Dietary Cholic Acid Exacerbates Liver Fibrosis in NASH Model of Sprague–Dawley Rats Fed a High-Fat and High-Cholesterol Diet
Abstract
:1. Introduction
2. Results
2.1. Body Weight, Cumulative Energy Intake, and Relative Organ Weight
2.2. Histopathological Findings of the Livers
2.3. Serum and Hepatic Biochemical Parameters
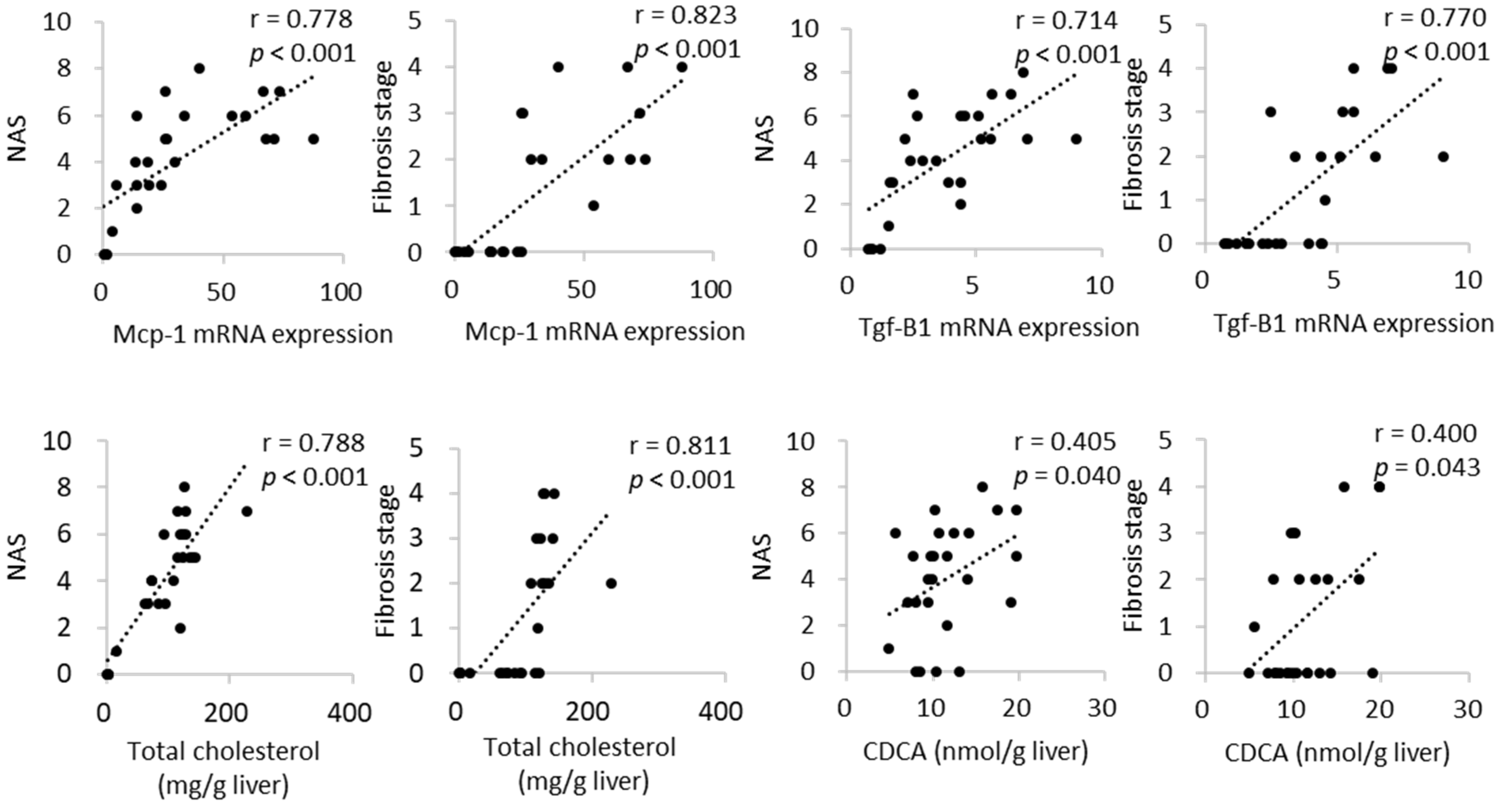
2.4. Hepatic mRNA Expression
2.5. Bile Acid Profile in the Liver
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Histopathological Assessment of the Livers
4.3. Serum and Tissue Biochemical Analysis
4.4. mRNA Quantification by Real-Time PCR
4.5. Determination of Bile Acid Levels in the Liver
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, P.; Zhang, Y.; Klaassen, C.D. Dose-Response of Five Bile Acids on Serum and Liver Bile Acid Concentrations and Hepatotoxicty in Mice. Toxicol. Sci. 2011, 123, 359–367. [Google Scholar] [CrossRef]
- Wu, W.; Liu, X.; Peng, X.; Xue, R.; Ji, L.; Shen, X.; Chen, S.; Gu, J.; Zhang, S. Bile acids override steatosis in farnesoid X receptor deficient mice in a model of non-alcoholic steatohepatitis. Biochem. Biophys. Res. Commun. 2014, 448, 50–55. [Google Scholar] [CrossRef]
- Watanabe, S.; Tsuneyama, K. Eicosapentaenoic acid attenuates hepatic accumulation of cholesterol esters but aggravates liver injury and inflammation in mice fed a cholate-supplemented high-fat diet. J. Toxicol. Sci. 2013, 38, 379–390. [Google Scholar] [CrossRef]
- Sanches, S.C.; Ramalho, L.N.; Augusto, M.J.; da Silva, D.M.; Ramalho, F.S. Nonalcoholic Steatohepatitis: A Search for Factual Animal Models. Biomed. Res. Int. 2015, 2015, 574832. [Google Scholar] [CrossRef]
- Schuppan, D.; Surabattula, R.; Wang, X.Y. Determinants of fibrosis progression and regression in NASH. J. Hepatol. 2018, 68, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zeng, X.Y.; Zhou, X.; Wang, H.; Jo, E.; Robinson, S.R.; Xu, A.; Ye, J.M. Dietary cholesterol induces hepatic inflammation and blunts mitochondrial function in the liver of high-fat-fed mice. J. Nutr. Biochem. 2016, 27, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Wouters, K.; Van Gorp, P.J.; Bieghs, V.; Gijbels, M.J.; Duimel, H.; Lütjohann, D.; Kerksiek, A.; Van Kruchten, R.; Maeda, N.; Staels, B.; et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology 2008, 48, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Van Rooyen, D.M.; Larter, C.Z.; Haigh, W.G.; Yeh, M.M.; Ioannou, G.; Kuver, R.; Lee, S.P.; Teoh, N.C.; Farrell, G.C. Hepatic Free Cholesterol Accumulates in Obese, Diabetic Mice and Causes Nonalcoholic Steatohepatitis. Gastroenterology 2011, 141, 1393–1403.e1395. [Google Scholar] [CrossRef] [PubMed]
- Ichimura-Shimizu, M.; Omagari, K.; Yamashita, M.; Tsuneyama, K. Development of a novel mouse model of diet-induced nonalcoholic steatohepatitis-related progressive bridging fibrosis. Biosci. Biotechnol. Biochem. 2021, 85, 941–947. [Google Scholar] [CrossRef]
- Ichimura, M.; Kawase, M.; Masuzumi, M.; Sakaki, M.; Nagata, Y.; Tanaka, K.; Suruga, K.; Tamaru, S.; Kato, S.; Tsuneyama, K.; et al. High-fat and high-cholesterol diet rapidly induces non-alcoholic steatohepatitis with advanced fibrosis in Sprague-Dawley rats. Hepatol. Res. 2015, 45, 458–469. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Hirsova, P.; Malhi, H.; Gores, G.J. Animal Models of Nonalcoholic Steatohepatitis: Eat, Delete, and Inflame. Dig. Dis. Sci. 2016, 61, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Fujita, K.; Tsuneyama, K.; Nose, M. Changes in liver lipidomics associated with sodium cholate-induced liver injury and its prevention by boiogito, a Japanese herbal medicine, in mice. Tradit. Kampo Med. 2016, 3, 9–19. [Google Scholar] [CrossRef]
- Day, C.P.; James, J.O. Steatohepatitis: A tale of two “hits”? Gastroenterol. Hepatol. 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Vergnes, L.; Phan, J.; Strauss, M.; Tafuri, S.; Reue, K. Cholesterol and Cholate Components of an Atherogenic Diet Induce Distinct Stages of Hepatic Inflammatory Gene Expression. J. Biol. Chem. 2003, 278, 42774–42784. [Google Scholar] [CrossRef]
- Miyazaki, T.; Sasaki, S.; Toyoda, A.; Shirai, M.; Ikegami, T.; Matsuzaki, Y.; Honda, A. Influences of Taurine Deficiency on Bile Acids of the Bile in the Cat Model. Taurine 2019, 11, 35–44. [Google Scholar]
- Honda, A.; Miyazaki, T.; Iwamoto, J.; Hirayama, T.; Morishita, Y.; Monma, T.; Ueda, H.; Mizuno, S.; Sugiyama, F.; Takahashi, S.; et al. Regulation of bile acid metabolism in mouse models with hydrophobic bile acid composition. J. Lipid Res. 2020, 61, 54–69. [Google Scholar] [CrossRef]
- Matsuzawa, N.; Takamura, T.; Kurita, S.; Misu, H.; Ota, T.; Ando, H.; Yokoyama, M.; Honda, M.; Zen, Y.; Nakanuma, Y.; et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology 2007, 46, 1392–1403. [Google Scholar] [CrossRef]
- Crespo Yanguas, S.; Cogliati, B.; Willebrords, J.; Maes, M.; Colle, I.; Van Den Bossche, B.; De Oliveira, C.P.M.S.; Andraus, W.; Alves, V.A.; Leclercq, I.; et al. Experimental models of liver fibrosis. Arch. Toxicol. 2016, 90, 1025–1048. [Google Scholar] [CrossRef]
- Teratani, T.; Tomita, K.; Suzuki, T.; Oshikawa, T.; Yokoyama, H.; Shimamura, K.; Tominaga, S.; Hiroi, S.; Irie, R.; Okada, Y.; et al. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology 2012, 142, 152–164.e110. [Google Scholar] [CrossRef]
- Perez, M.J.; Briz, O. Bile-acid-induced cell injury and protection. World J. Gastroenterol. 2009, 15, 1677. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhou, J.; Zhao, S.; Tian, C.; Wang, P.; Xu, C.; Chen, Y.; Cai, W.; Wu, J. Chenodeoxycholic acid activates NLRP3 inflammasome and contributes to cholestatic liver fibrosis. Oncotarget 2016, 7, 83951–83963. [Google Scholar] [CrossRef] [PubMed]
- Kwan, S.Y.; Jiao, J.; Qi, J.; Wang, Y.; Wei, P.; McCormick, J.B.; Fisher-Hoch, S.P.; Beretta, L. Bile Acid Changes Associated With Liver Fibrosis and Steatosis in the Mexican-American Population of South Texas. Hepatol. Commun. 2020, 4, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef]
- Gabbi, C.; Bertolotti, M.; Anzivino, C.; Macchioni, D.; Del Puppo, M.; Ricchi, M.; Carubbi, F.; Tagliafico, E.; Romagnoli, D.; Odoardi, M.R.; et al. Effects of bile duct ligation and cholic acid treatment on fatty liver in two rat models of non-alcoholic fatty liver disease. Dig. Liver Dis. 2012, 44, 1018–1026. [Google Scholar] [CrossRef]
- Savard, C.; Tartaglione, E.V.; Kuver, R.; Haigh, W.G.; Farrell, G.C.; Subramanian, S.; Chait, A.; Yeh, M.M.; Quinn, L.S.; Ioannou, G.N. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology 2013, 57, 81–92. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Fujita, K.; Iguchi, Y.; Une, M.; Watanabe, S. Ursodeoxycholic Acid Suppresses Lipogenesis in Mouse Liver: Possible Role of the Decrease in β-Muricholic Acid, a Farnesoid X Receptor Antagonist. Lipids 2017, 52, 335–344. [Google Scholar] [CrossRef]
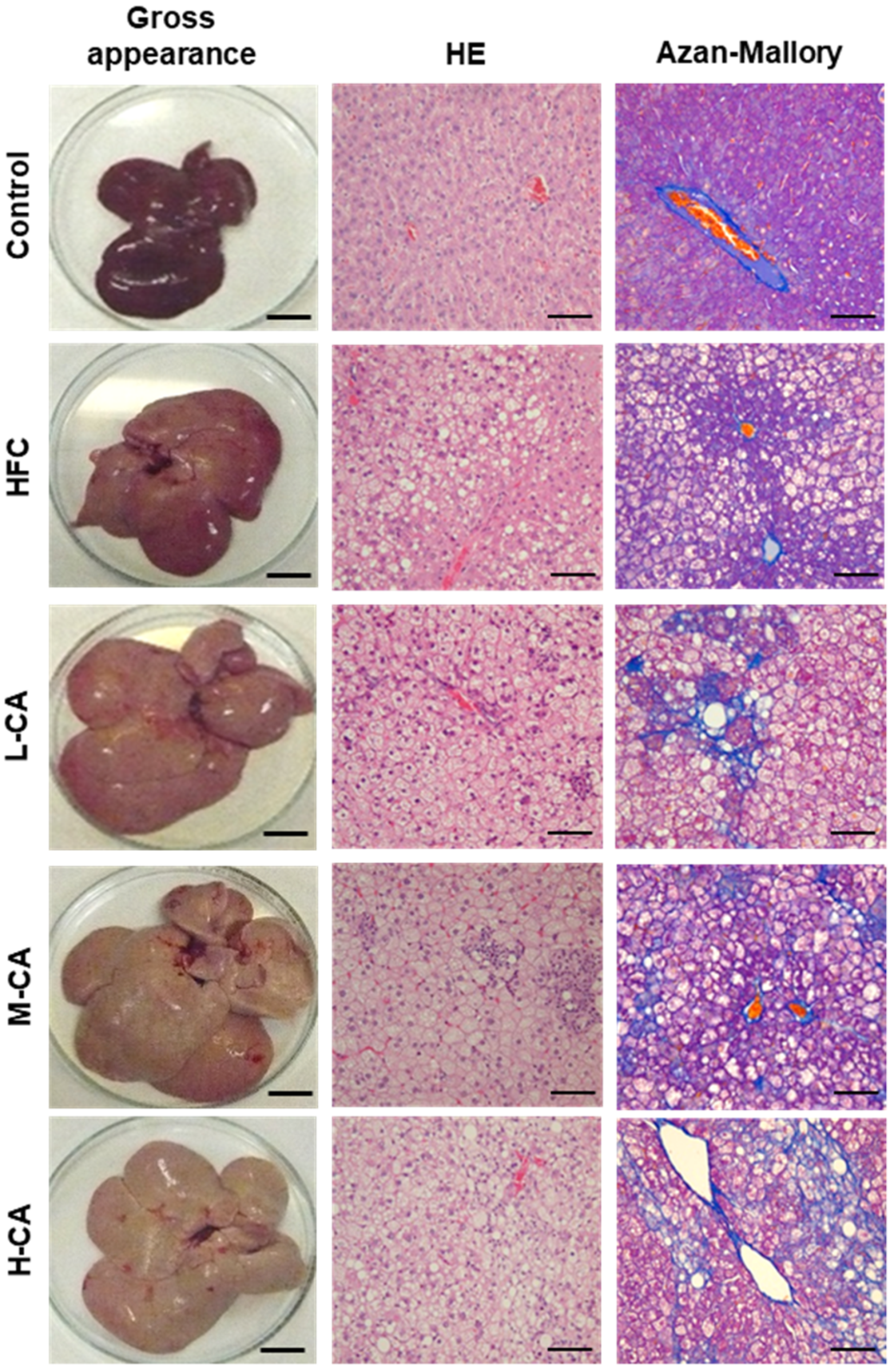


| Group | Control | HFC | L-CA | M-CA | H-CA |
|---|---|---|---|---|---|
| Cumulative energy intake (kcal) | 5711 ± 109 a | 7555 ± 141 b | 7369 ± 169 b,c | 7481 ± 102 b | 6832 ± 98 c |
| Final body weight (g) | 525 ± 11 a | 574 ± 13 a | 584 ± 19 a | 574 ± 18 a | 480 ± 12 b |
| Body weight gain (g) | 202 ± 8 a | 252 ± 10 a | 259 ± 17 a | 254 ± 16 a | 159 ± 13 b |
| Food efficacy (g/kcal) | 0.035 ± 0.001 a | 0.033 ± 0.001 a | 0.035 ± 0.002 a | 0.034 ± 0.002 a | 0.023 ± 0.002 b |
| Liver weight (g) | 17.7 ± 1.5 a | 28.5 ± 1.6 b | 32.7 ± 2.7 b | 42.0 ± 3.3 c | 42.2 ± 2.3 c |
| Liver weight/body weight (%) | 3.4 ± 0.2 a | 5.0 ± 0.2 b | 5.6 ± 0.4 b | 7.3 ± 0.5 c | 8.8 ± 0.2 c |
| Epididymal fat pad weight/body weight (%) | |||||
| 2.1 ± 0.1 a | 2.9 ± 0.2 b | 2.4 ± 0.2 a,b | 2.2 ± 0.3 a,b | 1.0 ± 0.2 c |
| Group/Rat No. | Steatosis | Lobular Inflammation | Hepatocyte Ballooning | NAFLD Activity Score | Fibrosis |
|---|---|---|---|---|---|
| Control-1 | 0 | 0 | 0 | 0 | 0 |
| Control-2 | 1 | 0 | 0 | 1 | 0 |
| Control-3 | 0 | 0 | 0 | 0 | 0 |
| Control-4 | 0 | 0 | 0 | 0 | 0 |
| Control-5 | 0 | 0 | 0 | 0 | 0 |
| HFC-1 | 2 | 2 | 0 | 4 | 0 |
| HFC-2 | 2 | 2 | 0 | 4 | 0 |
| HFC-3 | 2 | 1 | 0 | 3 | 0 |
| HFC-4 | 2 | 1 | 0 | 3 | 0 |
| HFC-5 | 2 | 1 | 0 | 3 | 0 |
| L-CA-1 | 3 | 2 | 0 | 5 | 0 |
| L-CA-2 | 1 | 1 | 0 | 2 | 0 |
| L-CA-3 | 3 | 3 | 0 | 6 | 0 |
| L-CA-4 | 3 | 3 | 0 | 6 | 1 |
| L-CA-5 | 2 | 1 | 0 | 3 | 0 |
| M-CA-1 | 3 | 2 | 0 | 5 | 2 |
| M-CA-2 | 3 | 1 | 1 | 5 | 3 |
| M-CA -3 | 2 | 3 | 0 | 5 | 3 |
| M-CA-4 | 3 | 3 | 1 | 7 | 3 |
| M-CA-5 | 3 | 1 | 0 | 4 | 2 |
| M-CA-6 | 3 | 3 | 0 | 6 | 2 |
| H-CA-1 | 3 | 2 | 0 | 5 | 4 |
| H-CA -2 | 3 | 3 | 0 | 6 | 2 |
| H-CA-3 | 2 | 3 | 2 | 7 | 2 |
| H-CA-4 | 3 | 3 | 2 | 8 | 4 |
| H-CA-5 | 3 | 3 | 1 | 7 | 4 |
| Group | Control | HFC | L-CA | M-CA | H-CA |
|---|---|---|---|---|---|
| Triglyceride (mg/dL) | 83 ± 20 | 70 ± 12 | 69 ± 6 | 76 ± 10 | 49 ± 7 |
| Total cholesterol (mg/dL) | 38 ± 2 a | 36 ± 3 a | 38 ± 5 a | 81 ± 6 b | 132 ± 16 c |
| Free cholesterol (mg/dL) | 16 ± 1 a | 12 ± 1 a | 13 ± 2 a | 30 ± 4 b | 63 ± 5 c |
| Glucose (mg/dL) | 144 ± 2 | 219 ± 41 | 196 ± 55 | 155 ± 11 | 134 ± 6 |
| Insulin (ng/mL) | 5.6 ± 1.1 | 8.7 ± 1.5 | 6.2 ± 0.6 | 6.0 ± 1.5 | 5.7 ± 1.5 |
| Leptin (ng/mL) | 8.1 ± 0.6 a | 15.0 ± 1.3 b | 9.7 ± 1.0 a,c | 5.9 ± 0.9 a,d | 2.6 ± 0.3 d |
| AST (IU/L) | 43 ± 15 a | 67 ± 7 a | 74 ± 7 a | 81 ± 32 a | 146 ± 32 b |
| ALT (IU/L) | 11 ± 4 a | 24 ± 4 a | 24 ± 1 a | 31 ± 9 a | 53 ± 11 b |
| Group | Control | HFC | L-CA | M-CA | H-CA |
|---|---|---|---|---|---|
| ω-MCA (nmol/g liver) | 3.91 ± 0.92 a | 1.68 ± 0.33 a,b | 1.07 ± 0.32 b | 0.99 ± 0.49 b | 0.53 ± 0.13 b |
| α-MCA (nmol/g liver) | 6.52 ± 1.72 | 8.04 ± 2.11 | 6.00 ± 1.92 | 6.25 ± 0.77 | 7.90 ± 1.09 |
| β-MCA (nmol/g liver) | 6.52 ± 1.72 | 47.23 ± 11.10 | 21.57 ± 4.93 | 20.59 ± 5.05 | 17.65 ± 0.93 |
| CA (nmol/g liver) | 74.99 ± 13.91 a,c | 44.13 ± 11.13 a,b,c | 27.23 ± 8.26 b | 33.55 ± 4.16 a,b | 92.80 ± 7.06 a,c |
| UDCA (nmol/g liver) | 2.40 ± 0.46 | 3.83 ± 1.34 | 2.98 ± 0.76 | 3.11 ± 0.61 | 2.81 ± 0.40 |
| HDCA (nmol/g liver) | 0.75 ± 0.12 a | 0.21 ± 0.03 b | 0.11 ± 0.04 b | 0.11 ± 0.06 b | 0.12 ± 0.04 b |
| CDCA (nmol/g liver) | 8.95 ± 1.34 a | 10.97 ± 2.08 a,b | 10.23 ± 1.50 a,b | 10.43 ± 0.83 a,b | 17.04 ± 1.36 b |
| DCA (nmol/g liver) | 18.40 ± 6.01 | 19.61 ± 5.10 | 12.94 ± 2.77 | 27.53 ± 7.41 | 14.55 ± 8.80 |
| LCA (nmol/g liver) | 0.23 ± 0.08 | 0.58 ± 0.21 | 0.11 ± 0.05 | 0.27 ± 0.09 | 0.11 ± 0.07 |
| Total bile acids (nmol/g liver) | 138.55 ± 23.23 | 136.28 ± 30.33 | 82.24 ± 16.76 | 102.83 ± 14.95 | 153.52 ± 16.34 |
| Forward Sequence | Reverse Sequence | |
|---|---|---|
| Col1a1 | GCGTAGCCTACATGGACCAA | AAGTTCCGGTGTGACTCGTG |
| Cpt-1a | ATCGACCGCCATCTCTTCTG | CCATGGCTCAGACAATACCTCC |
| Fasn | CAACATTGACGCCAGTTCCG | TTCGAGCCAGTGTCTTCCAC |
| Gapdh | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
| Mcp-1 | GCAGTTAATGCCCCACTCAC | TTGAGCTTGGTGACAAATACTACAG |
| Mttp | CAAGCTCAAGGCAGTGGTTG | AGCAGGTACATCGTGGTGTC |
| Tgf-β1 | CTTTGTACAACAGCACCCGC | TAGATTGCGTTGTTGCGGTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichimura-Shimizu, M.; Watanabe, S.; Kashirajima, Y.; Nagatomo, A.; Wada, H.; Tsuneyama, K.; Omagari, K. Dietary Cholic Acid Exacerbates Liver Fibrosis in NASH Model of Sprague–Dawley Rats Fed a High-Fat and High-Cholesterol Diet. Int. J. Mol. Sci. 2022, 23, 9268. https://doi.org/10.3390/ijms23169268
Ichimura-Shimizu M, Watanabe S, Kashirajima Y, Nagatomo A, Wada H, Tsuneyama K, Omagari K. Dietary Cholic Acid Exacerbates Liver Fibrosis in NASH Model of Sprague–Dawley Rats Fed a High-Fat and High-Cholesterol Diet. International Journal of Molecular Sciences. 2022; 23(16):9268. https://doi.org/10.3390/ijms23169268
Chicago/Turabian StyleIchimura-Shimizu, Mayuko, Shiro Watanabe, Yuka Kashirajima, Ami Nagatomo, Hitomi Wada, Koichi Tsuneyama, and Katsuhisa Omagari. 2022. "Dietary Cholic Acid Exacerbates Liver Fibrosis in NASH Model of Sprague–Dawley Rats Fed a High-Fat and High-Cholesterol Diet" International Journal of Molecular Sciences 23, no. 16: 9268. https://doi.org/10.3390/ijms23169268






