Optimization of Multilayer Films Composed of Chitosan and Low-Methoxy Amidated Pectin as Multifunctional Biomaterials for Drug Delivery
Abstract
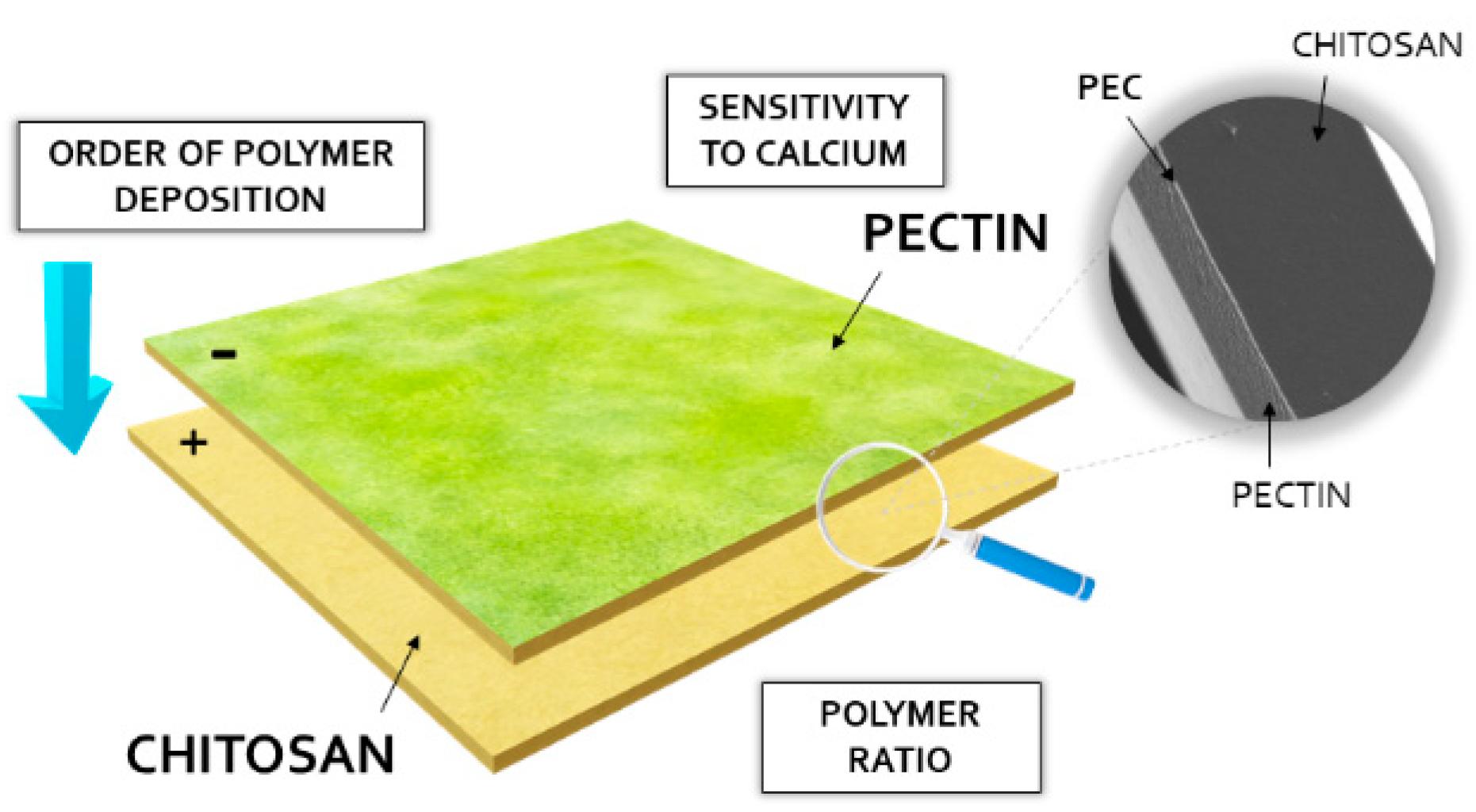
1. Introduction
2. Results and Discussion
2.1. Mechanical Properties
2.2. Films Morphology
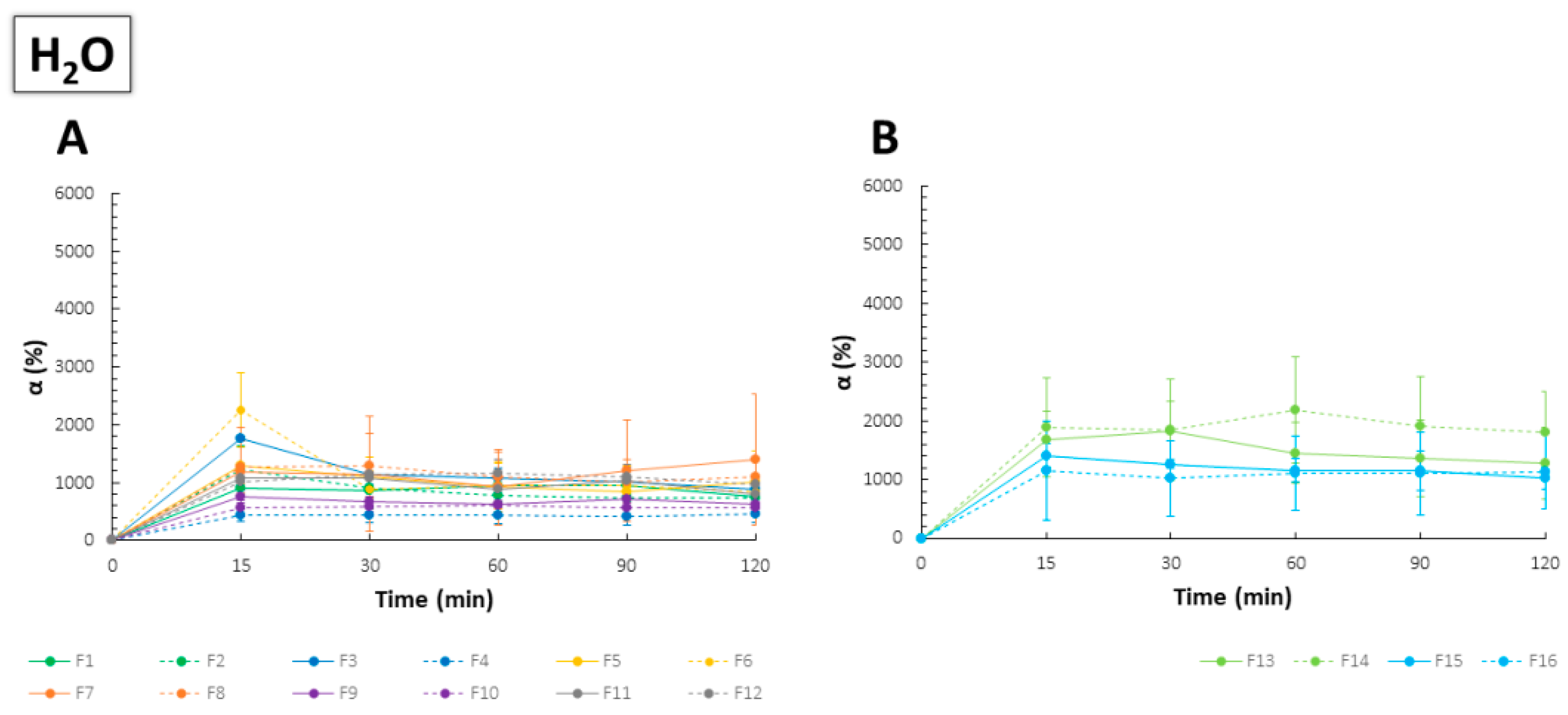
2.3. Swelling Capacity
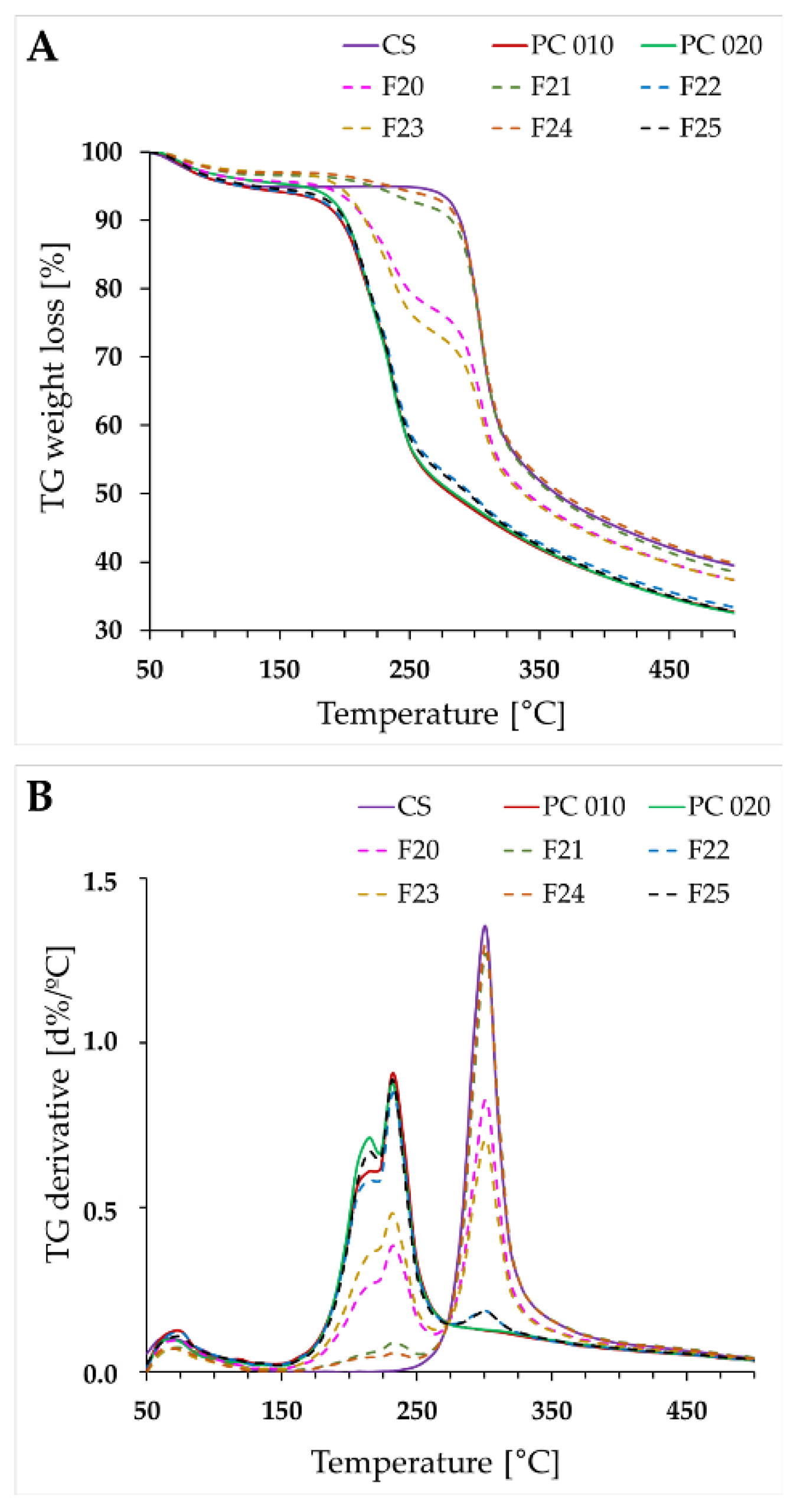
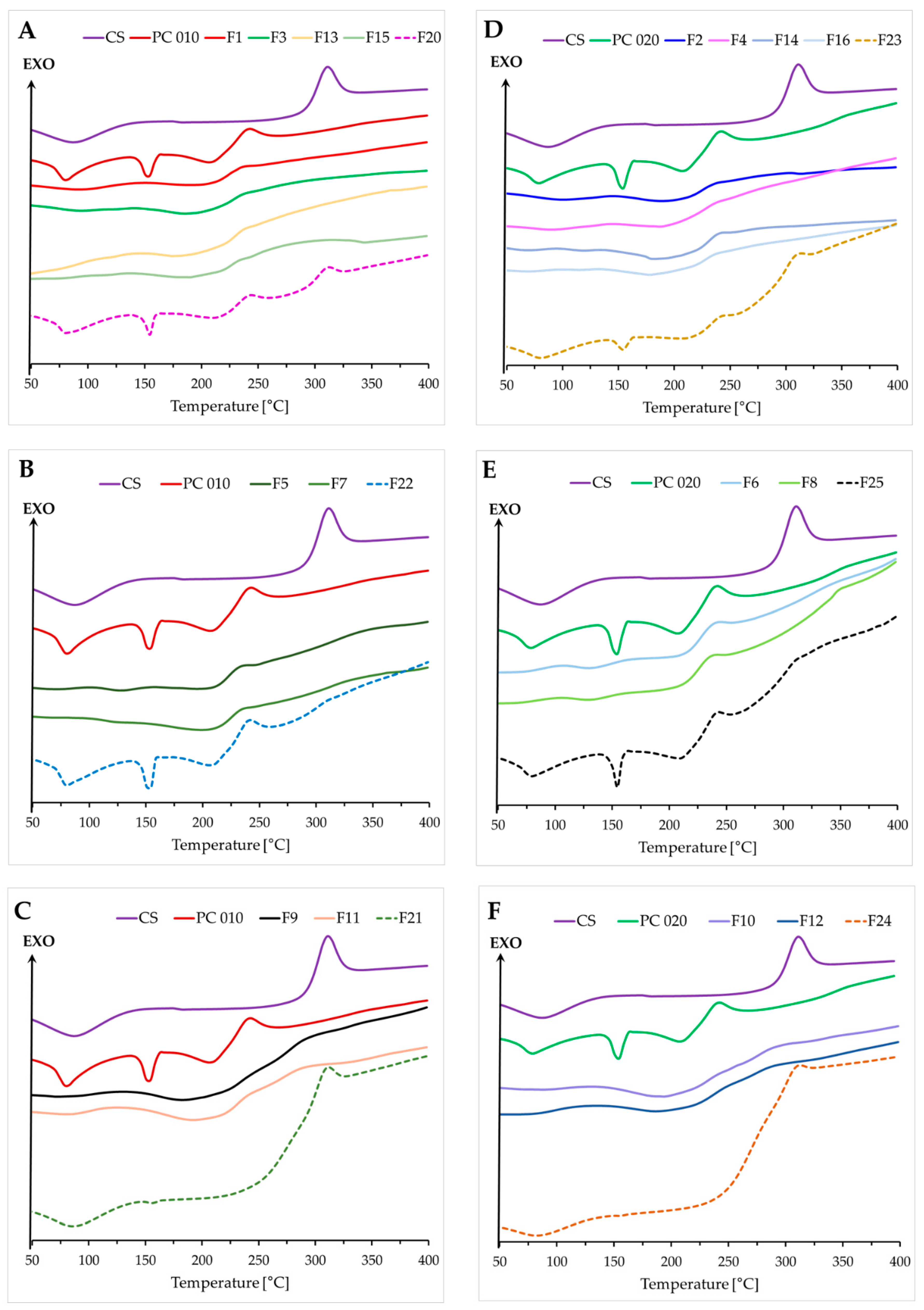
2.4. Thermal Characteristics
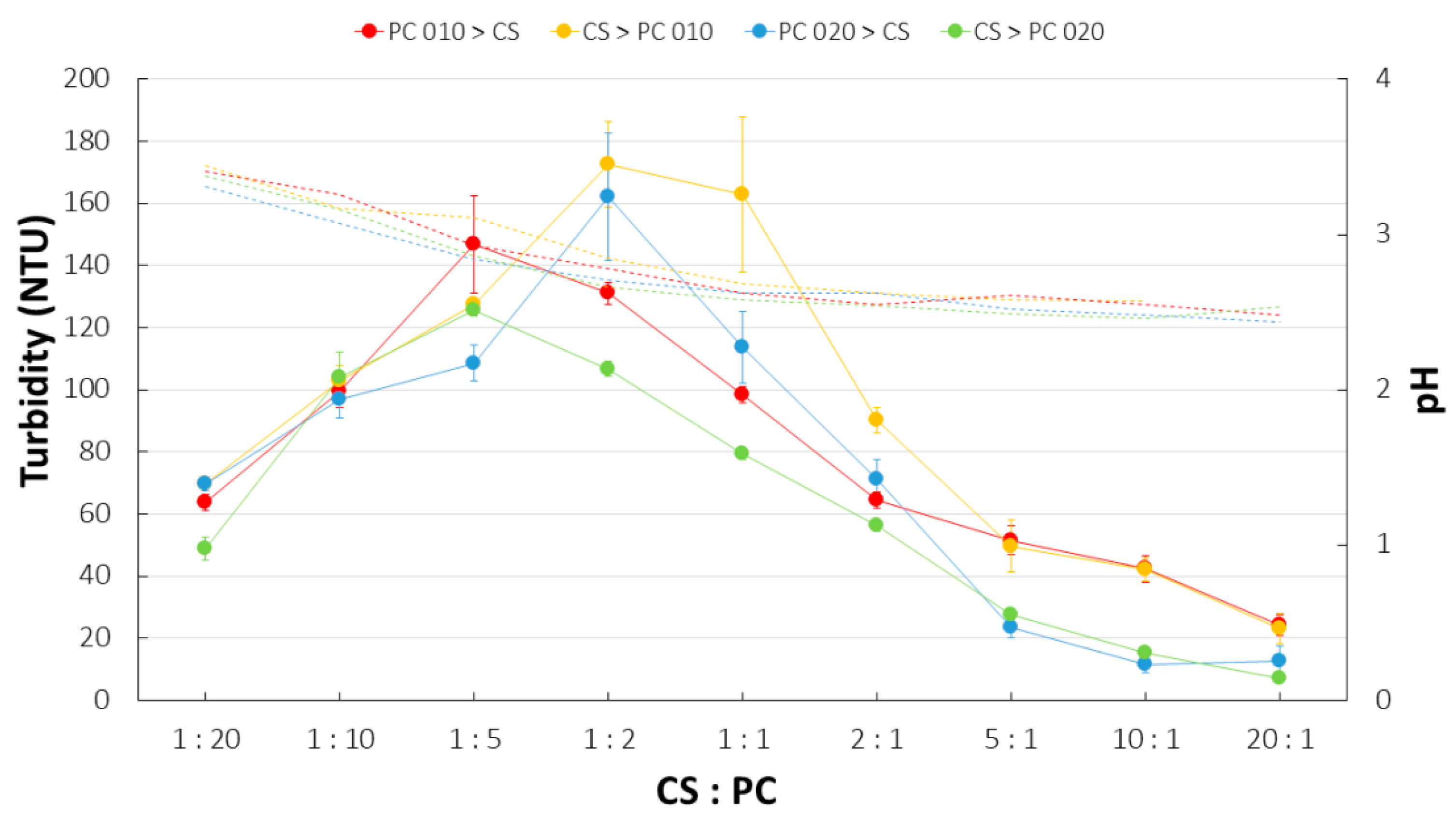
2.5. Turbidity
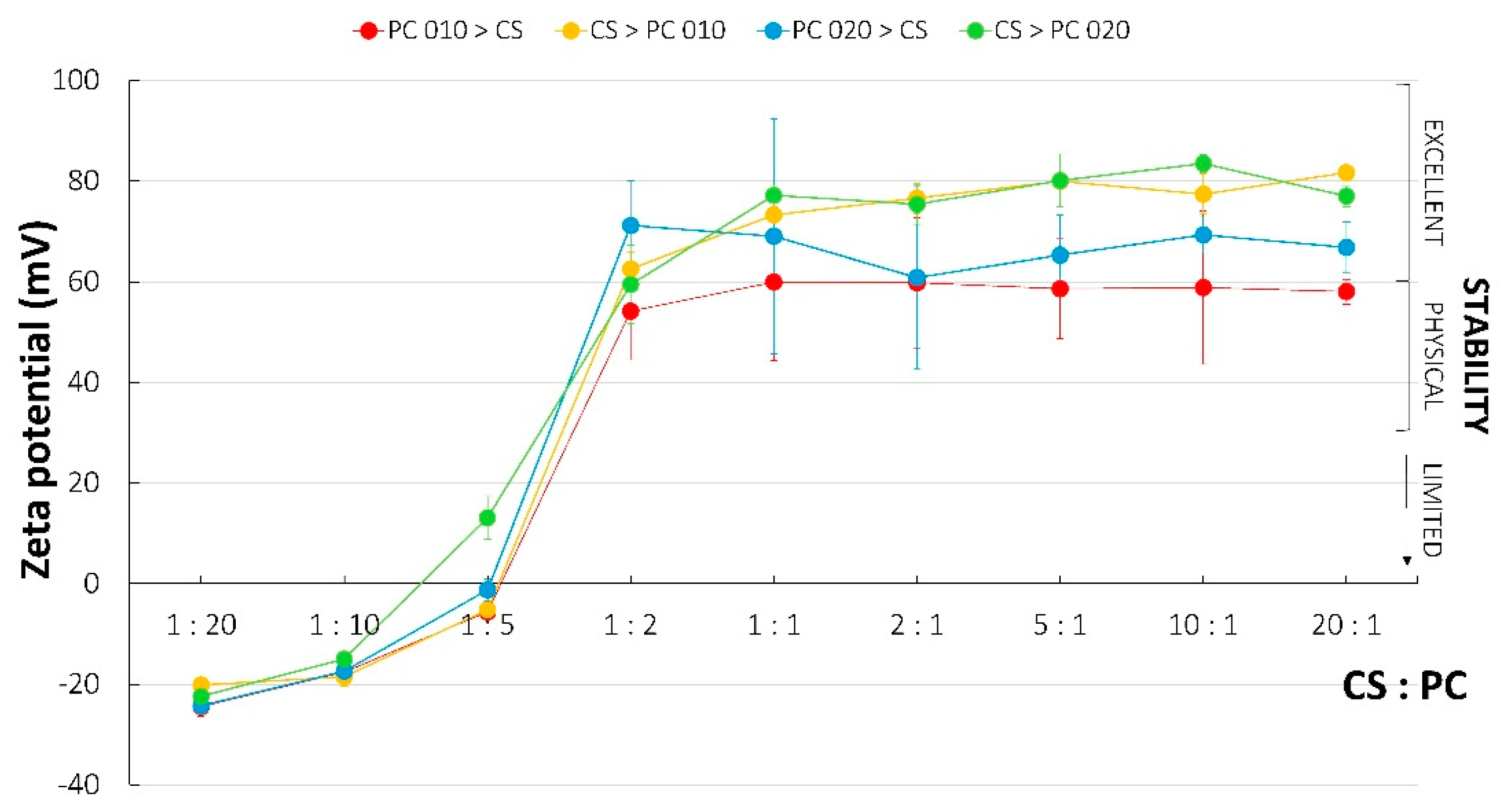
2.6. Zeta Potential
2.7. FTIR Assay
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of the Films
3.2.2. Evaluation of Mechanical Properties
3.2.3. SEM Analysis
3.2.4. Swelling and Disintegration Test
3.2.5. Thermal Analysis
3.2.6. Turbidity Test
3.2.7. Measurements of Zeta Potential
3.2.8. FTIR Analysis
3.2.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potaś, J.; Winnicka, K. The Potential of polyelectrolyte multilayer films as drug delivery materials. Int. J. Mol. Sci. 2022, 23, 3496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Caruso, F.; Dähne, L.; Decher, G.; De Geest, B.G.; Fan, J.; Feliu, N.; Gogotsi, Y.; Hammond, P.T.; Hersam, M.C.; et al. The future of layer-by-layer assembly: A tribute to ACS nano associate editor Helmuth Möhwald. ACS Nano 2019, 13, 6151–6169. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Mijangos, C.; Hernández, R. Polyelectrolyte multilayer films based on natural polymers: From fundamentals to bio-applications. Polymers 2021, 13, 2254. [Google Scholar] [CrossRef] [PubMed]
- Gamzazade, A.I.; Nasibov, S.M. Formation and properties of polyelectrolyte complexes of chitosan hydrochloride and sodium dextransulfate. Carbohydr. Polym. 2002, 50, 339–343. [Google Scholar] [CrossRef]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapall, V.R.M.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Magoń, M.S. Layered polyelectrolyte complexes: Physics of formation and molecular properties. J. Phys. Condens. Matter. 2003, 15, 1781–1808. [Google Scholar]
- Montenegro-Nicolini, M.; Morales, J.O. Overview and future potential of buccal mucoadhesive films as drug delivery systems for biologics. AAPS PharmSciTech 2017, 18, 3–14. [Google Scholar] [CrossRef]
- Chollakup, R.; Smitthipong, W.; Eisenbach, C.; Tirrell, M. Phase behavior and coacervation of aqueous poly(acrylic acid)-poly(allylamine) solutions. Macromolecules 2010, 43, 2518–2528. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Kumar, A.; Vimal, A.; Kumar, A. Why Chitosan? From properties to perspective of mucosal drug delivery. Int. J. Biol. Macromol. 2016, 91, 615–622. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Seo, S.J.; Moon, H.S.; Yoo, M.K.; Park, I.Y.; Kim, B.C.; Cho, C.S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Sundar-Raj, A.A.; Rubila, S.; Jayabalan, R.; Ranganathan, T.V. A review on pectin: Chemistry due to general properties of pectin and its pharmaceutical uses. Sci. Rep. 2012, 1, 550–553. [Google Scholar]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Nižić, L.; Potaś, J.; Winnicka, K.; Szekalska, M.; Erak, I.; Gretić, M.; Jug, M.; Hafner, A. Development, characterisation and nasal deposition of melatonin-loaded pectin/hypromellose microspheres. Eur. J. Pharm. Sci. 2020, 141, 105115. [Google Scholar] [CrossRef] [PubMed]
- Jug, M.; Kosalec, I.; Maestrelli, F.; Mura, P. Development of low methoxy amidated pectin—Based mucoadhesive patches for buccal delivery of triclosan: Effect of cyclodextrin complexation. Carbohydr. Polym. 2012, 90, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, J.; Dong, H.; Li, X.; Zhang, J.; Ramaswamy, S.; Xu, F. Pectin in biomedical and drug delivery applications: A review. Int. J. Biol; Macromol. 2021, 185, 49–65. [Google Scholar] [CrossRef]
- Murata, Y.; Maida, C.; Kofuji, K. Drug release profiles and disintegration properties of pectin films. Materials 2019, 12, 355. [Google Scholar] [CrossRef]
- Akhgari, A.; Farahmand, F.; Afrasiabi Garekani, H.; Sadeghi, F.; Vandamme, T. The effect of pectin on swelling and permeability characteristics of free films containing Eudragit RL and/or RS as a coating formulation aimed for colonic drug delivery. Daru 2010, 18, 91–96. [Google Scholar]
- Singh, S.; Jain, S.; Muthu, M.S.; Tiwari, S.; Tilak, R. Preparation and evaluation of buccal bioadhesive films containing clotrimazole. AAPS Pharm. Sci. Tech. 2008, 9, 660–667. [Google Scholar] [CrossRef]
- Tejada, G.; Barrera, M.G.; Piccirilli, G.N.; Sortino, M.; Frattini, A.; Salomón, C.J.; Lamas, M.C.; Leonardi, D. Development and evaluation of buccal films based on chitosan for the potential treatment of oral candidiasis. AAPS Pharm. Sci. Tech. 2017, 18, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Potaś, J.; Szymańska, E.; Basa, A.; Hafner, A.; Winnicka, K. Tragacanth gum/chitosan polyelectrolyte complexes-based hydrogels enriched with xanthan gum as promising materials for buccal application. Materials 2021, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Potaś, J.; Szymańska, E.; Wróblewska, M.; Kurowska, I.; Maciejczyk, M.; Basa, A.; Wolska, E.; Wilczewska, A.Z.; Winnicka, K. Multilayer films based on chitosan/pectin polyelectrolyte complexes as novel platforms for buccal administration of clotrimazole. Pharmaceutics 2021, 13, 1588. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, D.M.; Laxman, S.D. Buccal mucoadhesive films: A review. Sys. Rev. Pharm. 2017, 8, 31–38. [Google Scholar] [CrossRef]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al-Dhubiab, B.E.; Alhaider, I.A. In vitro techniques to evaluate buccal films. J. Control. Release 2013, 166, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Centkowska, K.; Ławrecka, E.; Sznitowska, M. Technology of orodispersible polymer films with micronized loratadine-influence of different drug loadings on film properties. Pharmaceutics 2020, 12, 250. [Google Scholar] [CrossRef]
- Mishra, R.; Soni, K.; Mehta, T. Mucoadhesive vaginal film of fluconazole using cross-linked chitosan and pectin. J. Therm. Anal. Calorim. 2017, 130, 1683–1695. [Google Scholar] [CrossRef]
- Tejada, G.; Piccirilli, G.N.; Sortino, M.; Salomón, C.J.; Lamas, M.C.; Leonardi, D. Formulation and in vitro efficacy of antifungal mucoadhesive polymeric matrices for the delivery of miconazole nitrate. Mater. Sci. Eng. C 2017, 79, 140–150. [Google Scholar] [CrossRef]
- Saporito, F.; Sandri, G.; Rossi, S.; Bonferoni, M.C.; Riva, F.; Malavasi, L.; Caramella, C.; Ferrari, F. Freeze dried chitosan acetate dressings with glycosaminoglycans and tranexamic acid. Carbohydr. Polym. 2018, 184, 408–417. [Google Scholar] [CrossRef]
- Sakloetsakun, D.; Preechagoon, D.; Bernkop-Schnürch, A.; Pongjanyakul, T. Chitosan-gum arabic polyelectrolyte complex films: Physicochemical, mechanical and mucoadhesive properties. Pharm. Dev. Technol. 2016, 21, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, F.; Bigucci, T.; Cerchiara, B.; Saladini, M.C.; Gallucci, F.; Cruciani, B.; Vitali, B. Luppi, Chitosan/alginate complexes for vaginal delivery of chlorhexidine digluconate. Carbohydr. Polym. 2013, 91, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Kononova, S.V.; Kruchinina, E.V.; Petrova, V.A.; Baklagina, Y.G.; Klechkovskaya, V.V.; Orekhov, A.S.; Vlasova, E.N.; Popova, E.N.; Gubanova, G.N.; Skorik, Y.A. Pervaporation membranes of a simplex type with polyelectrolyte layers of chitosan and sodium hyaluronate. Carbohydr Polym. 2019, 209, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Schlenoff, J.B.; Rmaile, A.H.; Bucur, C.B. Hydration contributions to association in polyelectrolyte multilayers and complexes: Visualizing hydrophobicity. J. Am. Chem. Soc. 2008, 130, 13589–13597. [Google Scholar] [CrossRef]
- Fares, H.M.; Schlenoff, J.B. Diffusion of sites versus polymers in polyelectrolyte complexes and multilayers. J. Am. Chem. Soc. 2017, 139, 14656–14667. [Google Scholar] [CrossRef]
- de Souza, R.F.B.; de Souza, F.C.B.; Moraes, A.M. Analysis of the performance of polysaccharide membranes in aqueous media as a tool to assist wound-dressing selection. J. Appl. Polym. Sci. 2017, 134, 45386–45397. [Google Scholar] [CrossRef]
- Chabala, L.F.G.; Cuartas, C.E.E.; López, M.E.L. Release behavior and antibacterial activity of chitosan/alginate blends with Aloe vera and silver nanoparticles. Mar. Drugs 2017, 15, 328. [Google Scholar] [CrossRef]
- Kassem, A.A.; Ismail, F.A.; Naggar, V.F.; Aboulmagd, E. Preparation and evaluation of periodontal films based on polyelectrolyte complex formation. Pharm. Dev. Technol. 2014, 20, 297–305. [Google Scholar] [CrossRef]
- Ghaffari, A.; Navaee, K.; Oskoui, M.; Bayati, K.; Rafiee-Tehrani, M. Preparation and characterization of free mixed-film of pectin/chitosan/Eudragit((R)) RS intended for sigmoidal drug delivery. Eur. J. Pharm. Biopharm. 2007, 67, 175–186. [Google Scholar] [CrossRef]
- Neufeld, L.; Bianco-Peled, H. Pectin-chitosan physical hydrogels as potential drug delivery vehicles. Int. J. Biol. Macromol. 2017, 101, 852–861. [Google Scholar] [CrossRef]
- Mura, C.; Nácher, A.; Merino, V.; Merino-Sanjuan, M.; Carda, C.; Ruiz, A.; Manconi, M.; Loy, G.; Fadda, A.M.; Diez-Sales, O. N-Succinyl-chitosan systems for 5-aminosalicylic acid colon delivery: In vivo study with TNBS-induced colitis model in rats. Int. J. Pharm. 2011, 416, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Mahbubul, I.M. 3-stability and dispersion characterization of nanofluid. In Micro and Nano Technologies, Preparation, Characterization, Properties and Application of Nanofluid; Mahbubul, I.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 47–112. [Google Scholar]
- Maciel, V.B.V.; Yoshida, C.M.P.; Franco, T.T. Chitosan/pectin polyelectrolyte complex as a pH indicator. Carbohydr. Polym. 2015, 132, 537–545. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopoeial Convention. United States Pharmacopoeia and National Formulary (USP 41—NF 36); United States Pharmacopoeial Convention: Rockville, MD, USA, 2016. [Google Scholar]
- Yüksel, N.; Dinç, E.; Onur, F.; Baykara, T. Influence of swelling degree on release of nicardipine hydrochloride from acrylic microspheres prepared by solvent evaporation method. Pharm. Dev. Technol. 1998, 3, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Strand, A.; Vähäsalo, L.; Ketola, A.; Salminen, K.; Retulainen, E.; Sundberg, A. In-situ analysis of polyelectrolyte complexes by flow cytometry. Cellulose 2018, 25, 3781–3795. [Google Scholar] [CrossRef]
- Jeganathan, B.; Prakya, V.; Deshmukh, A. Preparation and evaluation of diclofenac sodium tablet coated with polyelectrolyte multilayer film using hypromellose acetate succinate and polymethacrylates for pH-dependent, modified release drug delivery. AAPS PharmSciTech 2016, 17, 578–587. [Google Scholar] [CrossRef][Green Version]
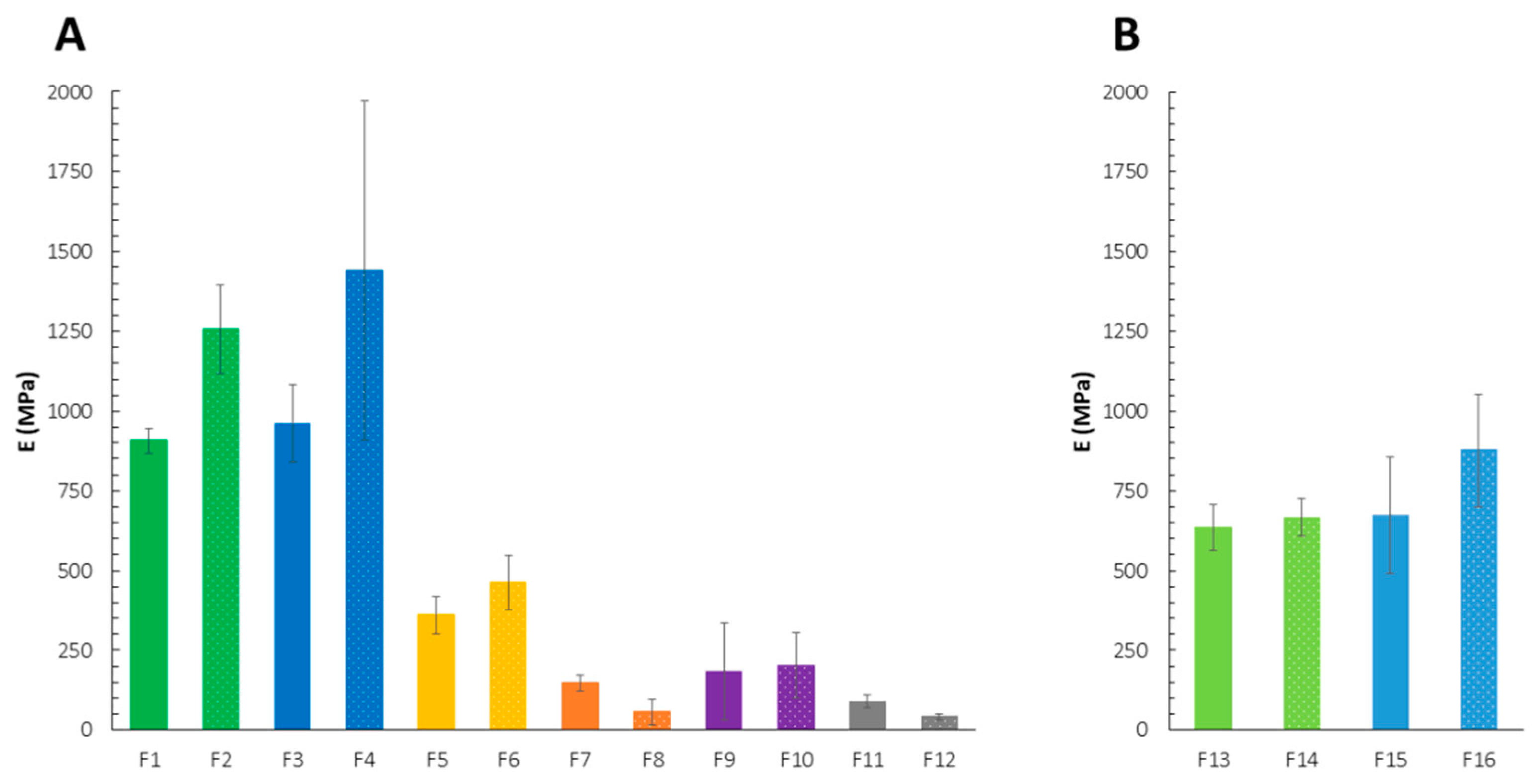
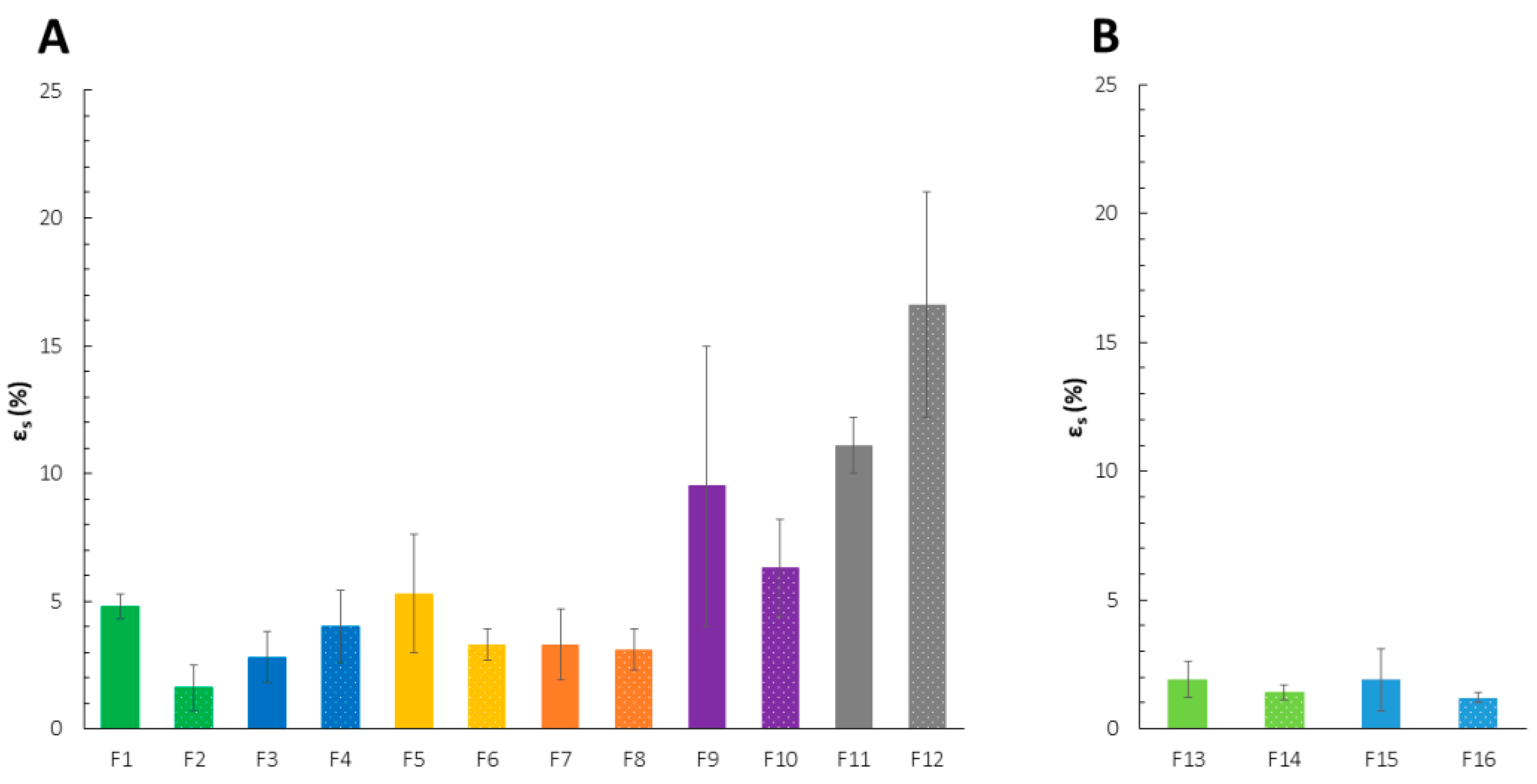
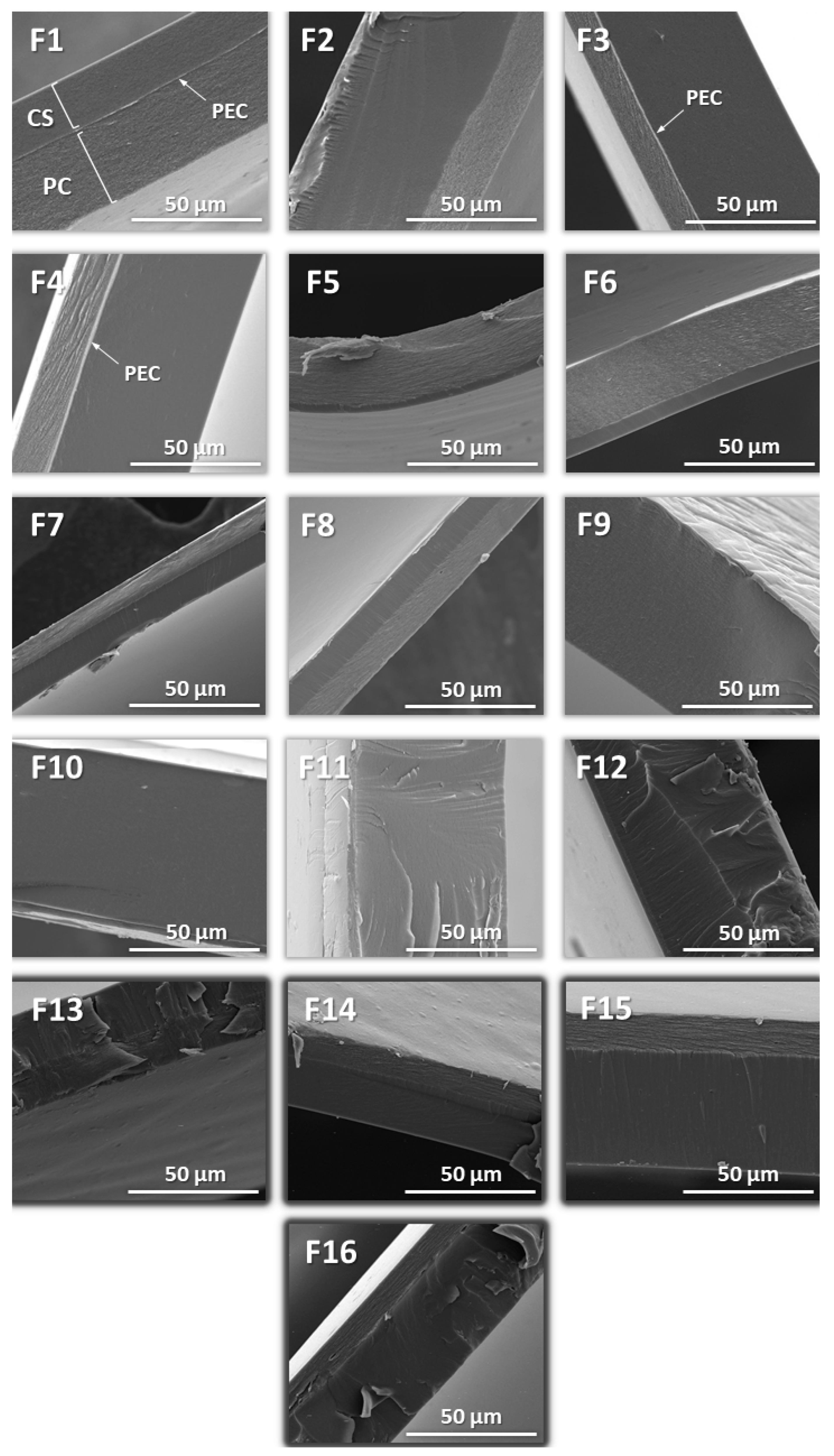
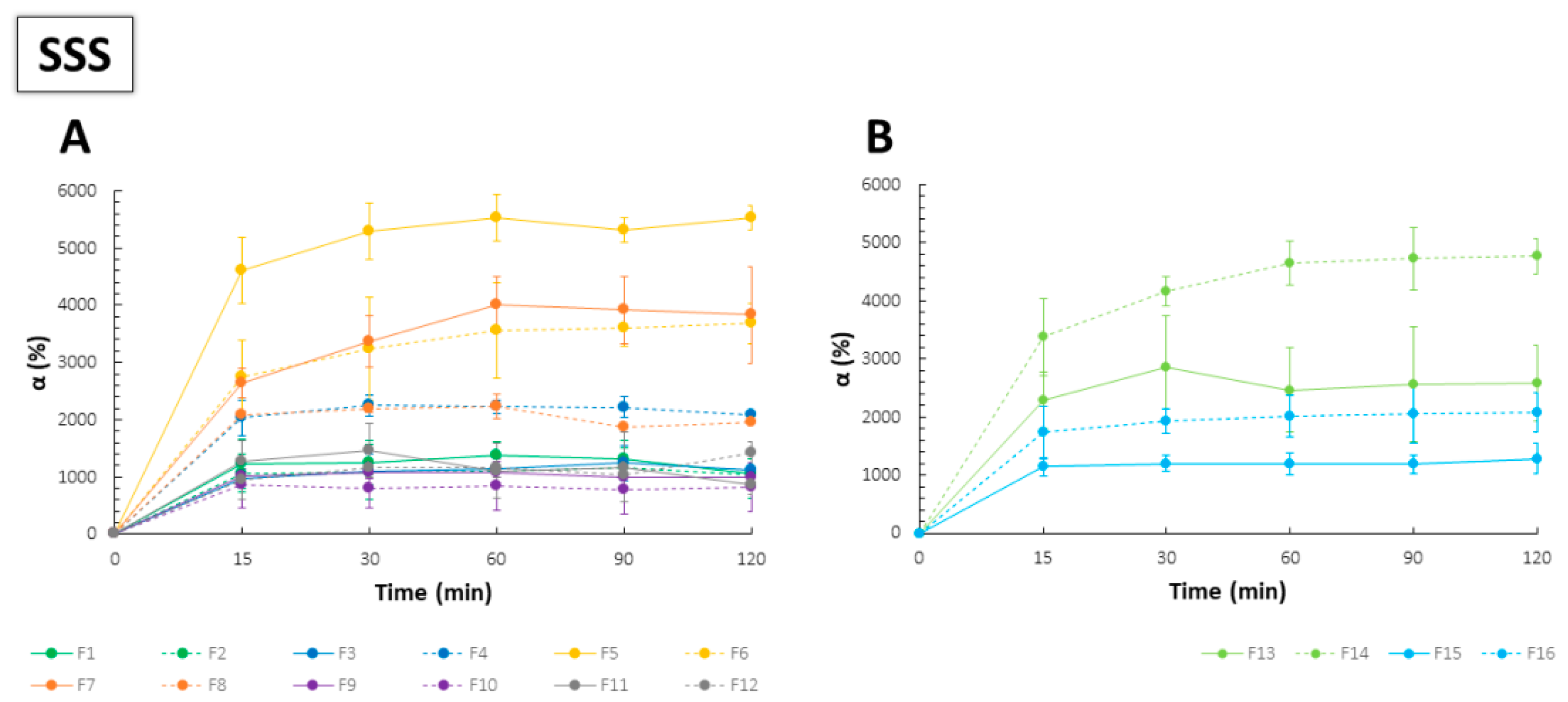
| Formulation | σs (N/mm2) | TR (N) |
|---|---|---|
| F1 | 28.2 ± 0.0 | 27.2 ± 2.7 |
| F2 | 16.6 ± 7.4 | 9.6 ± 2.5 |
| F3 | 22.9 ± 6.1 | 24.6 ± 12.0 |
| F4 | 44.4 ± 11.8 | 28.7 ± 2.9 |
| F5 | 14.8 ± 3.6 | 10.0 ± 2.5 |
| F6 | 13.7 ± 0.2 | 8.0 ± 0.3 |
| F7 | 4.3 ± 1.9 | 4.0 ± 1.4 |
| F8 | 1.5 ± 1.2 | 2.9 ± 0.7 |
| F9 | 9.6 ± 4.3 | 7.8 ± 4.0 |
| F10 | 8.4 ± 3.2 | 6.8 ± 3.5 |
| F11 | 6.9 ± 1.2 | 5.7 ± 0.6 |
| F12 | 6.7 ± 1.8 | 5.1 ± 1.8 |
| F13 | 11.1 ± 3.2 | 6.1 ± 1.9 |
| F14 | 9.8 ± 2.3 | 5.0 ± 0.6 |
| F15 | 15.9 ± 9.5 | 6.0 ± 3.6 |
| F16 | 10.0 ± 0.7 | 6.0 ± 0.4 |
| Formulation | CS:PC | I Layer | II Layer |
|---|---|---|---|
| F1 1 | 1:1 | 2%CS | 2%PC010 |
| F2 1 | 2%CS | 2%PC020 | |
| F3 1 | 2%PC010 | 2%CS | |
| F4 1 | 2%PC020 | 2%CS | |
| F5 | 1:10 | 0.2%CS | 2%PC010 |
| F6 | 0.2%CS | 2%PC020 | |
| F7 | 2%PC010 | 0.2%CS | |
| F8 | 2%PC020 | 0.2%CS | |
| F9 | 10:1 | 2%CS | 0.2%PC010 |
| F10 | 2%CS | 0.2%PC020 | |
| F11 | 0.2%PC010 | 2%CS | |
| F12 | 0.2%PC020 | 2%CS | |
| F13 1 | 1:1 | 1.1%CS | 1.1%PC010 |
| F14 1 | 1.1%CS | 1.1%PC020 | |
| F15 1 | 1.1%PC010 | 1.1%CS | |
| F16 1 | 1.1%PC020 | 1.1%CS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potaś, J.; Wilczewska, A.Z.; Misiak, P.; Basa, A.; Winnicka, K. Optimization of Multilayer Films Composed of Chitosan and Low-Methoxy Amidated Pectin as Multifunctional Biomaterials for Drug Delivery. Int. J. Mol. Sci. 2022, 23, 8092. https://doi.org/10.3390/ijms23158092
Potaś J, Wilczewska AZ, Misiak P, Basa A, Winnicka K. Optimization of Multilayer Films Composed of Chitosan and Low-Methoxy Amidated Pectin as Multifunctional Biomaterials for Drug Delivery. International Journal of Molecular Sciences. 2022; 23(15):8092. https://doi.org/10.3390/ijms23158092
Chicago/Turabian StylePotaś, Joanna, Agnieszka Zofia Wilczewska, Paweł Misiak, Anna Basa, and Katarzyna Winnicka. 2022. "Optimization of Multilayer Films Composed of Chitosan and Low-Methoxy Amidated Pectin as Multifunctional Biomaterials for Drug Delivery" International Journal of Molecular Sciences 23, no. 15: 8092. https://doi.org/10.3390/ijms23158092
APA StylePotaś, J., Wilczewska, A. Z., Misiak, P., Basa, A., & Winnicka, K. (2022). Optimization of Multilayer Films Composed of Chitosan and Low-Methoxy Amidated Pectin as Multifunctional Biomaterials for Drug Delivery. International Journal of Molecular Sciences, 23(15), 8092. https://doi.org/10.3390/ijms23158092









