Engineering Bio-Adhesives Based on Protein–Polysaccharide Phase Separation
Abstract
:1. Introduction
2. Results and Discussion
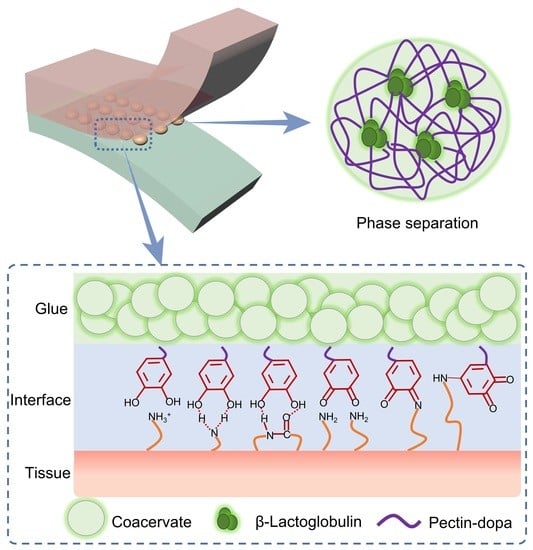
2.1. Design of the Glue-Type Bio-Adhesive Based on Protein–Polysaccharide Phase Separation
2.2. Phase Separation Based on Pectin-Dopa and β-Lactoglobulin
2.3. Adhesion Performance
2.4. Regulation of the Adhesion Strength and Biocompatibility of PD-L Bio-Glue
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Pectin-Dopa
3.3. Preparation of the Bio-Glue
3.4. Dynamic Light Scattering (DLS) Measurement
3.5. Light Microscopic Images
3.6. UV, FT-IR, and 1H-NMR Spectroscopy Measurements
3.7. Adhesion Strength Test
3.8. Cell Culture and Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuk, H.; Varela, C.E.; Nabzdyk, C.S.; Mao, X.; Padera, R.F.; Roche, E.T.; Zhao, X. Dry double-sided tape for adhesion of wet tissues and devices. Nature 2019, 575, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, E.A.; Molenaar, I.Q.; Porte, R.J.; De Boer, M.T. Topical haemostatic agents in liver surgery: Do we need them? Hpb 2009, 11, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Spotnitz, W.D.; Burks, S. Hemostats, sealants, and adhesives: Components of the surgical toolbox. Transfusion 2008, 48, 1502–1516. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yuk, H.; Wu, J.; Varela, C.E.; Chen, X.; Roche, E.T.; Guo, C.F.; Zhao, X. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 2020, 20, 229–236. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Ghobril, C.; Grinstaff, M.W. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: A tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef]
- Shin, J.; Choi, S.; Kim, J.H.; Cho, J.H.; Jin, Y.; Kim, S.; Min, S.; Kim, S.K.; Choi, D.; Cho, S.-W. Tissue Tapes—Phenolic Hyaluronic Acid Hydrogel Patches for Off-the-Shelf Therapy. Adv. Funct. Mater. 2019, 29, 1903863. [Google Scholar] [CrossRef]
- Xue, B.; Gu, J.; Li, L.; Yu, W.; Yin, S.; Qin, M.; Jiang, Q.; Wang, W.; Cao, Y. Hydrogel tapes for fault-tolerant strong wet adhesion. Nat. Commun. 2021, 12, 7156. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Yang, J. Design strategies and applications of tissue bioadhesives. Macromol. Biosci. 2013, 13, 271–288. [Google Scholar] [CrossRef]
- Duan, W.; Bian, X.; Bu, Y. Applications of Bioadhesives: A Mini Review. Front. Bioeng. Biotechnol. 2021, 9, 716035. [Google Scholar] [CrossRef]
- Zhu, W.; Chuah, Y.J.; Wang, D.A. Bioadhesives for internal medical applications: A review. Acta. Biomater. 2018, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Zhang, L.; Sun, G.; Sun, F.; Liu, J.; Yang, F.; Tang, P.; Wu, D. Tetra-PEG Based Hydrogel Sealants for In Vivo Visceral Hemostasis. Adv. Mater. 2019, 31, 1901580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, L.; Xiao, H.; Cong, H.; Wang, S. A reversible underwater glue based on photo- and thermo-responsive dynamic covalent bonds. Mater. Horiz. 2020, 7, 282–288. [Google Scholar] [CrossRef]
- Ramesh, M.; Kumar, L.R. Bioadhesives. In Green Adhesives: Preparation, Properties and Applications; Scrivener Publishing LLC: Beverly, MA, USA, 2020; pp. 145–164. [Google Scholar]
- Schricker, S.R. Bioadhesives. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–10. [Google Scholar]
- Leggat, P.A.; Smith, D.R.; Kedjarune, U. Surgical applications of cyanoacrylate adhesives: A review of toxicity. ANZ J. Surg. 2007, 77, 209–213. [Google Scholar] [CrossRef] [PubMed]
- MacGillivray, T.E. Fibrin Sealants and Glues. J. Cardiac Surg. 2003, 18, 480–485. [Google Scholar] [CrossRef]
- Moulay, S. Dopa/Catechol-Tethered Polymers: Bioadhesives and Biomimetic Adhesive Materials. Polym. Rev. 2014, 54, 436–513. [Google Scholar] [CrossRef]
- Forooshani, P.K.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. A Pol. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef]
- Balkenende, D.W.R.; Winkler, S.M.; Messersmith, P.B. Marine-inspired polymers in medical adhesion. Eur. Polym. J. 2019, 116, 134–143. [Google Scholar] [CrossRef]
- Matos-Perez, C.R.; White, J.D.; Wilker, J.J. Polymer Composition and Substrate Influences on the Adhesive Bonding of a Biomimetic, Cross-Linking Polymer. J. Am. Chem. Soc. 2012, 134, 9498–9505. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Wang, J.; Xu, Y.; Chen, Y.; Lu, Z.; Wang, W.; Xue, B.; Li, Y.; Cao, Y. Smart Adhesive Peptide Nanofibers for Cell Capture and Release. ACS Biomater. Sci. Eng. 2020, 6, 6800–6807. [Google Scholar] [CrossRef]
- Gebbie, M.A.; Wei, W.; Schrader, A.M.; Cristiani, T.R.; Dobbs, H.A.; Idso, M.; Chmelka, B.F.; Waite, J.H.; Israelachvili, J.N. Tuning underwater adhesion with cation–π interactions. Nat. Chem. 2017, 9, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.S.; Zeng, H.; Lu, Q.; Israelachvili, J.; Waite, J.H. Adhesion mechanism in a DOPA-deficient foot protein from green mussels. Soft Matter 2012, 8, 5640–5648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lei, H.; Qin, M.; Wang, W.; Cao, Y. Quantifying cation-π interactions in marine adhesive proteins using single-molecule force spectroscopy. Supramol. Mater. 2022, 1, 100005. [Google Scholar] [CrossRef]
- Deepankumar, K.; Guo, Q.; Mohanram, H.; Lim, J.; Mu, Y.; Pervushin, K.; Yu, J.; Miserez, A. Liquid-Liquid Phase Separation of the Green Mussel Adhesive Protein Pvfp-5 is Regulated by the Post-Translated Dopa Amino Acid. Adv. Mater. 2022, 34, e2103828. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Xue, B.; Lao, Y.; Wutthinitikornkit, Y.; Tian, R.; Zou, A.; Yang, L.; Wang, W.; Cao, Y.; Li, J. Structure and sequence features of mussel adhesive protein lead to its salt-tolerant adhesion ability. Sci. Adv. 2020, 6, eabb7620. [Google Scholar] [CrossRef]
- Li, Y.R.; Cheng, J.; Delparastan, P.; Wang, H.Q.; Sigg, S.J.; DeFrates, K.G.; Cao, Y.; Messersmith, P.B. Molecular design principles of Lysine-DOPA wet adhesion. Nat. Commun. 2020, 11, 3895. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y. The molecular mechanisms underlying mussel adhesion. Nano. Adv. 2019, 1, 4246–4257. [Google Scholar] [CrossRef]
- Delparastan, P.; Malollari, K.G.; Lee, H.; Messersmith, P.B. Direct Evidence for the Polymeric Nature of Polydopamine. Angew. Chem. Int. Ed. Engl. 2019, 58, 1077–1082. [Google Scholar] [CrossRef]
- Stewart, R.J.; Ransom, T.C.; Hlady, V. Natural underwater adhesives. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 757–771. [Google Scholar] [CrossRef]
- Xue, B.; Qin, M.; Wang, T.; Wu, J.; Luo, D.; Jiang, Q.; Li, Y.; Cao, Y.; Wang, W. Electrically Controllable Actuators Based on Supramolecular Peptide Hydrogels. Adv. Funct. Mater. 2016, 26, 9053–9062. [Google Scholar] [CrossRef]
- Ahn, B.K.; Lee, D.W.; Israelachvili, J.N.; Waite, J.H. Surface-initiated self-healing of polymers in aqueous media. Nat. Mater. 2014, 13, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.L.; Dubin, P.L.; Kayitmazer, A.B.; Turksen, S. Polyelectrolyte–protein complexes. Curr. Opin. Colloid Interface Sci. 2005, 10, 52–78. [Google Scholar] [CrossRef]
- Cousin, F.; Gummel, J.; Ung, D.; Boué, F. Polyelectrolyte-protein complexes: Structure and conformation of each specie revealed by SANS. Langmuir 2005, 21, 9675–9688. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Holkar, A.; Srivastava, S. Protein–Polyelectrolyte Complexes and Micellar Assemblies. Polymers 2019, 11, 1097. [Google Scholar] [CrossRef] [PubMed]
- Laneuville, S.I.; Paquin, P.; Turgeon, S.L. Effect of preparation conditions on the characteristics of whey protein—xanthan gum complexes. Food Hydrocoll. 2000, 14, 305–314. [Google Scholar] [CrossRef]
- Dubin, P.L.; Gao, J.; Mattison, K. Protein Purification by Selective Phase Separation with Polyelectrolytes. Sep. Purif. Methods 1994, 23, 1–16. [Google Scholar] [CrossRef]
- Singh, M.N.; Hemant, K.S.; Ram, M.; Shivakumar, H.G. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar]
- Lee, S.Y.; Ma, J.; Khoo, T.S.; Abdullah, N.; Nik Md Noordin Kahar, N.N.F.; Abdul Hamid, Z.A.; Mustapha, M. Polysaccharide-Based Hydrogels for Microencapsulation of Stem Cells in Regenerative Medicine. Front. Bioeng. Biotechnol. 2021, 9, 735090. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, R.; Zheng, B.; Guo, W.; Li, C.; He, W.; Wei, Y.; Du, Y.; Wang, H.; Wu, D.; et al. Highly Stretchable, Adhesive, Biocompatible, and Antibacterial Hydrogel Dressings for Wound Healing. Adv. Sci. 2021, 8, 2003627. [Google Scholar] [CrossRef]
- Gan, D.; Xing, W.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Fang, L.; Wang, K.; Lu, X. Plant-inspired adhesive and tough hydrogel based on Ag-Lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019, 10, 1487. [Google Scholar] [CrossRef]
- Wei, K.; Senturk, B.; Matter, M.T.; Wu, X.; Herrmann, I.K.; Rottmar, M.; Toncelli, C. Mussel-Inspired Injectable Hydrogel Adhesive Formed under Mild Conditions Features Near-Native Tissue Properties. ACS Appl. Mater. Interf. 2019, 11, 47707–47719. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Rana, D.; Shirzaei Sani, E.; Portillo-Lara, R.; Gifford, J.L.; Fares, M.M.; Mithieux, S.M.; Weiss, A.S. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials 2017, 139, 229–243. [Google Scholar] [CrossRef]
- Yang, B.; Song, J.; Jiang, Y.; Li, M.; Wei, J.; Qin, J.; Peng, W.; Lasaosa, F.L.; He, Y.; Mao, H.; et al. Injectable Adhesive Self-Healing Multicross-Linked Double-Network Hydrogel Facilitates Full-Thickness Skin Wound Healing. ACS Appl. Mater. Interf. 2020, 12, 57782–57797. [Google Scholar] [CrossRef]
- Cui, C.; Wu, T.; Chen, X.; Liu, Y.; Li, Y.; Xu, Z.; Fan, C.; Liu, W. A Janus Hydrogel Wet Adhesive for Internal Tissue Repair and Anti-Postoperative Adhesion. Adv. Funct. Mater. 2020, 30, 2005689. [Google Scholar] [CrossRef]
- Gunnarsson, S.B.; Bernfur, K.; Mikkelsenbc, A.; Cedervall, T. Analysis of nanoparticle biomolecule complexes. Nanoscale 2018, 1, 4246–4257. [Google Scholar] [CrossRef]
- Li, J.; Celiz, A.D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B.R.; Vasilyev, N.V.; Vlassak, J.J.; Suo, Z.; et al. Tough adhesives for diverse wet surfaces. Science 2017, 357, 378–381. [Google Scholar] [CrossRef]
- Guvendiren, M.; Messersmith, P.B.; Shull, K.R. Self-assembly and adhesion of DOPA-modified methacrylic triblock hydrogels. Biomacromolecules 2008, 9, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Oh, D.X.; Kim, S.; Seo, J.H.; Hwang, D.S.; Masic, A.; Han, D.K.; Cha, H.J. Mussel-mimetic protein-based adhesive hydrogel. Biomacromolecules 2014, 15, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-Inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wang, M.; Meng, L.; Chang, H.; Wang, B.; Xu, F.; Yang, J.; Wan, P. Mussel-Inspired Cellulose Nanocomposite Tough Hydrogels with Synergistic Self-Healing, Adhesive, and Strain-Sensitive Properties. Chem. Mater. 2018, 30, 3110–3121. [Google Scholar] [CrossRef]
- Han, L.; Liu, K.; Wang, M.; Wang, K.; Fang, L.; Chen, H.; Zhou, J.; Lu, X. Mussel-Inspired Adhesive and Conductive Hydrogel with Long-Lasting Moisture and Extreme Temperature Tolerance. Adv. Funct. Mater. 2018, 28, 1704195. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, Z.; Yu, W.; Xu, Z.; Li, Y.; Lai, J.; Li, Y.; Cao, Y.; Xue, B. Engineering Bio-Adhesives Based on Protein–Polysaccharide Phase Separation. Int. J. Mol. Sci. 2022, 23, 9987. https://doi.org/10.3390/ijms23179987
Bashir Z, Yu W, Xu Z, Li Y, Lai J, Li Y, Cao Y, Xue B. Engineering Bio-Adhesives Based on Protein–Polysaccharide Phase Separation. International Journal of Molecular Sciences. 2022; 23(17):9987. https://doi.org/10.3390/ijms23179987
Chicago/Turabian StyleBashir, Zoobia, Wenting Yu, Zhengyu Xu, Yiran Li, Jiancheng Lai, Ying Li, Yi Cao, and Bin Xue. 2022. "Engineering Bio-Adhesives Based on Protein–Polysaccharide Phase Separation" International Journal of Molecular Sciences 23, no. 17: 9987. https://doi.org/10.3390/ijms23179987