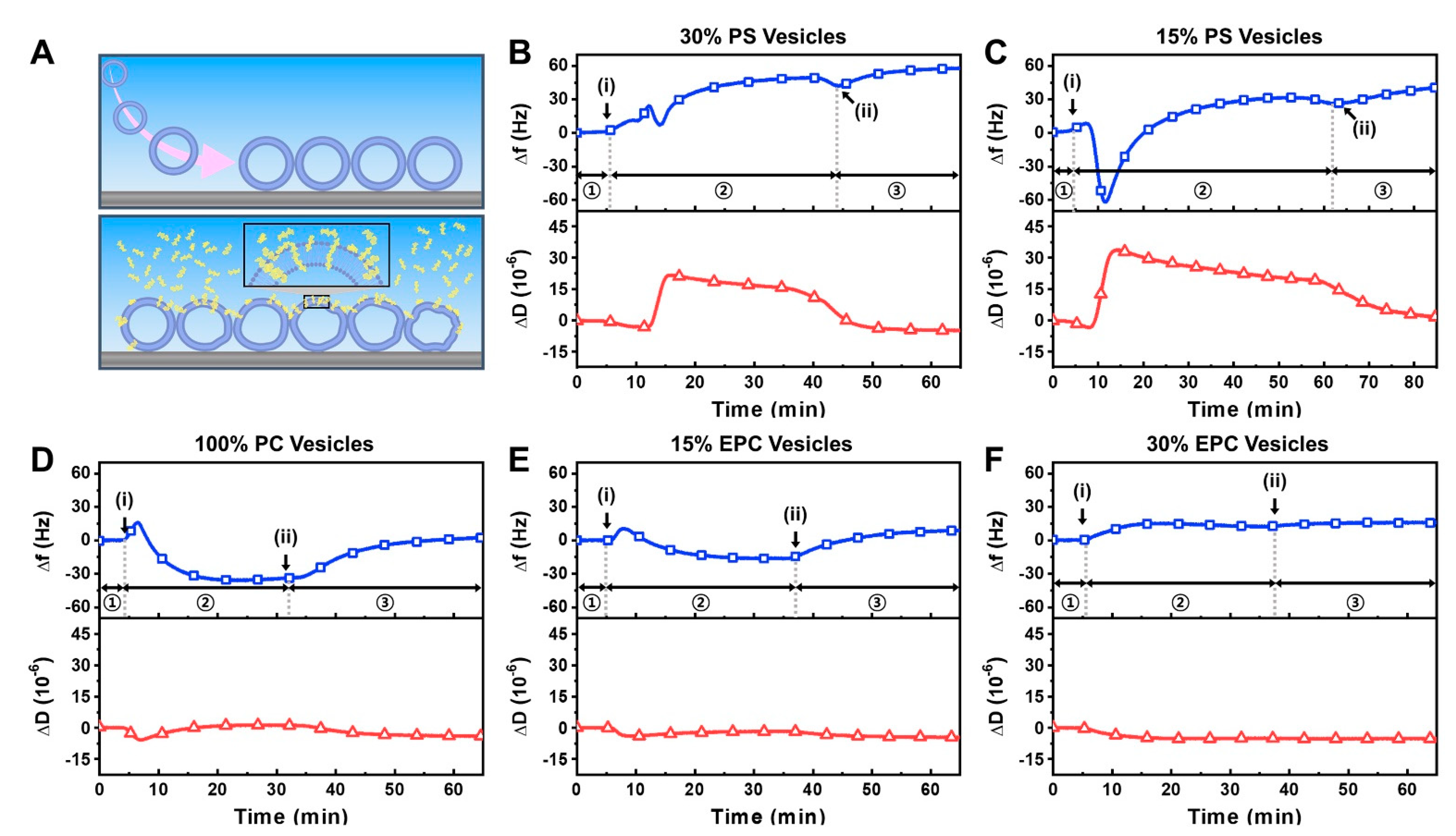
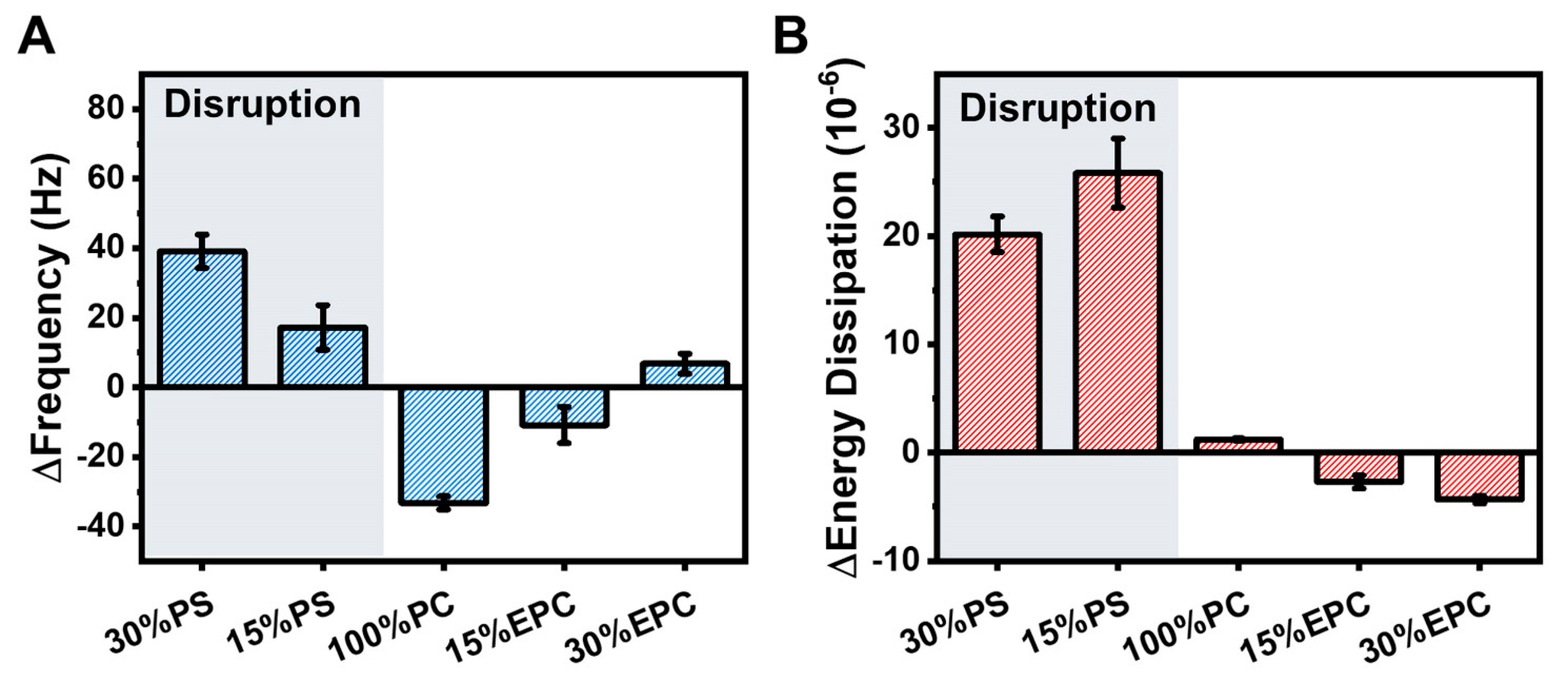
2.1. Intact Vesicle Platform
2.2. Supported Lipid Bilayer Platform
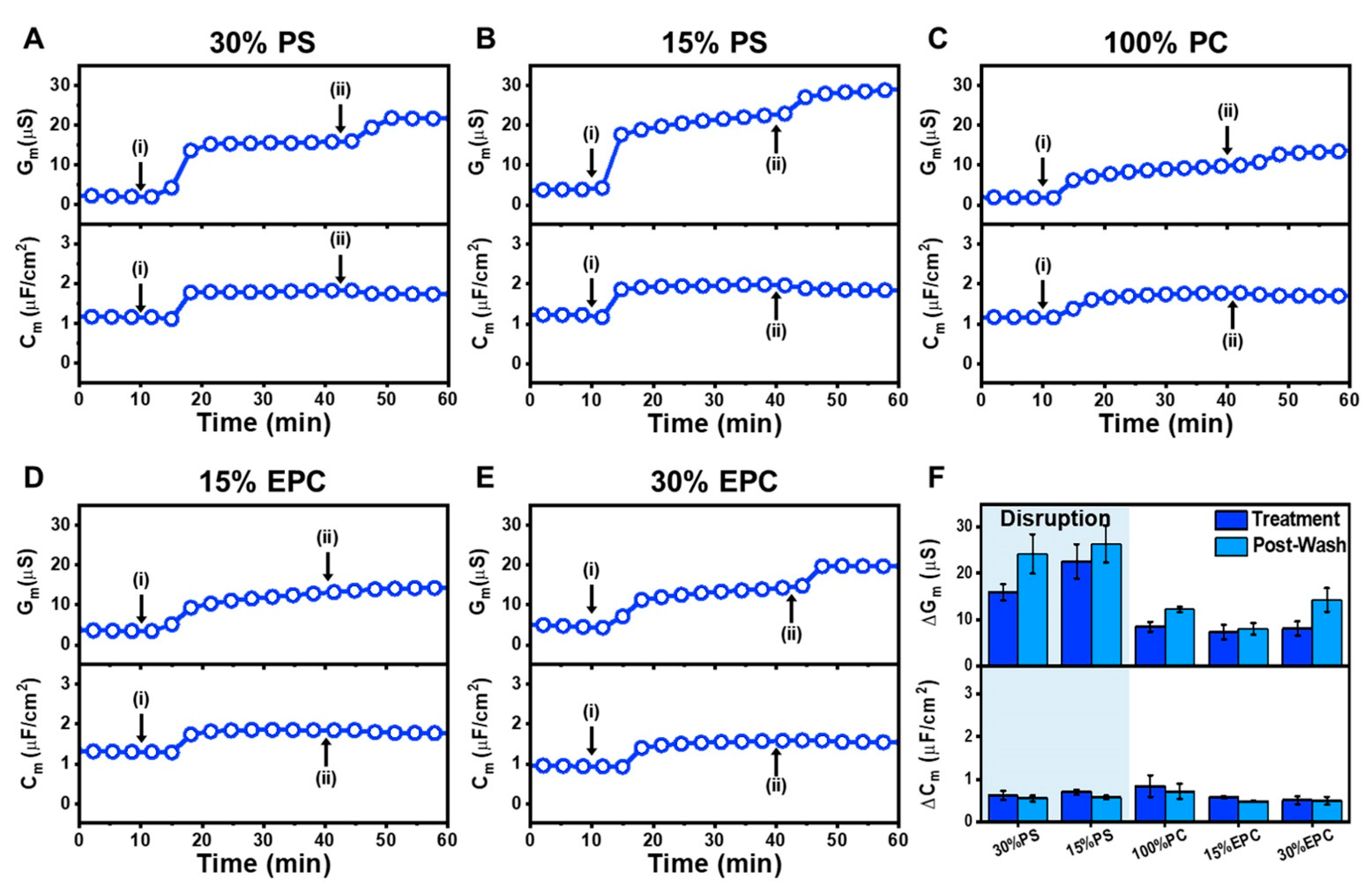
2.3. Tethered Bilayer Lipid Membrane Platform
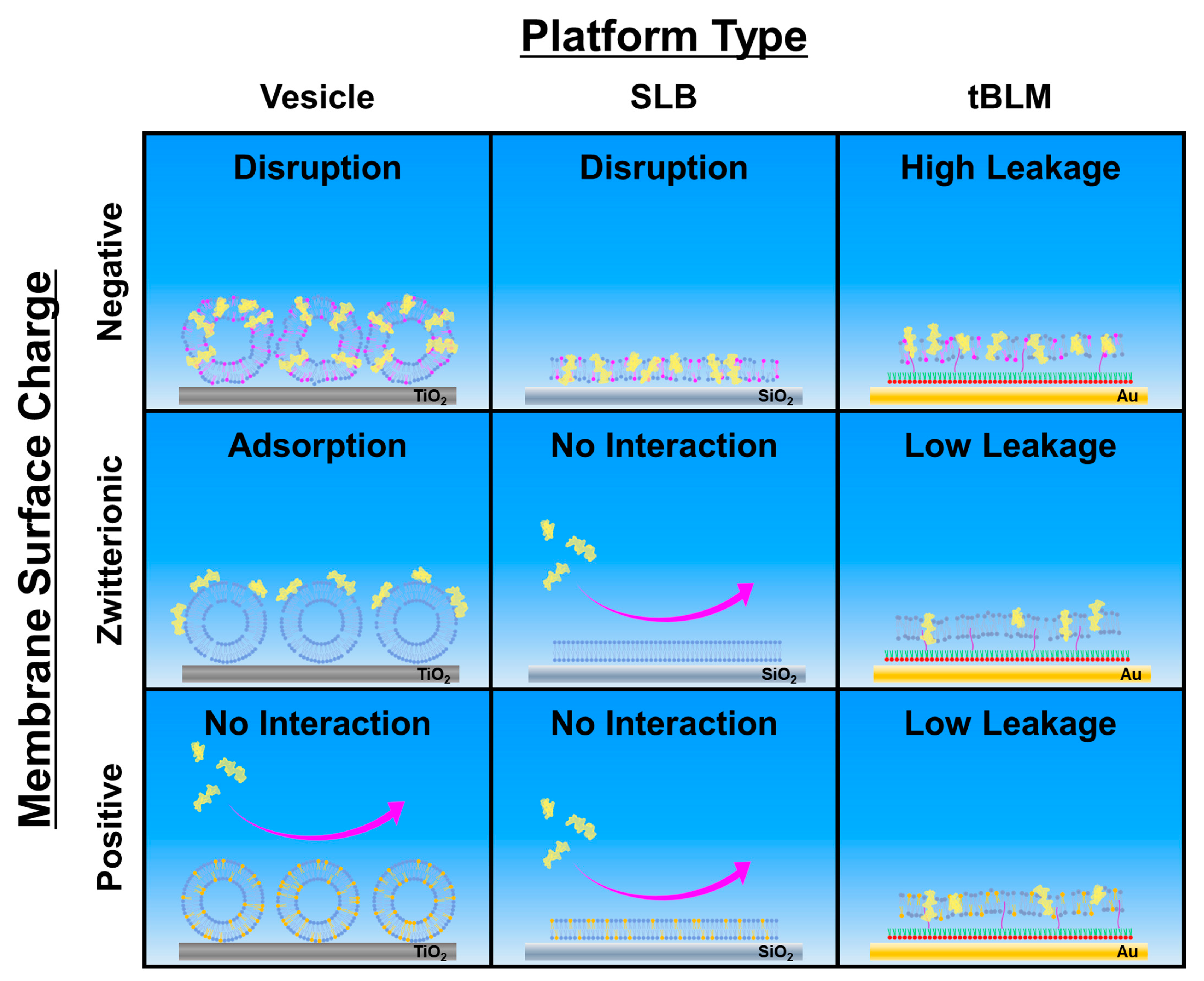
2.4. Mechanistic Analysis of Membrane-Peptide Interactions
3. Materials and Methods
3.1. Peptide
3.2. Vesicle Preparation
3.3. Circular Dichroism (CD) Spectroscopy
3.4. Dynamic Light Scattering (DLS)
3.5. Quartz crystal Microbalance-Dissipation (QCM-D)
3.6. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACP | Anticancer peptide |
| AMP | Antimicrobial peptide |
| CD | Circular dichroism |
| DLS | Dynamic light scattering |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| DOPS | 1,2-dioleoyl-sn-glycero-3-phospho-L-serine |
| DOEPC | 1,2-dioleoyl-sn-glycero-3-ethylphosphocholine |
| EIS | Electrochemical impedance spectroscopy |
| EPC | Ethylphosphatidylcholine |
| PC | Phosphatidylcholine |
| PS | Phosphatidylserine |
| QCM-D | Quartz crystal microbalance-dissipation |
| SLB | Supported lipid bilayer |
| tBLM | Tethered bilayer lipid membrane |
References
- Gabernet, G.; Müller, A.T.; Hiss, J.A.; Schneider, G. Membranolytic anticancer peptides. MedChemComm 2016, 7, 2232–2245. [Google Scholar] [CrossRef]
- Kardani, K.; Bolhassani, A. Antimicrobial/anticancer peptides: Bioactive molecules and therapeutic agents. Immunotherapy 2021, 13, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Adamczak, A. Anticancer activity of bacterial proteins and peptides. Pharmaceutics 2018, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Deslouches, B.; Di, Y.P. Antimicrobial peptides with selective antitumor mechanisms: Prospect for anticancer applications. Oncotarget 2017, 8, 46635. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Tuknait, A.; Anand, P.; Gupta, S.; Sharma, M.; Mathur, D.; Joshi, A.; Singh, S.; Gautam, A.; Raghava, G.P. CancerPPD: A database of anticancer peptides and proteins. Nucleic Acids Res. 2015, 43, D837–D843. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef]
- Haug, B.E.; Camilio, K.A.; Eliassen, L.T.; Stensen, W.; Svendsen, J.S.; Berg, K.; Mortensen, B.; Serin, G.; Mirjolet, J.-F.; Bichat, F. Discovery of a 9-mer cationic peptide (LTX-315) as a potential first in class oncolytic peptide. J. Med. Chem. 2016, 59, 2918–2927. [Google Scholar] [CrossRef]
- Gaspar, D.; Veiga, A.S.; Sinthuvanich, C.; Schneider, J.P.; Castanho, M.A. Anticancer peptide SVS-1: Efficacy precedes membrane neutralization. Biochemistry 2012, 51, 6263–6265. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2008, 1778, 357–375. [Google Scholar] [CrossRef]
- Kurrikoff, K.; Aphkhazava, D.; Langel, Ü. The future of peptides in cancer treatment. Curr. Opin. Pharmacol. 2019, 47, 27–32. [Google Scholar] [CrossRef]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with dual antimicrobial and anticancer activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Thundimadathil, J. Cancer treatment using peptides: Current therapies and future prospects. J. Amino Acids 2012, 2012, 967347. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur. J. Pharmacol. 2009, 625, 190–194. [Google Scholar] [CrossRef]
- Agrawal, P.; Bhagat, D.; Mahalwal, M.; Sharma, N.; Raghava, G.P. AntiCP 2.0: An updated model for predicting anticancer peptides. Briefings Bioinf. 2021, 22, bbaa153. [Google Scholar] [CrossRef]
- Connor, J.; Bucana, C.; Fidler, I.J.; Schroit, A.J. Differentiation-dependent expression of phosphatidylserine in mammalian plasma membranes: Quantitative assessment of outer-leaflet lipid by prothrombinase complex formation. Proc. Natl. Acad. Sci. USA 1989, 86, 3184–3188. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Matsuzaki, I.; Musangile, F.Y.; Takahashi, Y.; Iwahashi, Y.; Warigaya, K.; Kinoshita, Y.; Kojima, F.; Murata, S.-i. Measurement and visualization of cell membrane surface charge in fixed cultured cells related with cell morphology. PLoS ONE 2020, 15, e0236373. [Google Scholar]
- Huang, Y.-b.; Wang, X.-f.; Wang, H.-y.; Liu, Y.; Chen, Y. Studies on mechanism of action of anticancer peptides by modulation of hydrophobicity within a defined structural framework. Mol. Cancer Ther. 2011, 10, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application. Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef]
- Hu, C.; Huang, Y.; Chen, Y. Targeted modification of the cationic anticancer peptide HPRP-A1 with iRGD to improve specificity, penetration, and tumor-tissue accumulation. Mol. Pharm. 2018, 16, 561–572. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Sveinbjørnsson, B.; Camilio, K.A.; Haug, B.E.; Rekdal, Ø. LTX-315: A first-in-class oncolytic peptide that reprograms the tumor microenvironment. Future Med. Chem. 2017, 9, 1339–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilio, K.A.; Berge, G.; Ravuri, C.S.; Rekdal, Ø.; Sveinbjørnsson, B. Complete regression and systemic protective immune responses obtained in B16 melanomas after treatment with LTX-315. Cancer Immunol. Immunother. 2014, 63, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Camilio, K.A.; Rekdal, Ø.; Sveinbjörnsson, B. LTX-315 (Oncopore™) a short synthetic anticancer peptide and novel immunotherapeutic agent. Oncoimmunology 2014, 3, e29181. [Google Scholar] [CrossRef] [PubMed]
- Spicer, J.; Marabelle, A.; Baurain, J.-F.; Jebsen, N.L.; Jøssang, D.E.; Awada, A.; Kristeleit, R.; Loirat, D.; Lazaridis, G.; Jungels, C. Safety, antitumor activity, and T-cell responses in a dose-ranging phase I trial of the oncolytic peptide LTX-315 in patients with solid tumors. Clin. Cancer Res. 2021, 27, 2755–2763. [Google Scholar] [CrossRef]
- Zhou, H.; Forveille, S.; Sauvat, A.; Yamazaki, T.; Senovilla, L.; Ma, Y.; Liu, P.; Yang, H.; Bezu, L.; Müller, K. The oncolytic peptide LTX-315 triggers immunogenic cell death. Cell Death Dis. 2016, 7, e2134. [Google Scholar] [CrossRef]
- Camilio, K.A.; Wang, M.-Y.; Mauseth, B.; Waagene, S.; Kvalheim, G.; Rekdal, Ø.; Sveinbjørnsson, B.; Mælandsmo, G.M. Combining the oncolytic peptide LTX-315 with doxorubicin demonstrates therapeutic potential in a triple-negative breast cancer model. Breast Cancer Res. 2019, 21, 9. [Google Scholar] [CrossRef]
- Zhou, H.; Forveille, S.; Sauvat, A.; Sica, V.; Izzo, V.; Durand, S.; Müller, K.; Liu, P.; Zitvogel, L.; Rekdal, Ø. The oncolytic peptide LTX-315 kills cancer cells through Bax/Bak-regulated mitochondrial membrane permeabilization. Oncotarget 2015, 6, 26599. [Google Scholar] [CrossRef]
- Vitale, I.; Yamazaki, T.; Wennerberg, E.; Sveinbjørnsson, B.; Rekdal, Ø.; Demaria, S.; Galluzzi, L. Targeting cancer heterogeneity with immune responses driven by oncolytic peptides. Trends Cancer 2021, 7, 557–572. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Jackman, J.A.; Cho, N.-J. Model membrane platforms for biomedicine: Case study on antiviral drug development. Biointerphases 2012, 7, 18. [Google Scholar] [CrossRef]
- Jackman, J.A.; Goh, H.Z.; Zhdanov, V.P.; Knoll, W.; Cho, N.-J. Deciphering how pore formation causes strain-induced membrane lysis of lipid vesicles. J. Am. Chem. Soc. 2016, 138, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Andrä, J.; Böhling, A.; Gronewold, T.M.; Schlecht, U.; Perpeet, M.; Gutsmann, T. Surface acoustic wave biosensor as a tool to study the interaction of antimicrobial peptides with phospholipid and lipopolysaccharide model membranes. Langmuir 2008, 24, 9148–9153. [Google Scholar] [CrossRef] [PubMed]
- Mozsolits, H.; Wirth, H.-J.; Werkmeister, J.; Aguilar, M.-I. Analysis of antimicrobial peptide interactions with hybrid bilayer membrane systems using surface plasmon resonance. Biochim. Biophys. Acta Biomembr. 2001, 1512, 64–76. [Google Scholar] [CrossRef]
- Höök, F.; Kasemo, B. The QCM-D technique for probing biomacromolecular recognition reactions. In Piezoelectric Sensors; Janshoff, A., Steinem, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 425–447. [Google Scholar]
- Kanazawa, K.; Cho, N.-J. Quartz crystal microbalance as a sensor to characterize macromolecular assembly dynamics. J. Sens. 2009, 2009, 824947. [Google Scholar] [CrossRef]
- Dixon, M.C. Quartz crystal microbalance with dissipation monitoring: Enabling real-time characterization of biological materials and their interactions. J. Biomol. Tech. 2008, 19, 151. [Google Scholar]
- Cho, N.-J.; Frank, C.W.; Kasemo, B.; Höök, F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat. Protoc. 2010, 5, 1096–1106. [Google Scholar] [CrossRef]
- Alghalayini, A.; Garcia, A.; Berry, T.; Cranfield, C.G. The use of tethered bilayer lipid membranes to identify the mechanisms of antimicrobial peptide interactions with lipid bilayers. Antibiotics 2019, 8, 12. [Google Scholar] [CrossRef]
- Berry, T.; Dutta, D.; Chen, R.; Leong, A.; Wang, H.; Donald, W.A.; Parviz, M.; Cornell, B.; Willcox, M.; Kumar, N. Lipid membrane interactions of the cationic antimicrobial peptide chimeras melimine and cys-melimine. Langmuir 2018, 34, 11586–11592. [Google Scholar] [CrossRef]
- Cranfield, C.G.; Berry, T.; Holt, S.A.; Hossain, K.R.; Le Brun, A.P.; Carne, S.; Al Khamici, H.; Coster, H.; Valenzuela, S.M.; Cornell, B. Evidence of the key role of H3O+ in phospholipid membrane morphology. Langmuir 2016, 32, 10725–10734. [Google Scholar]
- Cranfield, C.G.; Cornell, B.A.; Grage, S.L.; Duckworth, P.; Carne, S.; Ulrich, A.S.; Martinac, B. Transient potential gradients and impedance measures of tethered bilayer lipid membranes: Pore-forming peptide insertion and the effect of electroporation. Biophys. J. 2014, 106, 182–189. [Google Scholar] [CrossRef]
- Sen, T.Z.; Jernigan, R.L.; Garnier, J.; Kloczkowski, A. GOR V server for protein secondary structure prediction. Bioinformatics 2005, 21, 2787–2788. [Google Scholar] [CrossRef] [PubMed]
- Milner-White, E.J.; Russell, M.J. Predicting the conformations of peptides and proteins in early evolution. Biol. Direct 2008, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919. [Google Scholar]
- Zan, G.H.; Jackman, J.A.; Cho, N.-J. AH peptide-mediated formation of charged planar lipid bilayers. J. Phys. Chem. B 2014, 118, 3616–3621. [Google Scholar] [CrossRef]
- Reimhult, E.; Höök, F.; Kasemo, B. Vesicle adsorption on SiO2 and TiO2: Dependence on vesicle size. J. Chem. Phys. 2002, 117, 7401–7404. [Google Scholar] [CrossRef]
- Reviakine, I.; Rossetti, F.F.; Morozov, A.N.; Textor, M. Investigating the properties of supported vesicular layers on titanium dioxide by quartz crystal microbalance with dissipation measurements. J. Chem. Phys. 2005, 122, 204711. [Google Scholar] [CrossRef]
- Mechler, A.; Praporski, S.; Atmuri, K.; Boland, M.; Separovic, F.; Martin, L.L. Specific and selective peptide-membrane interactions revealed using quartz crystal microbalance. Biophys. J. 2007, 93, 3907–3916. [Google Scholar] [CrossRef]
- John, T.; Abel, B.; Martin, L.L. The quartz crystal microbalance with dissipation monitoring (QCM-D) technique applied to the study of membrane-active peptides. Aust. J. Chem. 2018, 71, 543–546. [Google Scholar] [CrossRef]
- Martin, L.L.; Kubeil, C.; Piantavigna, S.; Tikkoo, T.; Gray, N.P.; John, T.; Calabrese, A.N.; Liu, Y.; Hong, Y.; Hossain, M.A. Amyloid aggregation and membrane activity of the antimicrobial peptide uperin 3.5. Pept. Sci. 2018, 110, e24052. [Google Scholar] [CrossRef]
- Losada-Pérez, P.; Khorshid, M.; Hermans, C.; Robijns, T.; Peeters, M.; Jiménez-Monroy, K.; Truong, L.; Wagner, P. Melittin disruption of raft and non-raft-forming biomimetic membranes: A study by quartz crystal microbalance with dissipation monitoring. Colloids Surf. B 2014, 123, 938–944. [Google Scholar] [CrossRef]
- Khorshid, M.; Losada-Pérez, P.; Wackers, G.; Yongabi, D.; Renner, F.U.; Thoelen, R.; Wagner, P. Real-time monitoring of interactions between Ebola fusion peptide and solid-supported phospholipid membranes: Effect of peptide concentration and layer geometry. Phys. Med. Biol. 2017, 4, 1–7. [Google Scholar]
- Liu, J.; Xiao, S.; Li, J.; Yuan, B.; Yang, K.; Ma, Y. Molecular details on the intermediate states of melittin action on a cell membrane. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2234–2241. [Google Scholar] [CrossRef]
- Lu, N.; Yang, K.; Yuan, B.; Ma, Y. Molecular response and cooperative behavior during the interactions of melittin with a membrane: Dissipative quartz crystal microbalance experiments and simulations. J. Phys. Chem. B 2012, 116, 9432–9438. [Google Scholar] [CrossRef] [PubMed]
- Ferhan, A.R.; Ma, G.J.; Jackman, J.A.; Sut, T.N.; Park, J.H.; Cho, N.-J. Probing the interaction of dielectric nanoparticles with supported lipid membrane coatings on nanoplasmonic arrays. Sensors 2017, 17, 1484. [Google Scholar] [CrossRef] [PubMed]
- McCubbin, G.A.; Praporski, S.; Piantavigna, S.; Knappe, D.; Hoffmann, R.; Bowie, J.H.; Separovic, F.; Martin, L.L. QCM-D fingerprinting of membrane-active peptides. Eur. Biophys. J. 2011, 40, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jackman, J.A.; Cho, N.-J. Comparing the membrane-interaction profiles of two antiviral peptides: Insights into structure–function relationship. Langmuir 2019, 35, 9934–9943. [Google Scholar] [CrossRef]
- Tan, S.W.; Jeon, W.-Y.; Yoon, B.K.; Jackman, J.A. Mechanistic Evaluation of Antimicrobial Lipid Interactions with Tethered Lipid Bilayers by Electrochemical Impedance Spectroscopy. Sensors 2022, 22, 3712. [Google Scholar] [CrossRef]
- Cranfield, C.G.; Henriques, S.T.; Martinac, B.; Duckworth, P.; Craik, D.J.; Cornell, B. Kalata b1 and kalata b2 have a surfactant-like activity in phosphatidylethanolomine-containing lipid membranes. Langmuir 2017, 33, 6630–6637. [Google Scholar] [CrossRef]
- Hatzakis, N.S.; Bhatia, V.K.; Larsen, J.; Madsen, K.L.; Bolinger, P.-Y.; Kunding, A.H.; Castillo, J.; Gether, U.; Hedegård, P.; Stamou, D. How curved membranes recruit amphipathic helices and protein anchoring motifs. Nat. Chem. Biol. 2009, 5, 835–841. [Google Scholar]
- Jackman, J.A.; Zan, G.H.; Zhdanov, V.P.; Cho, N.-J. Rupture of lipid vesicles by a broad-spectrum antiviral peptide: Influence of vesicle size. J. Phys. Chem. B 2013, 117, 16117–16128. [Google Scholar] [CrossRef]
- Shai, Y.; Oren, Z. From “carpet” mechanism to de-novo designed diastereomeric cell-selective antimicrobial peptides. Peptides 2001, 22, 1629–1641. [Google Scholar] [CrossRef]
- Park, S.; Avsar, S.Y.; Cornell, B.; Ferhan, A.R.; Jeon, W.-Y.; Chung, M.; Cho, N.-J. Probing the influence of tether density on tethered bilayer lipid membrane (tBLM)-peptide interactions. Appl. Mater. Today 2020, 18, 100527. [Google Scholar] [CrossRef]
- Kuroda, K.; Okumura, K.; Isogai, H.; Isogai, E. The human cathelicidin antimicrobial peptide LL-37 and mimics are potential anticancer drugs. Front. Oncol. 2015, 5, 144. [Google Scholar] [CrossRef] [PubMed]
- Nizalapur, S.; Ho, K.K.; Kimyon, Ö.; Yee, E.; Berry, T.; Manefield, M.; Cranfield, C.G.; Willcox, M.; Black, D.S.; Kumar, N. Synthesis and biological evaluation of N-naphthoyl-phenylglyoxamide-based small molecular antimicrobial peptide mimics as novel antimicrobial agents and biofilm inhibitors. Org. Biomol. Chem. 2016, 14, 3623–3637. [Google Scholar] [CrossRef]
- Oren, Z.; Lerman, J.C.; Gudmundsson, G.H.; Agerberth, B.; Shai, Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: Relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 1999, 341, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Shahmiri, M.; Cornell, B.; Mechler, A. Phenylalanine residues act as membrane anchors in the antimicrobial action of Aurein 1.2. Biointerphases 2017, 12, 05G605. [Google Scholar] [CrossRef]
- Yu, T.T.; Nizalapur, S.; Ho, K.K.; Yee, E.; Berry, T.; Cranfield, C.G.; Willcox, M.; Black, D.S.; Kumar, N. Design, Synthesis and Biological Evaluation of N-Sulfonylphenyl glyoxamide-Based Antimicrobial Peptide Mimics as Novel Antimicrobial Agents. ChemistrySelect 2017, 2, 3452–3461. [Google Scholar] [CrossRef]
- Aroui, S.; Kenani, A. Cell-penetrating peptides: A challenge for drug delivery. In Cheminformatics and Its Applications; Stefaniu, A., Rasul, A., Hussain, G., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Afonin, S.; Frey, A.; Bayerl, S.; Fischer, D.; Wadhwani, P.; Weinkauf, S.; Ulrich, A.S. The cell-penetrating peptide TAT (48–60) induces a non-lamellar phase in DMPC membranes. ChemPhysChem 2006, 7, 2134–2142. [Google Scholar] [CrossRef]
- Troeira Henriques, S.; Quintas, A.; Bagatolli, L.A.; Homblé, F.; Castanho, M.A. Energy-independent translocation of cell-penetrating peptides occurs without formation of pores. A biophysical study with pep-1. Mol. Membr. Biol. 2007, 24, 282–293. [Google Scholar] [CrossRef]
- Bobone, S.; Piazzon, A.; Orioni, B.; Pedersen, J.Z.; Nan, Y.H.; Hahm, K.S.; Shin, S.Y.; Stella, L. The thin line between cell-penetrating and antimicrobial peptides: The case of Pep-1 and Pep-1-K. J. Pept. Sci. 2011, 17, 335–341. [Google Scholar] [CrossRef]
- Hadianamrei, R.; Tomeh, M.A.; Brown, S.; Wang, J.; Zhao, X. Rationally designed short cationic α-helical peptides with selective anticancer activity. J. Colloid Interface Sci. 2022, 607, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, L.S.; Lan, Y.; Abbate, V.; Ruh, E.; Bui, T.T.; Wilkinson, L.J.; Kanno, T.; Jumagulova, E.; Kozlowska, J.; Patel, J. Conformational flexibility determines selectivity and antibacterial, antiplasmodial, and anticancer potency of cationic α-helical peptides. J. Biol. Chem. 2012, 287, 34120–34133. [Google Scholar] [CrossRef] [PubMed]
- Hadianamrei, R.; Tomeh, M.A.; Brown, S.; Wang, J.; Zhao, X. Correlation between the secondary structure and surface activity of β-sheet forming cationic amphiphilic peptides and their anticancer activity. Colloids Surf. B 2022, 209, 112165. [Google Scholar] [CrossRef] [PubMed]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid composition of the cancer cell membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [PubMed]
- Utsugi, T.; Schroit, A.J.; Connor, J.; Bucana, C.D.; Fidler, I.J. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991, 51, 3062–3066. [Google Scholar] [PubMed]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Beaven, G.H.; Holiday, E. Ultraviolet absorption spectra of proteins and amino acids. Adv. Protein Chem. 1952, 7, 319–386. [Google Scholar]
- Grimsley, G.R.; Pace, C.N. Spectrophotometric determination of protein concentration. Curr. Protoc. Protein Sci. 2003, 33, 3.1.1–3.1.9. [Google Scholar] [CrossRef]
- Hazra, C.; Samanta, T.; Mahalingam, V. A resonance energy transfer approach for the selective detection of aromatic amino acids. J. Mater. Chem. C 2014, 2, 10157–10163. [Google Scholar] [CrossRef]
- Phung, C.D.; Nguyen, B.L.; Jeong, J.H.; Chang, J.H.; Jin, S.G.; Choi, H.G.; Ku, S.K.; Kim, J.O. Shaping the “hot” immunogenic tumor microenvironment by nanoparticles co-delivering oncolytic peptide and TGF-β1 siRNA for boosting checkpoint blockade therapy. Bioeng. Transl. Med. 2022, e10392. [Google Scholar] [CrossRef]
- Jackman, J.A.; Zhao, Z.; Zhdanov, V.P.; Frank, C.W.; Cho, N.-J. Vesicle adhesion and rupture on silicon oxide: Influence of freeze–thaw pretreatment. Langmuir 2014, 30, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Tabaei, S.R.; Jackman, J.A.; Kim, M.; Yorulmaz, S.; Vafaei, S.; Cho, N.-J. Biomembrane fabrication by the solvent-assisted lipid bilayer (SALB) method. J. Vis. Exp. 2015, 106, e53073. [Google Scholar] [CrossRef]
- Sut, T.N.; Yoon, B.K.; Jeon, W.-Y.; Jackman, J.A.; Cho, N.-J. Supported lipid bilayer coatings: Fabrication, bioconjugation, and diagnostic applications. Appl. Mater. Today 2021, 25, 101183. [Google Scholar] [CrossRef]
- Jackman, J.A.; Saravanan, R.; Zhang, Y.; Tabaei, S.R.; Cho, N.J. Correlation between membrane partitioning and functional activity in a single lipid vesicle assay establishes design guidelines for antiviral peptides. Small 2015, 11, 2372–2379. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J.; Fasman, G.D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 1969, 8, 4108–4116. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.J.; Ferhan, A.R.; Jackman, J.A.; Cho, N.-J. Conformational flexibility of fatty acid-free bovine serum albumin proteins enables superior antifouling coatings. Commun. Mater. 2020, 1, 45. [Google Scholar] [CrossRef]
- Cranfield, C.; Carne, S.; Martinac, B.; Cornell, B. The assembly and use of tethered bilayer lipid membranes (tBLMs). In Methods in Membrane Lipids; Springer: Berlin/Heidelberg, Germany, 2015; pp. 45–53. [Google Scholar]
- Hoiles, W.; Gupta, R.; Cornell, B.; Cranfield, C.; Krishnamurthy, V. The effect of tethers on artificial cell membranes: A coarse-grained molecular dynamics study. PLoS ONE 2016, 11, e0162790. [Google Scholar]
- Alghalayini, A.; Jiang, L.; Gu, X.; Yeoh, G.H.; Cranfield, C.G.; Timchenko, V.; Cornell, B.A.; Valenzuela, S.M. Real-time monitoring of heat transfer between gold nanoparticles and tethered bilayer lipid membranes. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183334. [Google Scholar] [CrossRef]
- Alghalayini, A.; Jiang, L.; Gu, X.; Yeoh, G.H.; Cranfield, C.G.; Timchenko, V.; Cornell, B.A.; Valenzuela, S.M. Tethered bilayer lipid membranes to monitor heat transfer between gold nanoparticles and lipid membranes. J. Vis. Exp. 2020, 166, e61851. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, D.J.; Sut, T.N.; Tan, S.W.; Yoon, B.K.; Jackman, J.A. Biophysical Characterization of LTX-315 Anticancer Peptide Interactions with Model Membrane Platforms: Effect of Membrane Surface Charge. Int. J. Mol. Sci. 2022, 23, 10558. https://doi.org/10.3390/ijms231810558
Koo DJ, Sut TN, Tan SW, Yoon BK, Jackman JA. Biophysical Characterization of LTX-315 Anticancer Peptide Interactions with Model Membrane Platforms: Effect of Membrane Surface Charge. International Journal of Molecular Sciences. 2022; 23(18):10558. https://doi.org/10.3390/ijms231810558
Chicago/Turabian StyleKoo, Dong Jun, Tun Naw Sut, Sue Woon Tan, Bo Kyeong Yoon, and Joshua A. Jackman. 2022. "Biophysical Characterization of LTX-315 Anticancer Peptide Interactions with Model Membrane Platforms: Effect of Membrane Surface Charge" International Journal of Molecular Sciences 23, no. 18: 10558. https://doi.org/10.3390/ijms231810558




